Abstract
Background
Hypnotics have a role in the management of acute insomnia; however, the efficacy and safety of pharmacological interventions in the management of chronic insomnia is unclear.
Objective
The objective of this paper is to conduct a systematic review of the efficacy and safety of drug treatments for chronic insomnia in adults.
Data Sources
Twenty-one electronic databases were searched, up to July 2006.
Study Selection
Randomized double-blind, placebo-controlled trials were eligible. Quality was assessed using the Jadad scale. Data were pooled using the random effects model.
Data Synthesis
One hundred and five studies were included in the review. Sleep onset latency, as measured by polysomnography, was significantly decreased for benzodiazepines (BDZ), (weighted mean difference: −10.0 minutes; 95% CI: −16.6, −3.4), non-benzodiazepines (non-BDZ) (−12.8 minutes; 95% CI: −16.9, −8.8) and antidepressants (ADP) (−7.0 minutes; 95% CI: −10.7, −3.3). Sleep onset latency assessed by sleep diaries was also improved (BDZ: −19.6 minutes; 95% CI: −23.9, −15.3; non-BDZ: −17.0 minutes; 95% CI: −20.0, −14.0; ADP: −12.2 minutes; 95% CI: −22.3, −2.2). Indirect comparisons between drug categories suggest BDZ and non-BDZ have a similar effect. All drug groups had a statistically significant higher risk of harm compared to placebo (BDZ: risk difference [RD]: 0.15; non-BDZ RD: 0.07; and ADP RD: 0.09), although the most commonly reported adverse events were minor. Indirect comparisons suggest that non-BDZ are safer than BDZ.
Conclusions
Benzodiazepines and non-benzodiazepines are effective treatments in the management of chronic insomnia, although they pose a risk of harm. There is also some evidence that antidepressants are effective and that they pose a risk of harm.
KEY WORDS: drug, treatment, chronic, insomnia
INTRODUCTION
Insomnia, defined as the inability to initiate or maintain sleep or lack of restorative sleep, is common, with an estimated prevalence of 9–12% in adults.1,2 Chronic insomnia has a significant impact on society because it is associated with frequent use of health care services,3,4 chronic health problems,5,6 increased medication use,3,4 and perceived poor health.7 Growing evidence suggests that chronic, unremitting insomnia may predispose individuals to the development of psychiatric disorders and lead to substantial economic burden.2,8,9
Treatment options for chronic insomnia include pharmacological agents and non-pharmacological agents. Pharmacological agents, particularly benzodiazepines (BDZ) and non-benzodiazepines (non-BDZ), are effective in the management of acute insomnia and endorsed by the National Institute of Health10,11, but their role in the management of chronic insomnia is uncertain because of concerns about physical dependence, withdrawal, and rebound insomnia, and long-term safety. The Food and Drug Administration recently approved Eszopiclone (Lunesta), a non-BDZ hypnotic, for management of chronic insomnia in adults. This is the only drug not limited to short-term usage. Much less data are available for antidepressants (ADP).
A number of meta-analyses have evaluated the role of pharmacological agents versus behavioral therapy for insomnia, but only one has focused on chronic insomnia.12–16 Our goal was to conduct a meta-analysis of randomized controlled trials of the efficacy and safety of BDZ, non-BDZ, and ADP in the management of chronic insomnia in adults.
METHODS
Search Strategy
This review is derived from an Evidence Report on manifestations and management of chronic insomnia in adults17 and covered the period up to July 2006. A research librarian conducted a comprehensive search for published literature in 21 electronic databases, including MEDLINE® (1966–2006), EMBASE (1988–2006), CINAHL (1982–2006), PsycINFO (1985–2006), Cochrane Central Register of Controlled Trials (1950–2006), International Pharmaceutical Abstracts (1970–2006), Science Citation Index Expanded (via Web of Science®, 1900–2006), and Biosis Previews (1969–2006). A combination of subject headings and keywords were adapted for each database, based on the following terms: insomnia, sleep initiation, and maintenance disorders, sleep onset delay (or latency), early awakening, sleeplessness, time zone change, jet lag, random, clinical trial, and placebo. We also searched for unpublished trials through ClinicalTrials.gov, Current Controlled Trials, and OCLC PapersFirst. We did not seek unpublished data from pharmaceutical manufacturers. Complete search strategies are available from the original Evidence Report or the corresponding author.
Study Selection
All titles and abstracts identified by the search were screened independently by two reviewers for potential relevance and were retrieved. Two reviewers independently assessed the manuscripts for inclusion using predetermined criteria. To assess the efficacy of drug treatments for chronic insomnia, we included English-language reports of randomized, double-blind, placebo-controlled trials (RCTs) that: (1) involved human adult participants suffering from chronic insomnia; (2) compared a drug treatment to placebo; and (3) reported on at least one of the following outcomes: sleep onset latency (amount of time between lying down to sleep and the onset of sleep); wakefulness after sleep onset (amount of time spent awake in bed following the first attainment of sleep); sleep efficiency (amount of time spent asleep as a percentage of the total time spent in bed); sleep quality (perceived quality of sleep); total sleep time, quality of life, or adverse events. A study population was considered to suffer from chronic insomnia if the majority of participants met at least one of the following criteria: (1) they had a sleep disturbance (either sleep initiation or maintenance problem) of 4 weeks or more; (2) they were described as having a chronic or long-standing or persistent sleep disturbance; and/or (3) they attended a sleep disorders clinic. The 4-week cut point for chronic insomnia was considered long enough to eliminate studies involving transient insomnia and short enough to include studies involving persistent insomnia. To assess the safety of drug treatments for chronic insomnia in adults, we included studies that met the criteria for the efficacy review and reported on adverse events. Disagreements regarding inclusion were resolved through discussion. The primary reason for exclusion of articles was documented.
Quality Assessment
Included studies were assessed for methodological quality using the validated Jadad scale,18 which evaluates randomization, blinding, and reporting of dropouts and withdrawals. This scale provides an overall maximum score of five. In addition, we assessed concealment of allocation19 as “adequate”, “inadequate”, and “unclear”. Two reviewers assessed study quality independently with any disagreements resolved through discussion.
Data Extraction
Data were extracted using a standardized form that captured details of study design, population, intervention, and outcomes. Trained reviewers extracted relevant data, and a second reviewer verified data extracted. Disagreements were resolved through discussion.
Data Analysis
Benzodiazepines act by nonselective activation of the BDZ receptor subtypes of the gamma-aminobutyric acid (GABA) receptor complex. The newer non-BDZ agents are much more selective for the BDZ receptor subtypes (GABAA) and have reportedly fewer side effects. In light of these differences, BDZ and non-BDZ were analyzed separately. Antidepressants were analyzed as a separate group. The data for four sleep outcomes (sleep onset latency, wakefulness after sleep onset, sleep efficiency, and total sleep time) were analyzed based on method of measurement (e.g., polysomnography: an overnight, monitored sleep period in a laboratory, which provides objective measures of sleep and sleep diary: a log of subjective estimates of sleep).
A priori, sleep onset latency was considered to be the primary outcome of the review, as it is an important measure of sleep-initiation insomnia and the most frequently reported outcome in the insomnia literature. Wakefulness after sleep onset was defined as the secondary outcome of the review, as it is an important measure of sleep-maintenance insomnia.
For continuous outcomes (e.g., sleep onset latency), studies were combined using a weighted mean difference (WMD) with the exception of sleep quality and quality of life, for which studies were combined using a standardized mean difference. For dichotomous outcomes (e.g., adverse events), studies were combined using a risk difference. Data were extracted in both the first and second period for all crossover trials. All meta-analyses were performed in RevMan 4.2.5 (Update Software 2004). Point estimates with corresponding 95% confidence intervals were computed for each outcome using the random effects model.
