Abstract
Background
One approach to improving outcomes for minority diabetics may be through better self-care. However, minority patients may encounter barriers to better self-care even within settings where variations in quality of care and insurance are minimized.
Objective
The objective of the study was to evaluate racial differences in long-term glucose self-monitoring and adherence rates in an HMO using evidence-based guidelines for self-monitoring.
Design
Retrospective cohort study using 10 years (1/1/1993–12/31/2002) of electronic medical record data was used.
Patients
Patients were 1,732 insured adult diabetics of black or white race newly initiated on hypoglycemic therapy in a large multi-specialty care group practice.
Measurements
Outcomes include incidence and prevalence of glucose self-monitoring, intensity of use, and rate of adherence to national recommended standards.
Results
We found no evidence of racial differences in adjusted initiation rates of glucose self-monitoring among insulin-treated patients, but found lower rates of initiation among black patients living in low-income areas. Intensity of glucose self-monitoring remained lower among blacks than whites throughout follow-up [IRR for insulin = 0.41 (0.27–0.62); IRR for oral hypoglycemic = 0.75 (0.63, 0.90)], with both groups monitoring well below recommended standards. Among insulin-treated patients, <1% of blacks and <10% of whites were self-monitoring 3 times per day; 36% of whites and 10% of blacks were self-monitoring at least once per day.
Conclusions
Adherence to glucose self-monitoring standards was low, particularly among blacks, and racial differences in self-monitoring persisted within a health system providing equal access to services for diabetes patients. Early and continued emphasis on adherence among black diabetics may be necessary to reduce racial differences in long-term glucose self-monitoring.
KEY WORDS: racial disparities, self-management, diabetes, HMO
BACKGROUND
Persistent self-management practice is a key component to effective diabetes care. Appropriate self-management including drug therapy, self-monitoring of blood glucose, and changes in diet and exercise can improve glycemic control, an important risk factor for microvascular complications.1–6 Improving self-care practices and performance among minority diabetes patients may lead to improved outcomes. Evidence suggests that minorities with diabetes may receive a lower quality of care and experience greater barriers to effective self-management,7–16 despite being at greater risk for complication, disability, and mortality than white diabetes patients.1–6
Intensive drug therapy improves glycemic control and reduces the risk of microvascular and other diabetes-related complications.17–20 While frequent self-monitoring of blood glucose (SMBG) in combination with insulin therapy can lead to reduced glycemic levels, the optimal frequency or timing of SMBG to produce clinical benefits for patients using oral hypoglycemic medications remain unclear.21–25 However, routine SMBG is the recommended standard of self-care for all diabetes patients based on American Diabetes Association (ADA) guidelines.26
Understanding the role of race in self-monitoring and other aspects of self-management is incomplete, and strong longitudinal evidence on racial differences in diabetes self-management practice is limited.7–10,16 In a recent longitudinal study of diabetes management,27 we found that despite receiving care in a managed care setting where equal access to services and high quality of care are provided, blacks had consistently higher HbA1c levels than whites, and these racial differences in diabetes control did not diminish over time. These findings suggest that variations in other areas of diabetes care, such as in self-management, may play a larger role in disparities in diabetes outcomes than differences in quality of care.
In this study, we evaluated whether differences exist between blacks and whites in long-term self-monitoring practices and whether these differences change over time in an HMO. We used 5-year follow-up of newly treated diabetes patients to assess racial differences in initiation of SMBG and subsequently examined race differences in prevalence, sustained intensity of SMBG, and in rates of compliance with ADA recommendations for appropriate use of SMBG among newly treated diabetics who initiated SMBG. Given previous findings, we hypothesized that racial differences may exist at the onset of receiving a new diabetes drug therapy, and racial differences in self-monitoring practice would persist over time.
RESEARCH DESIGN AND METHODS
Study Setting and Data Sources
Harvard Vanguard Medical Associates (HVMA) is a large multi-specialty care group comprised of 14 health centers serving over 300,000 people in the Boston area. During this study period, >95% of all HVMA patients were insured by Harvard Pilgrim Health Care (HPHC).
