Abstract
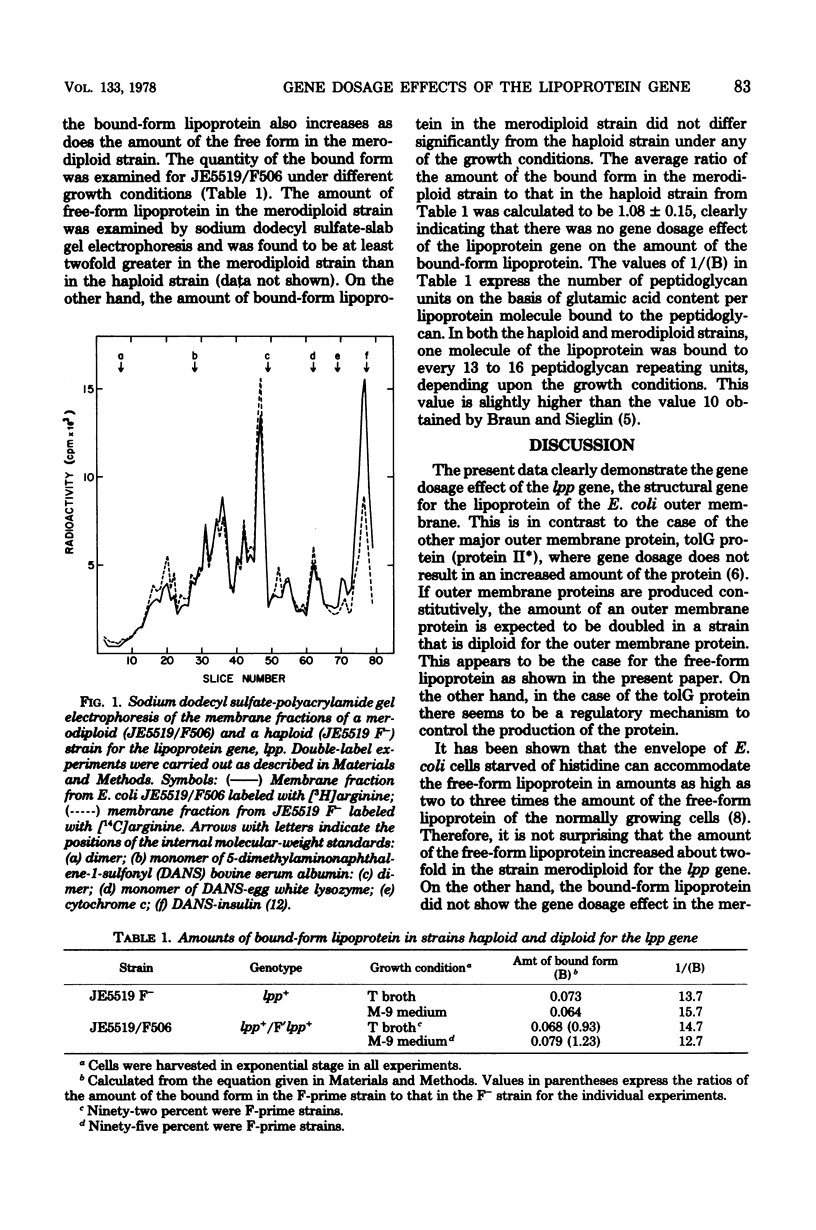
The gene dosage effects of the structural gene (lpp) for the lipoprotein of the Escherichia coli outer membrane were examined. A novel F-prime factor containing the lpp gene was constructed. The amount of the free-form lipoprotein in the merodiploid strain carrying the F-prime factor was found to be about two times as great as that in the corresponding haploid strain. On the other hand, the amount of the bound-form lipoprotein, which is vovalently linked to the peptidoglycan, was not significantly different in the merodiploid strain as compared with the corresponding haploid strain. The present results suggest that the lpp gene is expressed constitutively in contrast to another major protein of the E. coli outer membrane, tolG protein (protein II, D. B. Datta et al., J. Bacteriol. 128:834-841, 1976). The F-prime factor isolated may include a portion of the E. coli chromosome (located between 33 and 36 min on the genetic map) that is not covered by any other F-prime factor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyersmann D., Messer W., Schlicht M. Mutants of Escherichia coli B-r defective in deoxyribonucleic acid initiation: dnaI, a new gene for replication. J Bacteriol. 1974 Jun;118(3):783–789. doi: 10.1128/jb.118.3.783-789.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbeer C., Woodrow M. L., Khalifah L. I. Transport of vitamin B12 in Escherichia coli: common receptor system for vitamin B12 and bacteriophage BF23 on the outer membrane of the cell envelope. J Bacteriol. 1976 Mar;125(3):1032–1039. doi: 10.1128/jb.125.3.1032-1039.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Sieglin U. The covalent murein-lipoprotein structure of the Escherichia coli cell wall. The attachment site of the lipoprotein on the murein. Eur J Biochem. 1970 Apr;13(2):336–346. doi: 10.1111/j.1432-1033.1970.tb00936.x. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta D. B., Krämer C., Henning U. Diploidy for a structural gene specifying a major protein of the outer cell envelope membrane from Escherichia coli K-12. J Bacteriol. 1976 Dec;128(3):834–841. doi: 10.1128/jb.128.3.834-841.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirashima A., Inouye M. Specific biosynthesis of an envelope protein of Escherichia coli. Nature. 1973 Apr 6;242(5397):405–407. doi: 10.1038/242405a0. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Suzuki H., Nishimura Y., Yasuda S. On the process of cellular division in Escherichia coli: a mutant of E. coli lacking a murein-lipoprotein. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1417–1420. doi: 10.1073/pnas.74.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Guthrie J. P. A mutation which changes a membrane protein of E. coli. Proc Natl Acad Sci U S A. 1969 Nov;64(3):957–961. doi: 10.1073/pnas.64.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M. Internal standards for molecular weight determinations of proteins by polyacrylamide gel electrophoresis. Applications to envelope proteins of Escherichia coli. J Biol Chem. 1971 Aug 10;246(15):4834–4838. [PubMed] [Google Scholar]
- Inouye M., Shaw J., Shen C. The assembly of a structural lipoprotein in the envelope of Escherichia coli. J Biol Chem. 1972 Dec 25;247(24):8154–8159. [PubMed] [Google Scholar]
- Inouye M. Unlinking of cell division from deoxyribonucleic acid replication in a temperature-sensitive deoxyribonucleic acid synthesis mutant of Escherichia coli. J Bacteriol. 1969 Sep;99(3):842–850. doi: 10.1128/jb.99.3.842-850.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Lee N., Inouye M., Wu H. C., Suzuki H., Nishimura Y., Iketani H., Hirota Y. Amino acid replacement in a mutant lipoprotein of the Escherichia coli outer membrane. J Bacteriol. 1977 Oct;132(1):308–313. doi: 10.1128/jb.132.1.308-313.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novel M., Novel G. Regulation of beta-glucuronidase synthesis in Escherichia coli K-12: constitutive mutants specifically derepressed for uidA expression. J Bacteriol. 1976 Jul;127(1):406–417. doi: 10.1128/jb.127.1.406-417.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Nishimura Y., Iketani H., Campisi J., Hirashima A. Novel mutation that causes a structural change in a lipoprotein in the outer membrane of Escherichia coli. J Bacteriol. 1976 Sep;127(3):1494–1501. doi: 10.1128/jb.127.3.1494-1501.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