All estimates of efficacy were assessed for heterogeneity using the I-squared statistic.20 For our primary outcome, we explored heterogeneity in subgroup and sensitivity analyses using the following variables: type of drug treatment, presence of psychiatric illness, length of treatment (short-term or long-term defined as ≤4 weeks and >4 weeks, respectively), age (adult 18–65 or elderly >65 years), gender, study quality (low, moderate, or high defined as Jadad scores of 0–1, 2–3, and4–5, respectively). Method of measurement of sleep outcome (polysomnography or sleep diary) was analyzed post hoc. A chi-square statistic was used to test for significant heterogeneity reduction in subgroups.21
While active interventions were compared to placebo in the primary analyses, these interventions were compared by indirect comparisons in a secondary analysis.22
We tested for publication bias on our primary outcome using a funnel plot, both visually and quantitatively, with the rank correlation test,23 bias test,24 and trim and fill method.25
RESULTS
There were 20,086 records identified from database searches and 761 full-length manuscripts assessed for potential inclusion in the review. One hundred and five studies were included in the review: 52 on BDZ, 48 on non-BDZ and 8 studies on antidepressants. Figure 1 describes the flow of studies through the selection process.
Figure 1.
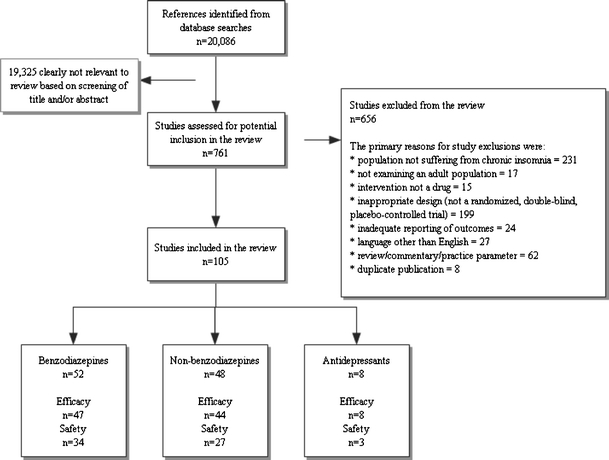
Study selection and retrieval
Study Characteristics
Some studies examined more than one drug group and were included in multiple analyses. Detailed characteristics of each study appear in Tables 1, 2 and 3.
Table 1.
Study Characteristics for Trials Comparing Benzodiazepines and Placebo in Adults with Chronic Insomnia
| First Author/Year | Sample Size, n, enrolled | Mean age ± SD or (range), in years | Number of Females/Males or (Percentage Female/Male) | Psychiatric Illness | Quality Score (Jadad) | Intervention | ||
|---|---|---|---|---|---|---|---|---|
| Drug | Dose, mg | Duration | ||||||
| Aden GC/1983 | 57 | 47 ± NS (23–59) | 29/21 | NS | 2 | Quazepam | 30 | 5 nights |
| Allain H/1998* | 84 | 54.3 ± 11.0 | NS (67.9/32.1) | NS | 4 | Triazolam; zolpidem | 0.125; 10 | 4 nights |
| Beary MD/1984* | 6 | NS (23–35) | 6/0 | NS | 3 | Temazepam | 20 | NS |
| Botter PA/1983† | 40 | Part 1: 1 mg loprazolam: 44.3 ± 8; PL: 40.6 ± 7.7 Part2: 2 mg loprazolam: 46.1 ± 7.2; PL: 44.8 ± 7.1 | 25/15 | Anxiety neoroses | 4 | Loprazolam | 1, 2 | 7 nights |
| Bowen AJ/1978 | 120 | NS (18–60) | 13/5 | NS | 3 | Triazolam | 0.5 | 2 nights |
| Campbell RD/1987 | 71 | 38 ± 2 | 25/31 | NS | 4 | Flurazepam; zopiclone | 30; 7.5 | 3 weeks for each TR |
| Cohn JB/1983 | 53 | 41.5 ± NS (18–60) | 38/15 | Depression | 4 | Triazolam | 0.25 | 4 nights |
| Cohn JB/1984 | 41 | 41.4 ± 10.2 | 18/12 | Various psychiatric conditions | 3 | Triazolam; lorazepam | 0.5; 2 | 4 days for each TR |
| Cohn JB/1991 | 223 | NS (18–65) | NS | NS | 3 | Estazolam; flurazepam | 1, 2; 3 | 7 nights |
| Dominguez RA/1985 | 67 | NS (20–60) | NS | NS | 3 | Brotizolam | 0.25–0.5 | 21 nights |
| Dominguez RA/1986 | 74 | 46.6 ± NS (21–65) | NS (46/54) | NS | 3 | Estazolam; flurazepam | 2; 30 | 7 nights |
| Drake CL/2000 | 93 | Study 1: 41.6 ± 9.5; study 2: 38.1 ± 11.1 | 38/45 | NS | 4 | Zaleplon; triazolam | Study 1: 10, 40; 0.25, study 2: 20, 60; 0.25 | 2 nights for each TR |
| Dujardin K/1998* | 12 | NS (40–62) | 0/12 | NS | 3 | Zolpidem; flunitrazepam | 10; 1 | 3 weeks |
| Elie R/1990* | 44 | 76 ± 1.3 | 33/11 | NS | 3 | Zopiclone; triazolam | 5, 7.5; 0.125; 0.25 | 3 weeks |
| Ferguson JM/1991 | 120 | 43.4 ± 10.9 | NS (56/44) | major depression | 4 | Estazolam | 2 | 7 nights |
| Fillingim JM/1982 | 75 | 81 ± NS (NS) | NS (89/11) | NS | 5 | Temazepam; flurazepam | 30; 30 | 4 nights |
| Fleming J/1995 | 144 | 33–37 ± NS‡(21–60) | NS (48/52)‡ | NS | 3 | Zolpidem; flurazepam | 10, 20; 30 | 3 nights |
| Goethe, JW/1982 | 69 | NS (19–60) | 50/19 | NS | 2 | Quazepam | 15 | 5 nights |
| Hajak G/1994† | 1,507 | 51 ± 11 | 939/566 | NS | 5 | Zopiclone; flunitrazepam triazolam | 7.5; 1.0; 0.25 | 28 days |