Using HVMA’s electronic medical record system, we captured data from 1/1/1992–12/31/2001 from all ambulatory and inpatient encounters, including pharmacy contacts and laboratory test results, diagnoses, procedures, and therapies. The electronic medical record system also contained enrollment data including date of birth, gender, specific months of membership, and census block group of member’s residence (which were linked to socioeconomic characteristics of the neighborhood such as income and education).
Under the pharmacy benefit, HVMA/HPHC members could fill prescriptions for up to 3 months’ supply from in-house pharmacies for a modest co-payment (median co-payment for blacks and whites = $5.00) throughout the study period. This benefit provided members with a strong incentive to use the pharmacies within the health care system under study, increasing the likelihood that dispensing information is complete.
All records for emergency room visits, hospitalizations, and other services received outside the HVMA system were recorded in a linked claims database. Previous studies have documented the reliability and validity of these linked longitudinal data.28,29
Study Cohort
To be eligible for inclusion in the study, patients had to be at least 18 years old, black or white race identified by medical personnel, and newly treated with an oral hypoglycemic or insulin. Patients must have had at least 1 year of continuous enrollment in HVMA leading up to the first diabetes drug dispensing with no prior SMBG. Because recommended standards of SMBG differ for patients on oral hypoglycemic versus patients on insulin therapy, patients who switched from oral hypoglycemic to insulin would expect to have fairly abrupt increases in their glucose self-monitoring; therefore, patients who switched from an oral hypoglycemic to insulin at any point during the 5-year follow-up period (=8% of black and white patients) were excluded. Women with gestational diabetes at any point during the study period were also excluded. The resulting study sample consisted of 2,500 patients, of whom, 661 (26%) were black. For multivariate analyses of the relative difference between blacks and whites in prevalence of SMBG, intensity of SMBG, and prevalence of patients meeting recommended targets of SMBG use, 768 patients with less than 12 months of follow-up after initiation of SMBG were excluded, leaving an analytical sample of 1,732 patients.
Key Predictor and Outcome Measures
Patient race was visually identified by medical personnel and available for 70% of the larger patient population from which this study cohort was identified. The validity of race data in this setting has been previously documented.34 Fixed effect covariates included gender, age, and census-derived median neighborhood household income and educational attainment.
Main outcome measures included incidence of SMBG (time to first dispensing of test strips after diabetes drug dispensing), cumulative probability of ever self-monitoring in a given year, prevalence of current SMBG (≥1 test strip in a given year), intensity of SMBG (mean number of test strips), and adherence to SMBG standards of care (% meeting ADA recommended use). To measure self-monitoring, we used dispensed test strips, a more valid indicator of actual self-monitoring than self-report.4,10,17 As described in our previous work,33 dispensed test strips were counted by distributing them evenly over the days between dispensings (or over 60 days after the dispensing date if no subsequent dispensing occurred within that period). ADA-recommended rates of SMBG were 3 or more strips per day for those using insulin or combination therapy and 1 or more strips per day for those on oral hypoglycemic medications. In a subsidiary analysis, we also measured adherence to a lower standard of 1 or more strips per day for those on insulin therapy.
Because changes in body mass index (BMI) or glycemic levels may precipitate decisions to initiate monitoring, we used last recorded BMI and HbA1c level in the quarter before the month in which initiation of SMBG was estimated. For multivariate statistical models, prevalence of SMBG use, intensity of SMBG, and prevalence of SMBG use recommended by guidelines represent annual summary outcome measures. Therefore, we used annualized covariates for the same year to characterize service utilization and health status.
Using a previously validated method,30 we assessed comorbidity by counting the average monthly number of non-diabetes medicines taken by each patient in a year using the first eight digits of the American Hospital Formulary Services code. We also included as a control for severity of illness a monthly and annual indicator of whether the patient had a diabetes-related hospitalization or emergency room visit (ICD-9 = 250.XX). To adjust for differences in patient involvement with the care system, we controlled for average monthly number of outpatient physician visits per year.