| Hartmann E/1983* | 106 | l-tryptophan: 38 ± NS, secobarbital: 41 ± NS, flurazepam: 46 ± NS, PL: 39 ± NS; (18-71) | 48/48 | NS | 3 | l-tryptophan; secobarbital; flurazepam | 1,000; 100; 30 | 7 nights |
| Heidrich H/1981 | 62 | Loremetazepam: 44.6 ± 2; PL: 46 ± 2 | NS (67/33) | NS | 4 | Loremetazepam | 2 | 2 weeks |
| Jacobson AF/1986 | 57 | Brotizolam: 72 ± NS, PL: 69 ± NS; (60–82) | NS | NS | 3 | Brotizolam | 0.125 | 4 nights |
| Lara RH/1983 | 36 | NS (22–65) | 24/12 | NS | 3 | Quazepam | 15 | 5 nights |
| Leppik IE/1997 | 457 | 69 ± NS (59–85) | NS (63/37) | NS | 3 | Zolpidem; triazolam; temazepam | 5; 0.125; 15 | 4 weeks |
| Mamelak M/1987* | 30 | 50 ± NS (32–60) | 21/9 | NS | 4 | Flurazepam; zopiclone | 30; 7.5 | 12 days |
| Mamelak M/1989* | 36 | NS (60–72) | NS | NS | 2 | Brotizolam; flurazepam | 0.25; 15 | 14 nights |
| McAlpine CJ/1984† | 190 | NS (18–94) | 90/57 | NS | 4 | Loprazolam; nitrazepam | 1.0; 5.0 | 7 nights |
| Melo de Paula A/1984 | 60 | 1 mg lormetazepam: 29.3 ± NS (20–55), 2 mg lormetazepam: 30.4 ± NS (21–45), flurazepam: 30.6 ± NS (19–41), PL: 27.9 ± NS (20–41) | 42/16 | NS | 3 | Lormetazepam; flurazepam | 1, 2; 30 | 2 weeks |
| Mendels J/1983 | 80 | Quazepam: 47 ± NS (20–58); PL: 45 ± NS (22–60) | 19/41 | NS | 3 | Quazepam | 15 | 5 nights |
| Minnekeer RJ/1988† | 205 | Quazepam: 53.2 ± 14.5, flunitrazepam: 55.4 ± 12.5, PL: 54.9 ± 13.7 | 130/74 | NS | 4 | Quazepam; flunitrazepam | 15; 2 | 4 weeks |
| Mitler MM/1984* | 21 | Flurazepam: 45.5 ± NS (31–61), traizolam: 43.8 ± NS (27–59), PL: 37.7 ± NS (27–57) | 17/4 | Personality disorder | 3 | Flurazepam; traizolam | 30; 0.5 | 37 nights |
| Morin CM/1999* | 78 | 65 ± 7 | 50/28 | NS | 2 | Temazepam; cognitive/behavioral therapy (alone and combined) | 7.5–30 | 8 weeks |
| Nair NPV/1990† | 60 | 46.9 ± 1.4 | 28/32 | NS | 3 | Zopiclone; flurazepam | 3.75, 7.5, 11.25, 15; 30 | 7 days |
| Reeves RL/1977 | 41 | Triazolam: 68.6 ± NS, flurazepam: 69.6 ± NS, PL: 70.4 ± NS (NS) | 27/14 | NS | 4 | Triazolam; flurazepam | 0.25; 15 | 28 nights |
| Rickels K/1986 | 63 | 46 ± 12 | NS (63/37) | NS | 4 | Brotizolam | 0.5 mg | 2 week |
| Riemann D/2002 | 65 | Lormetazepam: 45.3 ± 10.3, trimipramine: 47.0 ± 10.8, PL: 48.8 ± 11.6 | 23/32 | NS | 3 | Lormetazepam; trimipramine | 1; 25–200 | 28 days |
| Roehrs T/1983* | 12 | 33.3 ± 8.0 | 8/4 | NS | 3 | Brotizolam | 0.25; 0.5 | 1 week |
| Roth T/1979 | 16 | NS (18–65) | 0/16 | NS | 4 | Quazepam | 25 mg | 1 night |
| Roth TG/1997 | 30 | 65.9 ± 4.6 | 15/15 | NS | 2 | Quazepam | 7.5; 15 | 7 nights |
| Sastre-y-Hernandez M/1988 | 60 | NS (20–76) | 36/24 | NS | 4 | Lormetazepam | 1 | 1 week |
| Scharf MB/1990 | 75 | Estazolam: 0.4 ± 13.5, flurazepam: 42.8 ± 13.9, PL: 41.3 ± 13.0 | NS | NS | 4 | Estazolam; flurazepam | 2; 30 | 7 nights |
| Seidel WF/1985* | 12 | NS (21–60) | NS | NS | 2 | Buspirone–triazolam; buspirone–flurazepam; placebo–flurazepam; placebo–triazolam; buspirone–placebo | Placebo or buspirone: 5 at 0900 hours, 5 at 1400 hours, 10 at 2100 hours; 30 flurazepam; 0.5 triazolam | 4 nights |
| Staner L/2005* | 23 | 38.8 ± 2.0 | 14/9 | NS | 2 | Zolpidem; zopiclone; lormetazepam | 10; 7.5; 1 | 8 nights |
| Steens RD/1993* | 24 | 58.2 ± 5.5 | 9/15 | NS | 3 | Zolpidem; triazolam | 5, 10; 0.25 | 1 night for each treatment |
| Stip E/1999* | 60 | 42.6 ± 1.6 | 21/29 | NS | 4 | Zopiclone; temazepam | 7.5; 30 | 3 weeks |
| Tietz EI/1981* | 15 | 41.2 ± 16.8 | 0/15 | NS | 3 | Quazepam | 7.5, 15, 30; 45 | 5 nonconsecutive weeks |
| Tuk B/1997* | 21 | NS (18–78) | 15/6 | NS | 3 | Temazepam | 20 | 2 occasions with at least 1 week between occasions |
| Viukari M/1983* | 39 | Group A: 73.2 ± 2.9, group B: 75.1 ± 1.5 | 20/17 | Various psychiatric conditions | 4 | Flunitrazepam; nitrazepam | 1; 5 | 2 weeks |
| Walsh JK/1984 | 379 | 41.1 ± NS (19–65) | NS (52/48) | NS | 3 | Estazolam | 1; 2 | 7 nights |
| Walsh JK/1998 | 132 | 5 mg zaleplon: 38.9 ± 10.3, 10 mg zaleplon: 39.6 ± 10.0, triazolam: 39.3 ± 11.7, PL: 43.1 ± 9.0 | 77/55 | NS | 4 | Zaleplon; triazolam | 5, 10; 0.25 | 14 nights |
| Winsauer HJ/1984 | 60 | NS (60–90) | 39/21 | NS | 3 | Quazepam | 15 | 5 nights |
| Wu R/2006* | 77 | 38 ± 12 | 41/36 | NS | 2 | Temazepam | 7.5 to 30 | 8 weeks |
Table 2.
Study Characteristics for Trials Comparing Non-benzodiazepines and Placebo in Adults with Chronic Insomnia
| First Author/Year | Sample Size, n enrolled | Mean age ± SD or (range), in years | Number of Females/Males or (Percentage Female/Male) | Psychiatric Illness | Quality Score (Jadad) | Intervention | ||
|---|---|---|---|---|---|---|---|---|
| Drug | Dose, mg | Duration | ||||||
| Allain H/1998* | 84 | 54.3 ± 11.0 | NS (67.9/32.1) | NS | 4 | Triazolam; zolpidem | 0.125; 10 | 4 nights |
| Allain H/2001 | 245 | Zolpidem: 45.6 ± 9.6, PL: 46.7 ± 11.5 | 188/57 | NS | 3 | Zolpidem | 10 | 4 weeks |
| Asnis GM/1999 | 273 | Zolpidem: 41.6 ± 1.2, PL: 41.6 ± 1.0 | 150/40 | Various psychiatric conditions | 3 | Zolpidem | 10 | 4 weeks |
| Campbell RD/1987 | 71 | 38 ± 2 | 25/31 | NS | 4 | Flurazepam; zopiclone | 30; 7.5 | 3 weeks for each TR |
| Chaudoir PJ/1983 | 30 | 50 ± NS (35–65) | 18/7 | NS | 3 | Zopiclone | 7.5 | 7 nights |
| Deacon S/2005* | 26 | NS (18–65) | NS | NS | 3 | Gaboxadol | 5; 15 | 6 nights |
| Declerk A/1999 | 22 | 54 ± NS (NS) | 17/5 | NS | 3 | Zolpidem | 10 | 1 week |
| Drake CL/2000 | 93 | Study 1: 41.6 ± 9.5, study 2: 38.1 ± 11.1 | 38/45 | NS | 4 | Zaleplon; triazolam | Study 1: 10, 40; 0.25, study 2: 20, 60; 0.25 | 2 nights for each TR |
| Dujardin K/1998* | 12 | NS (40–62) | 0/12 | NS | 3 | Zolpidem; flunitrazepam | 10; 1 | 3 weeks |
| Elie R/1990* | 44 | 76 ± 1.3 | 33/11 | NS | 3 | Zopiclone; triazolam | 5, 7.5; 0.125, 0.25 | 3 weeks |
| Elie R/1999 | 615 | 5 mg zaleplon: 42.5 ± 12.9, 10 mg zaleplon: 42.6 ± 12.5, 20 mg zaleplon: 42.6 ± 12.2, 10 mg zolpidem: 44.3 ± 12.5, PL: 42.1 ± 12.0 | 370/204 | NS | 3 | Zaleplon; zolpidem | 5, 10, 20; 10 | 4 weeks |
| Farber R/2006* | 702 | 46 ± NS | 428/274 | NS | 2 | Indiplon | 10; 20 | 3 months |
| Fleming J/1995 | 144 | 33–37 ± NS†(21–60) | NS (48/52) | NS | 3 | Zolpidem; flurazepam | 10, 20; 30 | 3 nights |
| Fry J/2000 | 595 | 5 mg zaleplon: 43 ± 12, 10 mg zaleplon: 40 ± 10, 20 mg zaleplon: 41 ± 13, 10 mg zolpidem: 42 ± 11, PL: 43 ± 12 | 342/244 | NS | 3 | Zaleplon; zolpidem | 5, 10, 20; 10 | 28 nights |
| Gelinas B/1985* | 32 | 40.9 ± 2.19 | 16/10 | NS | 4 | Zopiclone | 7.5 | 3 weeks |
| Goldenberg F/1994 | 524 | Zopiclone: 42.5 ± 8.6; PL: 43.3 ± 9.2 | 291/167 | NS | 4 | Zopiclone | 7.5 | 14 nights—as needed for 4 weeks |
| Hajak G/1994‡ | 1,507 | 51 ± 11 | 939/566 | NS | 5 | Zopiclone; flunitrazepamtriazolam | 7.5; 1.0; 0.25 | 28 days |
| Hedner J/2000 | 437 | 5 mg zaleplon: 72.5 ± 5.9, 10 mg zaleplon: 72.5 ± 6.3, Pl: 72.5 ± 6.8 | 285/137 | NS | 3 | Zaleplon | 5, 10 | 2 weeks |
| Hermann WM/1993 | 25 | NS (25–65) | 9/12 | NS | 3 | Zolpidem | 10 | 2 weeks |
| Jacobs GD/2004* | 63 | TR: 45.4 ± 9.3, PL: 46.6 ± 10.1 (25–64) | 44/19 | NS | 3 | Zolpidem | 10 × 28 nights; 5 × 14 nights | 6 weeks |
| Krystal AD/2003 | 788 | 44 ± 11 | 498/290 | NS | 3 | Eszopiclone | 3 | 6 months |
| Lahmeyer, H/1997 | 178 | 44.9 ± NS (19–61) | 81/64 | NS | 4 | Zolpidem | 10, 15 | 31 nights |
| Lamphere JK/1989* | 12 | 36 ± 10 | 3/9 | NS | 2 | Zopiclone | 2.5, 5.0, 7.5, 10, 15 | 6 weeks |
| Lankford J/2005* | 229 | NS (65–85) | NS | NS | 2 | Indiplon | 15 | 2 weeks |
| Leppik IE/1997 | 457 | 69 ± NS (59–85) | NS (63/37) | NS | 3 | Zolpidem; triazolam; temazepam | 5; 0.125; 15 | 4 weeks |
| Mamelak M/1987* | 30 | 50 ± NS (32–60) | 21/9 | NS | 4 | Flurazepam; zopiclone | 30; 7.5 | 12 days |
| Monchesky TC/1986* | 99 | Zopiclone: 47.1 ± 1.7, PL: 46.6 ± 1.8 | 65/26 | NS | 4 | Zopiclone | 7.5 | 4 weeks |
| Monti JM/1996* | 12 | Zolpidem: 41.2 ± 3.9, PL: 47.3 ± 5.7 | 10/2 | NS | 3 | Zolpidem | 10 | 27 nights |
| Monti JM/2000* | 12 | Zolpidem: 53.8 ± 1.8, Pl: 50.0 ± 5.3 | 12/0 | NS | 3 | Zolpidem | 10 | 15 nights |
| Nair NPV/1990‡ | 60 | 46.9 ± 1.4 | 28/32 | NS | 3 | Zopiclone; flurazepam | 3.75, 7.5, 11.25, 15, 30 | 7 days |
| Perlis ML/2004* | 199 | 41.0 ± 12.8 (18–64) | 141/58 | NS | 3 | Zolpidem | 10 | 12 weeks |
| Scharf MB/1994 | 75 | 38 ± NS (22–60) | 48/27 | NS | 3 | Zolpidem | 10, 15 | 5 weeks |
| Scharf M/2005 | 231 | 72.3 ± 4.9 (65–85) | 133/98 | NS | 2 | Eszopiclone | 1; 2 | 2 weeks |
| Schnitzer T/2005 | 153 | TR: 52.3 ± 8.1, PL: 51.8 ± 9.5 | 133/20 | NS | 3 | Eszopiclone | 3 | 4 weeks |
| Shaw SH/1992‡ | 119 | 10 mg zolpidem: 74.9 ± 1.0, 20 mg zolpidem: 72.9 ± 1.0, PL: 75.7 ± 0.8 | 81/38 | Various psychiatric conditions | 3 | Zolpidem | 10, 20 | 21 days |
| Sivertsen B/200648 | 48 | 60.8 ± 5.4 | 22/24 | NS | 4 | Zopiclone | 7.5 | 6 weeks |
| Soares CN/2005 | 410 | TR: 49.3 ± 4.1, PL: 48.9 ± 3.9 (40–60) | 410/0 | NS | 3 | Eszopiclone | 3 | 4 weeks |
| Staner L/2005* | 23 | 38.8 ± 2.0 | 14/9 | NS | 2 | Zolpidem; zopiclone; lormetazepam | 10; 7.5; 1 | 8 nights |
| Steens RD/1993* | 24 | 58.2 ± 5.5 | 9/15 | NS | 3 | Zolpidem; triazolam | 5, 10; 0.25 | 1 night for each TR |
| Stip E/1999* | 60 | 42.6 ± 1.6 | 21/29 | NS | 4 | Zopiclone; temazepam | 7.5; 30 | 3 weeks |
| Walsh JK/1998 | 132 | 5 mg zaleplon: 38.9 ± 10.3, 10 mg zaleplon: 39.6 ± 10.0, triazolam: 39.3 ± 11.7 | 77/55 | NS | 4 | Zaleplon; triazolam | 5, 10; 0.25 | 14 nights |
| Walsh JK/1998 | 589 | 42 ± NS (21–65) | 193/85 | NS | 3 | Zolpidem; trazodone | 10; 50 | 14 nights |
| Walsh JK/2000* | 54 | 67.5 ± NS (60–79) | 17/31 | NS | 3 | Zaleplon | 2, 5, 10 | 2 nights |
| Walsh JK/ 2000* | 163 | Zolpidem: 43.2 ± 1.2, PL: 45.0 ± 1.3 | 115/48 | NS | 4 | Zolpidem | 10 | 8 weeks |
| Walsh JK/2002* | 365 | Zolpidem: 43.2 ± 1.2, PL: 45.0 ± 1.3 | 115/48 | NS | 3 | Zolpidem | 10 | 4 weeks |
| Walsh JK/2004* | 194 | 40.2 ± 11.8 | 128/66 | NS | 2 | Indiplon | 10; 20 | 5 weeks |
| Walsh JK/2005 | 358 | NS (65–80) | NS | NS | 2 | Indiplon | 5; 10 | 2 weeks |
| Zammit GK/2004 | 308 | TR 2 mg: 40.6 ± 11.5, TR 3 mg: 38 ± 11.7, PL: 40.8 ± 11.8 (21–64) | 199/109 | NS | 3 | Eszopiclone | 3; 3 | 44 nights |
Table 3.