Statistical Analysis
We performed all statistical analyses using SAS V8.02 (Cary, NC SAS, Inc 2000). All analyses were stratified by oral hypoglycemic and insulin therapy. All models were constructed separately for these 2 therapy groups. Patients on multiple oral hypoglycemic or metformin therapy were grouped with oral hypoglycemic-treated patients for all analyses.
We used chi-square and t tests to compare racial differences in demographic and clinical status during the 12 months after initiation of diabetes drug therapy (baseline period). Bivariate correlates of race (p < 0.20) were included in our main multivariate models (Cox survival, generalized estimating equation models).
To examine racial differences in SMBG initiation, we produced Kaplan–Meier estimates and unadjusted time to SMBG, comparing blacks to whites. We used multivariate Cox survival regression models to test whether race was an independent predictor of initiation of SMBG for both drug therapy cohorts,31 adjusting for fixed and time-varying patient-level characteristics. For the Cox survival models, we employed time lags of either 1 month or quarter for key time-varying covariates, including BMI, glycemic levels (HbA1c), number of non-diabetic medications, number of HbA1c tests, number of physician visits, and severity of illness. Follow-up time for these analyses was expressed as months from first diabetes medication dispensing. Patients who never initiated SMBG by the end of follow-up or whose enrollment in HVMA/HPHC ended before SMBG initiation occurred were censored.
Adjusting for fixed and time varying covariates, we then used generalized estimating equations (GEE) for the remainder of our analyses. The first was a logit GEE to determine whether prevalence of SMBG after initiation of SMBG differed between blacks and whites within insulin- and oral-hypoglycemic-treated patients. Among patients who initiated self-monitoring, we compared the intensity of SMBG between race groups over time using Poisson GEE models. Patients who discontinued self-monitoring continued to contribute to these analyses until disenrollment from HVMA/HPHC or end of follow-up. Lastly, in a subsidiary analysis among the cohort of insulin-treated patients for whom the efficacy of SMBG is well documented17–20, we fit logit GEE models to examine racial differences in rates of adherence using the evidence-based ADA standard of SMBG (i.e., ≥3 times per day) and a more relaxed standard of 1 or more strips per day. The main terms of interest in all our GEE models were interaction terms between race and temporal variables that captured the differential relationships between blacks and whites over the 5 years after SMBG initiation. We also tested for race and SES interactions using likelihood ratio and chi-square tests.
RESULTS
Description of Study Cohort by Race
As shown in Table 1, about 74% of patients were white and 26% were black. Among new oral-hypoglycemic-treated patients, black patients were more likely to be female, younger, and with poorer glycemic control, but had fewer hospitalizations and non-diabetes medications. Black patients who initiated treatment on insulin therapy had greater numbers of hospitalizations and emergency room visits than white patients. Most patients who initiated SMBG did so within the first month of drug therapy.
Table 1.
Race Differences in Baseline* Demographic and Clinical Status among Newly Drug-Treated Diabetes Patients Stratified by Type of Drug Therapy
| Oral hypoglycemic†N = 2,131 (85.2%) | Any insulin N = 369 (14.8%) | |||
|---|---|---|---|---|