Study Characteristics for Trials Comparing Antidepressants and Placebo in Adults with Chronic Insomnia
| First Author/Year | Sample Size, n, enrolled | Mean Age ± SD or (Range), in years | Number of Females/Males or (Percentage Female/Male) | Psychiatric Illness | Quality Score (Jadad) | Intervention | ||
|---|---|---|---|---|---|---|---|---|
| Drug | Dose, mg | Duration | ||||||
| Haffmans PMJ/1999* | 7 | 44 ± NS (NS) | 3/4 | Previous severe major depression | 4 | Trazodone | 150–250 | 7 nights |
| Hajak G/1996* | 15 | 41.3 ± 9.5 | 3/7 | NS | 3 | Doxepin | 25 | 5 weeks |
| Hajak G/2001 | 47 | Doxepin: 47.6 ± 11.3, PL: 47.4 ± 16.8 | 36/11 | NS | 3 | Doxepin | 25–50 | 4 weeks |
| Hornyak M/2005 | 40 | TR: 45.3 ± 13, PL: 51.3 ± 9.4 | NS | NS | 2 | Doxepin | 25 to 50 | 4 weeks |
| Negri L/1997* | 100 | 42.95 ± 13.22 | 70/30 | Anxiety alone and mild depressive symptoms | 2 | Pivagabine | 900 | 30 days |
| Riemann D/2002 | 65 | Lormetazepam: 45.3 ± 10.3, trimipramine: 47.0 ± 10.8, PL: 48.8 ± 11.6 | 23/32 | NS | 3 | lormetazepam; trimipramine | 1;25–200 | 28 days |
| Rodenbeck A/2003* | 10 | 41.3 ± 9.5 | 3/7 | NS | 3 | Doxepin | 25 | 1 night |
| Walsh JK/1998 | 589 | 42 ± NS (21–65) | 193/85 | NS | 3 | Zolpidem; trazodone | 10, 50 | 14 nights |
All studies were RCTs, and the majority (BDZ: 36/52; non-BDZ: 42/48; ADP: 5/8) had a parallel design with all others described as crossover. Of the studies reporting funding source, the majority of BDZ (20/22) and non-BDZ (27/30) received funds from private sources. Four studies on ADP were funded through private sources. Study quality across drug groups was either moderate (BDZ: 32/52; non-BDZ: 24/48; ADP: 7/8) or high (BDZ: 20/52; non-BDZ: 24/48; ADP: 1/8).
Most studies on BDZ and non-BDZ had a short treatment length (≤4 weeks) and involved non-elderly adults (42/52 and 37/48, respectively). For ADP, six studies involved short-term treatment, and all had populations of non-elderly adults of either gender. Nearly all of the BDZ and non-BDZ studies involved populations of both genders (44/52 and 45/48, respectively). Most of the populations reported in studies did not have a psychiatric disorder (BDZ: 47/52; non-BDZ: 46/48; ADP: 6/8). The mean duration of insomnia was similar for studies on both BDZ and non-BDZ, ranging from 1.1 months to 17.7 years. The mean duration of insomnia was reported in three of eight studies on ADP; ranging from 10.7–11.2 years.
Efficacy
The efficacy analysis included 47, 44, and 8 relevant studies for BDZ, non-BDZ, and ADP, respectively. The combined WMD showed that BDZ, non-BDZ, and ADP had significantly shorter sleep onset latency times compared to placebo when measured by polysomnography (WMD: −10.0 minutes; 95% CI: −16.6, −3.4; WMD: −12.8 minutes; 95% CI: −16.9, −8.8; WMD: −7.0 minutes; 95% CI: −10.7, −3.3, respectively) or sleep diary (WMD: −19.6 minutes; 95% CI: −23.9, −15.3; WMD −17.0 minutes; 95% CI: −20.0, −14.0; WMD: −12.2 minutes; 95% CI: −22.3, −2.2, respectively) (Table 4). The improvements measured by sleep diary were more prominent for all three drug groups. There was heterogeneity among studies on BDZ and non-BDZ for both measures of sleep onset latency times, but the direction of the estimate was fairly consistent for BDZ. Nine out of 11 comparisons had point estimates that favored BDZ for polysomnography, while all 26 sleep diary trials had point estimates that favored BDZ (Fig. 2). For non-BDZ, all 12 studies for polysomnography and all 34 studies for sleep diary showed a point estimate that favored non-BDZ (Fig. 3). For ADP, there was moderate heterogeneity among studies in the polysomnography group and negligible heterogeneity in the sleep diary group (Fig. 4).
Table 4.
Pooled Efficacy Outcomes of Treatment Versus Placebo
| Outcomes | Number of Studies | Point Estimate (95% CI) | Heterogeneity (I2) (%) |
|---|---|---|---|
| Sleep onset latency (WMD) | |||
| Benzodiazepine | |||
| Polysomnography | 11 | −10.0 min (−16.6, −3.4) | 72.6 |
| Sleep Diary | 26 | −19.6 min (−23.9, −15.3) | 55.5 |
| Non-benzodiazepines | |||
| Polysomnography | 12 | −12.8 min (−16.9, −8.8) | 39.3 |
| Sleep Diary | 34 | −17.0 min (−20.0, −14.0) | 64.8 |
| Antidepressants | |||
| Polysomnography | 4 | −7.0 min (−10.7, −3.3) | 34.1 |
| Sleep Diary | 2 | −12.2 min (−22.3, −2.2) | 0 |
| Wakefulness after sleep onset (WMD) | |||
| Benzodiazepine | |||
| Polysomnography | 5 | −16.7 min (−25.3, −8.1) | 0 |
| Sleep Diary | 4 | −39.9 min (−71.0, −8.8) | 68.2 |
| Non-benzodiazepines | |||
| Polysomnography | 3 | −7.0 min (−14.6, 0.7) | 0 |
| Sleep Diary | 12 | −15.0 min (−22.3, −7.7) | 66.5 |
| Antidepressants | |||
| Polysomnography | 2 | −12.2 min (−17.5, −7.0) | 0 |
| Sleep Diary | 1 | −7.1 min (−19.1, 4.9) | NA |
| Sleep efficiency (WMD) | |||
| Benzodiazepine | |||
| Polysomnography | 8 | 7.4% (5.2, 9.6) | 0 |
| Sleep Diary | 5 | 7.9% (3.3, 12.5) | 69.0 |
| Non-benzodiazepines | |||
| Polysomnography | 7 | 4.7% (3.1, 6.2) | 0 |
| Sleep Diary | 4 | 5.0% (1.5, 8.6) | 0 |
| Antidepressants | |||
| Polysomnography | 5 | 13.6% (9.5, 17.7) | 0 |
| Sleep Diary | 0 | NA | NA |
| Total sleep time (WMD) | |||
| Benzodiazepine | |||
| Polysomnography | 9 | 32.7 min (16.0, 49.4) | 66.3 |
| Sleep Diary | 12 | 52.6 min (38.8, 66.5) | 58.9 |
| Non-benzodiazepines | |||
| Polysomnography | 9 | 11.4 min (−0.5, 23.2) | 33.6 |
| Sleep Diary | 25 | 31.5 min (25.6, 37.5) | 54.3 |
| Antidepressants | |||
| Polysomnography | 4 | 79.6 min (48.8, 110.3) | 56.1 |
| Sleep Diary | 1 | −54.3 min (−109.8, 1.2) | NA |
| Sleep quality (SMD) | |||
| Benzodiazepine | 24 | 0.79 SD (0.65, 0.92) | 47.0 |
| Non-benzodiazepines | 23 | 0.47 SD (0.37, 0.56) | 51.3 |
| Antidepressants | 4 | 0.59 SD (0.28, 0.90) | 35.4 |
| Quality of life (SMD) | |||
| Non-benzodiazepines | 2 | 0.38 SD (0.19, 0.57) | 29.0 |
| Adverse events (RD) | |||
| Benzodiazepine | 34 | 0.15 SD (0.10, 0.20) | 69.6 |
| Non-benzodiazepines | 27 | 0.07 SD (0.04, 0.11) | 66.8 |
| Antidepressants | 3 | 0.09 SD (0.01, 0.18) | 0 |
CI Confidence interval, NA not applicable, RD risk difference, SMD standardized mean difference, WMD weighted mean difference
Figure 2.
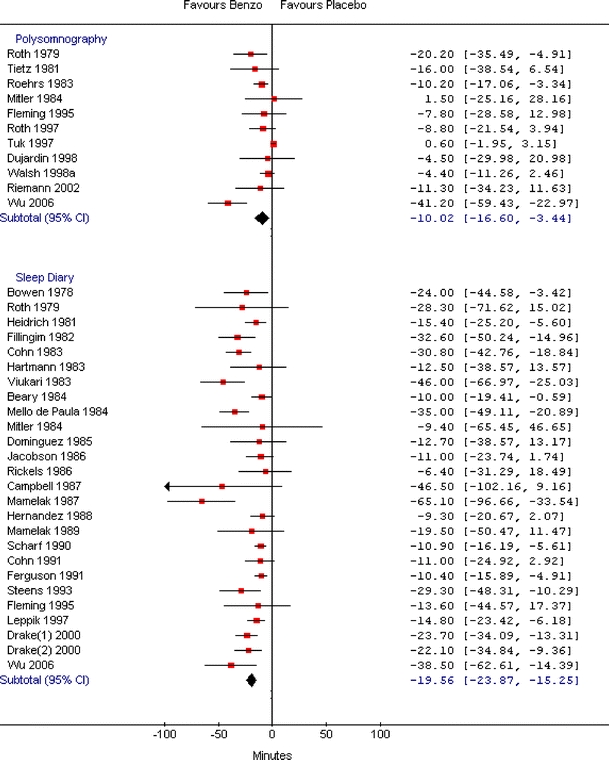
Meta-graph of sleep onset latency (minutes) in adults with chronic insomnia: benzodiazepines versus placebo
Figure 3.
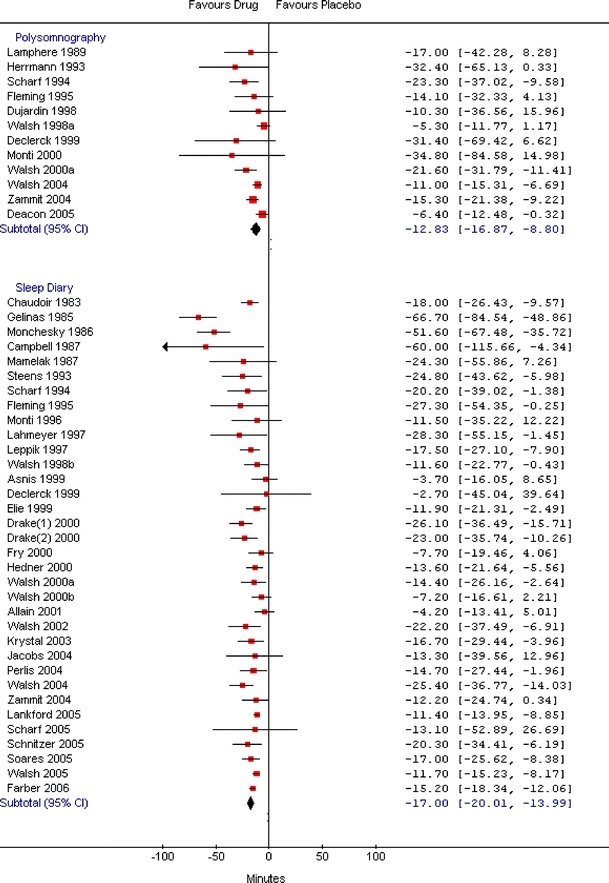
Meta-graph of sleep onset latency (minutes) in adults with chronic insomnia: non-benzodiazepines versus placebo
Figure 4.
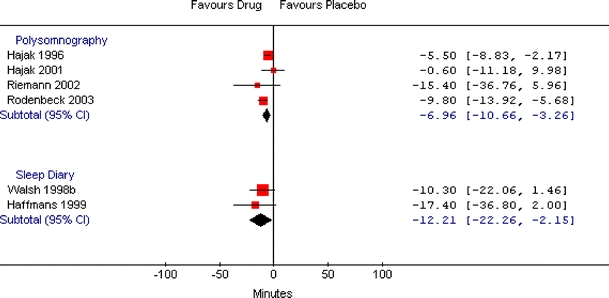
Meta-graph of sleep onset latency (minutes) in adults with chronic insomnia: antidepressants versus placebo
We conducted meta-analyses for wakefulness after sleep onset, sleep efficiency, total sleep time, and sleep quality, subcategorized by polysomnography and sleep diary for BDZ, non-BDZ, and ADP. All results were statistically significant and favored BDZ and non-BDZ with the exception of the polysomnography studies measuring wakefulness after sleep onset and total sleep time, which were marginally nonsignificant (Table 4). In contrast, for ADP, polysomnography results significantly favored ADP, but sleep diary results were fewer and nonsignificantly favored ADP for wakefulness after sleep onset and nonsignificantly favored placebo for total sleep time (Table 4).
Subgroup and Sensitivity Analyses We conducted subgroup analyses of sleep onset latency for drug type, psychiatric illness, length of treatment, age, and gender for BDZ, non-BDZ, and ADP. Heterogeneity was significantly reduced for polysomnography for BDZ studies when subgrouping by type of drug, length of treatment, and gender; while for sleep diary, it was only significantly reduced for type of drug.Heterogeneity was significantly reduced when we subgrouped by age for both polysomnography and sleep diary for non-BDZ studies, although the effects were opposite (elderly patients seemed to benefit more when sleep onset latency was measured by polysomnography, but less when measured by sleep diary). Drug type was the only other subgroup to show a heterogeneity reduction (sleep diary only).There was insufficient data to conduct subgroup analyses for gender and age for ADP studies. There were no significant differences in the effect of ADP among subgroupings of any other category.We also conducted sensitivity analyses based on study quality for each group of drugs, BDZ, non-BDZ, and ADP. This subgrouping of BDZ drugs did not significantly reduce heterogeneity in either polysomnography or sleep diary. Both methods of measurement showed a heterogeneity reduction for non-BDZ, but the effects were opposite (high quality studies showed more benefit when measured with sleep diary, but less benefit when measured by polysomnography). Finally, the subgrouping for ADP did not significantly reduce heterogeneity, and the efficacy estimates were not significantly different between subgroupings.
Safety
To analyze the safety of BDZ, non-BDZ, and ADP, there were 34, 27, and 3 studies included, respectively. The combined risk difference showed that all drug groups had significantly more adverse events than the placebo group (Table 4). There was substantial heterogeneity among studies in the BDZ and non-BDZ groups, but it was negligible in the ADP group. The most commonly reported adverse events in studies were somnolence (n = 27), headache (n = 18), dizziness (n = 16), nausea (n = 11), and fatigue (n = 11) in the BDZ group. There were no reports of falls, injury, or death. In the non-BDZ group, the most commonly reported adverse events were headache (n = 16), dizziness (n = 14), nausea (n = 13), and somnolence (n = 13). Accidental injury was reported in one study; however, there was no significant difference in the frequency of this event between non-BDZ and placebo groups. Finally, for ADP, the most commonly reported adverse events were somnolence (n = 4), headache (n = 3), dizziness (n = 3), and nausea (n = 3). There were no reports of falls, injury, or death.
Indirect Comparisons of the Three Interventions Table 5 shows the results of indirect comparison of the three drug groups. Compared to non-BDZ, BDZ showed a larger benefit on sleep diary measures of sleep onset latency, but non-BDZ was favored when measured by polysomnography—neither value was statistically significant. Non-BDZ was significantly safer than BDZ. Compared to ADP, the only significant result was that non-BDZ was significantly more efficacious in terms of sleep onset latency when measured by polysomnography—the result when measured by sleep diary still favored non-BDZ; but it was not significant.
Table 5.
Indirect Comparisons of Pharmacological Treatment Categories
| Comparison | Difference in SOL (min)/Risk Difference | 95% Confidence Interval (min) | Difference Favors | Significant Difference (Yes or No) |
|---|---|---|---|---|
| Sleep onset latency as measured by polysomnography | ||||
| BDZ versus non-BDZ | 2.8 | −4.9, 10.5 | non-BDZ | No |
| BDZ versus ADP | −3.1 | −10.6, 4.5 | BDZ | No |
| non-BDZ versus ADP | −5.9 | −11.3, −0.4 | non-BDZ | Yes |
| Sleep onset latency as measured by sleep diary | ||||
| BDZ versus non-BDZ | −2.6 | −7.8, 2.7 | BDZ | No |
| BDZ versus ADP | −7.4 | −18.3, 3.6 | BDZ | No |
| non-BDZ versus ADP | −4.8 | −15.3, 5.7 | non-BDZ | No |
| Adverse events | ||||
| BDZ versus non-BDZ | 0.08 | 0.02, 0.14 | non-BDZ | Yes |
| BDZ versus ADP | 0.06 | −0.04, 0.16 | ADP | No |
| non-BDZ versus ADP | −0.02 | −0.12, 0.08 | non-BDZ | No |
ADP Antidepressants, BDZ benzodiazepines, min minutes, non-BDZ non-benzodiazepines, SOL sleep onset latency
DISCUSSION
The review suggests that BDZ and non-BDZ are effective treatments for chronic insomnia, either measured by polysomnography or sleep diary. The analysis also suggests that ADP may have a role in the management of chronic insomnia. The three drug groups pose a risk of harm. The results of the review may be more relevant to the short-term treatment of chronic insomnia because only two studies evaluated long-term efficacy of the treatments. More studies are needed on the long-term efficacy and safety of these agents for chronic insomnia. There seem to be minor differences between drug groups, particularly between BDZ and non-BDZ, but it is difficult to evaluate the clinical importance of these differences because we did not compare drugs according to potency, half-life, or dosage. However, indirect comparisons suggest that non-BDZ are safer than BDZ.
There was strong evidence of publication bias in the pooled estimates for sleep onset latency in the BDZ category of intervention by the graphical test24 and visual inspection of the funnel plot,26 and for the non-BDZ category of intervention by the rank correlation test,23 graphical test,24 and visual inspection of the funnel plot.26 We were not able to assess publication bias in the ADP group. The majority of studies received funds from private sources (51/56), suggesting that negative results were less likely to be published. Thus, the true estimate of efficacy may be lower than the estimate calculated in the current analysis.
The majority of studies included in the review investigated hypnotics, the most commonly prescribed class of medication for insomnia. Our finding that these drugs are effective treatments for chronic insomnia is consistent with other meta-analyses.12–15 Three meta-analyses reporting effect sizes found reliable improvements in sleep parameters using hypnotics in patients with chronic insomnia.12,14,15 Holbrook et al. found that BDZ decreased sleep latency and increased sleep duration, although the latter effect was not statistically significant.13 Data show modest to poor correlations between subjective reports and objective findings in insomnia research. The tendency is to overestimate sleep latency and underestimate total sleep time.1 However, our analysis showed no statistically significant difference in results based on method of measurement (polysomnography versus sleep diary). Another meta-analysis comparing effectiveness of newer non-BDZ and BDZ reported consistent differences between drugs,16 in contrast to our analyses. Despite the heterogeneity in the pooled estimates for BDZ and non-BDZ in the current review, virtually all of the studies favored the intervention over placebo.
Compared to BDZ and non-BDZ, there were substantially fewer studies on ADP, although the review provides some evidence that ADP, particularly doxepin and trazadone, may be effective treatments for chronic insomnia. Despite the paucity of data with respect to the safety and efficacy of ADP relative to BDZ and non-BDZ, these agents are prescribed with increasing frequency for insomnia compared to hypnotics.27 Further studies are needed to establish long-term safety and efficacy to determine if they are equivalent to BDZ and non-BDZ.
The BDZ, non-BDZ, and ADP had a significantly greater risk of harm than placebo. The adverse events most commonly reported among studies included headache, drowsiness, dizziness, and nausea. Medications for insomnia are often used in the elderly, and BDZ have been shown to increase the risk of injury and decrease cognitive function in this group.13,15,28 None of the studies addressed safety issues related to concurrent medication use in the elderly treated for insomnia, which may be worth exploring in future studies. In the current review, we analyzed overall adverse events, rather than specific adverse events such as tolerance and rebound insomnia. However, another meta-analysis reviewing the effects of hypnotics on rebound insomnia and tolerance suggests that pharmacological profiles of medications are important considerations with respect to side effects, and insufficient data for some agents did not allow for conclusions to be drawn regarding their long-term safety.29
The results of subgroup analyses with respect to the relationship between method of measurement and effect estimates were inconsistent across treatment categories: although the effect of BDZ was more pronounced in the same direction, favoring medication, over placebo, when measured by sleep diary compared to polysomnography. No significant difference was found in the effect of either the non-BDZ or ADP with respect to the method of outcome measurement. This finding may point to underpowered analyses or a lack of evidence of a true relationship between treatment effect and measurement method in these studies.
The results of this review must be interpreted with caution when applying the evidence to clinical practice for several reasons. Although it appears that these results are generalizable to insomnia patients, translating these findings to the clinical setting is not straightforward. Patients with medical or psychiatric comorbidities were excluded in most of the studies. However, in clinical practice, many insomnia patients have either medical or psychiatric comorbidity; thus, it is difficult to extrapolate these findings to these populations. In addition, this meta-analysis also was not able to answer the clinically relevant question of the long-term effects of these medications. Furthermore, the drugs were analyzed in groups irrespective of their differences in half life, potency or dosage, and direct comparisons between drugs were not made. Nevertheless, the data show that sleep parameters do improve with the use of these agents. Additional large-scale randomized trials are needed to determine the efficacy and safety of these interventions across various subsets of the chronic insomnia population. In addition, more studies are needed to explore the long-term efficacy and safety of these drugs because the current results may be relevant to short-term treatment of insomnia only.
Limitations of this review include the lack of identification of unpublished data, such as data from pharmaceutical manufacturers. Furthermore, outcomes other than sleep onset latency were under-reported in the insomnia literature, despite the relevance of outcomes such as wakefulness after sleep onset, night wakings, and sleep quality to this condition.
Further research related to treatment of chronic insomnia in adults should address (1) the effect of drug treatments for chronic insomnia on quality of life and daytime functioning, (2) the safety and efficacy of pharmacotherapy for chronic insomnia in high risk or vulnerable groups such as the elderly, (3) the use of a consistent definition of chronic insomnia to determine prevalence rates and compare treatment effects among studies, (4) the long-term efficacy and safety of drug treatments for the management of chronic insomnia, and (5) the development of a threshold for a clinically significant treatment effect in the management of chronic insomnia, such that statistically significant findings can be put into clinical context.
Acknowledgment
We are grateful to members of the technical expert panel for providing input on the direction of the review.
Financial Support This study was conducted under contract to the Agency for Healthcare Research and Quality (Contract No.C400_000_021), Rockville, MD., funded by the Office of Medical Applications of Research, National Institutes of Health, Bethesda, MD. The study was presented at the NIH State_of_the_Science Conference on Manifestations and Management of Chronic Insomnia in Adults in June 2005.
Disclaimer The authors of this article are responsible for its contents, including any clinical or treatment recommendations. No statement in this article should be construed as an official position of the Agency for Healthcare Research and Quality, the Office of Medical Applications of Research or the U.S. Department of Health and Human Services.
Conflict of Interest The corresponding author declares, on behalf of all authors, that there are no conflicts of interest to disclose.None disclosed
Footnotes
NB participated in writing the proposal; planning, overseeing, and contributing to the systematic review process; and writing and editing the manuscript.
BV participated in writing the proposal, contributed to the systematic review process, performed all statistical analyses, and participated in writing and editing the manuscript.
CF planned and conducted the search strategy, wrote the literature search section and related appendices, and reviewed and edited the manuscript.
LB participated in data extraction, data verification and quality assessment; creation of evidence tables; formatting and editing the manuscript; and data management.
MT participated in data extraction, data verification, and quality assessment.
MO participated in data extraction, data verification, and quality assessment.
TPK participated in developing the proposal and provided methodological expertise.
MW participated in writing the proposal, contributed to the systematic review process, provided clinical expertise, and participated in writing the manuscript.
References
- 1.Sateia MJ, Doghramji K, Hauri P, Morin CM. Evaluation of chronic insomnia. An American Academy of Sleep Medicine Review. Sleep 2000;23:1–66. [PubMed]
- 2.Ford D, Kamerow D. Epidemiological study of sleep disturbances and psychiatric disorders: an opportunity for prevention. JAMA 1989;262:1479–84. [DOI] [PubMed]
- 3.Simon GE, VonKorff M. Prevalence, burden, and treatment of insomnia in primary care. Am J Psychiatry. 1997;54:1417–23. [DOI] [PubMed]
- 4.Leger D, Guilleminault C, Bader G, Levy E, Paillard M. Medical and socio-professional impact of insomnia. Sleep 2002;25:625–9. [PubMed]
- 5.Ohayon M. Epidemiological study on insomnia in the general population. Sleep 1996;19(3 suppl):S7–15. [DOI] [PubMed]
- 6.Roberts RE, Shema SJ, Kaplan GA. Prospective data on sleep complaints and associated risk factors in an older cohort. Psychosom Med. 1999;61:188–96. [DOI] [PubMed]
- 7.Foley DJ, Monjan A, Simonsick EM, Wallace RB, Blazer DG. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep 1999;22(suppl 2):S366–72. [PubMed]
- 8.Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbances and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411–18. [DOI] [PubMed]
- 9.Wiessman MM, Greenwald A, NinoMurcia G, Dement WC. The morbidity of insomnia uncomplicated by psychiatric disorders. Gen Hosp Psychiatry. 1997;19:245–50. [DOI] [PubMed]
- 10.NIH 1983. Drugs and Insomnia: The Use of Medications To Promote Sleep. NIH Consens Statement Online 1983 Nov 5–17 [cited 2005 08 29];4(10):1–19.
- 11.NIH 1990. The Treatment of Sleep Disorders of Older People. NIH Consens Statement Online 1990 Mar 26–28 [cited 2005 08 29];8(3):1–22. [PubMed]
- 12.Nowell PD, Mazumdar S, Buysse DJ, Dew MA, Kupfer DJ. Benzodiazepines and zolpidem for chronic insomnia: meta-analysis of treatment efficacy. J Am Med Assoc. 1997;278:2170–7. [DOI] [PubMed]
- 13.Holbrook AM, Crowther R, Lotter A, Cheng C, King D. Meta-analysis of benzodiazepine use in the treatment of insomnia. Can Med Assoc J. 2000;162:225–33. [PMC free article] [PubMed]
- 14.Smith MT, Perlis ML, Park A, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159:5–11. [DOI] [PubMed]
- 15.Glass J, Lanctot K, Hermmnn N, Sproule BA, Busto U. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ 2005;331:1169. [DOI] [PMC free article] [PubMed]
- 16.Dunbar Y, Dodd S, Strobl J, Boland A, Dickson R, Wally T. Comparative efficacy of newer hypnotic agents for the short-term management of insomnia: a systematic review and meta-analysis. Human Psychopharmacol Clin Exp. 2004;19:305–322. [DOI] [PubMed]
- 17.Buscemi N, Vandermeer B, Friesen C, et al. Manifestations and management of chronic insomnia in adults. Evid Rep Technol Assess (Summ). 2005 Jun;(125):1–10. [DOI] [PMC free article] [PubMed]
- 18.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. [DOI] [PubMed]
- 19.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Am Med Assoc. 1995;273:408–12. [DOI] [PubMed]
- 20.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. [DOI] [PubMed]
- 21.Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Eggar M, Smith GD, Altman DG, eds. Systematic Reviews in Health Care: Meta-analysis in context. 3rd ed. London: BMJ Publishing Group; 2001:285–312.
- 22.Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683–691. [DOI] [PubMed]
- 23.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [DOI] [PubMed]
- 24.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–34. [DOI] [PMC free article] [PubMed]
- 25.Duval S, Tweedie R. A nonparametric “tim and fill” method of accounting for publication bias in meta-analysis. J Am Med Assoc. 2000;95:89–98.
- 26.Light RJ, Pillemer DB. Summing up. The science of reviewing research. Cambridge, MA: Harvard University Press; 1984.
- 27.Walsh JK, Schweitzer PK. Ten-year trends in the pharmacological treatment of insomnia. Sleep 1999;22(3):371–5. [PubMed]
- 28.Panneman MJ, Goettsch WG, Kramarz P, Herings RM. The costs of benzodiazepine-associated hospital-treated fall Injuries in the EU: a Pharmo study. Drugs Aging. 2003;20:833–9. [DOI] [PubMed]
- 29.Soldatos CR, Dikeos DG, Whiehead A. Tolerance and rebound insomnia with rapidly eliminated hypnotics: a meta-analysis of sleep laboratory studies. Int Clin Psychopharmacol. 1999;14(5):287–303. [PubMed]