| Black (n = 519) | White (n = 1,612) | Black (n = 142) | White (n = 227) | |
| Demographic Characteristics | ||||
| Male | 51.5% | 60.2%‡ | 47.2% | 45.8% |
| Mean age (SD) | 46(11) | 54(13)‡ | 44(14) | 46(15) |
| Census-derived SES measures | ||||
| Living in neighborhood where median household income < poverty level | 32.3% | 10.2%‡ | 36.6% | 11.0%‡ |
| Living in neighborhood where >75% residents have < a high school degree | 50.1% | 19.2%‡ | 56.5% | 16.5%‡ |
| Health service utilization | ||||
| Mean # of MD visits(SD) | 5(2) | 5(2) | 6(3) | 5(3) |
| Mean # of lab tests(SD) | 3(1) | 3(1)§ | 3(2) | 3(2) |
| Clinical characteristics | ||||
| Mean HbA1c values (SD)// | 8.8 (2.2) | 7.8 (1.6)‡ | 8.3 (1.8) | 8.5 (1.9) |
| Body mass index (BMI)¶ | ||||
| Overweight (30–<40) | 44.4% | 48.1% | 37.1% | 38.4% |
| Obese (40+) | 12.8% | 12.6% | 15.2% | 12.3% |
| Mean BMI (SD)¶ | 32.5 (7.1) | 32.7 (7.1) | 31.9 (8.8) | 31.2 (7.6) |
| Comorbidities | ||||
| Any diabetes-related hospitalizations | 8.7% | 13.7%§ | 36.6% | 26.9%§ |
| Any diabetes-related emergency room visits | 9.8% | 12.0% | 35.2% | 24.2%§ |
| Monthly mean # non-diabetic AHFS# dispensed (SD) | 2.4 (1.1) | 2.7 (1.4)‡ | 3.1 (1.7) | 3.3 (1.9) |
| Self-monitoring of blood glucose (SMBG) | ||||
| Timing of initiation of SMBG** | ||||
| Within month of 1st diabetes drug | 74.5% | 63.0%‡ | 80.0% | 74.4% |
| Between months 2 and 12 | 11.8% | 18.9%‡ | 13.1% | 15.1% |
Data are expressed as % patients or mean (SD).
*Baseline = 12 months following first drug dispensing; assignment to drug therapy group based on drug use at baseline
†Includes metformin and combination therapy (% with census-derived SES measures = oral: 88.4% of blacks and 93.7% of whites; Insulin: 87.3% of blacks and 90.7% of whites)
‡p < 0.001
§p < 0.05
//Among those with a baseline HbA1c test (% with HbA1c data = Oral: 93.4% of blacks and 93.2% of whites; Insulin: 97.2% of blacks and 86.8% of whites)
¶Among those with a baseline BMI (% with BMI data = Oral: 70.7% of blacks and 70.9% of whites; Insulin: 73.9% of blacks and 64.3% of whites)
#AHFS = American Hospital Formulary Services
**Among those who initiated SMBG during the study period
Effects of Race on SMBG Over Time
Figure 1 shows race differences in unadjusted cumulative rates of trials of SMBG during 5 years after first diabetes medication dispensing. As expected, insulin-treated patients initiated SMBG sooner and at higher rates of SMBG use than patients on oral hypoglycemic therapy. Blacks were as likely as whites to initiate SMBG when on insulin therapy. However, within the oral-hypoglycemic-treated group, the unadjusted rate of initiation of SMBG among blacks compared with whites remained higher over time [HR = 1.2 (1.07–1.35)]. After adjusting for covariates, race alone no longer was a significant predictor of initiation among oral-hypoglycemic-treated patients [Table 2, HR = 1.08 (0.80–1.46)]. The interaction between race and income was significant (p = 0.01), suggesting that the effect on initiation of SMBG of living in a neighborhood where the median household income falls below poverty level varied by race.
Figure 1.
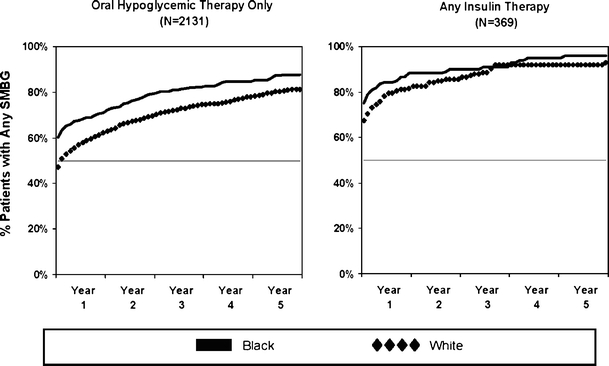
Race differences in time from first diabetes medication until first SMBG stratified by oral-hypoglycemic- and insulin-treated patients. Year 1 represents start of drug therapy for each newly diagnosed diabetes patient. Patient assignment to drug treatment group based on drug use in the 12 months of first drug therapy.
Table 2.
Multivariate Models for Initiation of SMBG, Prevalence of SMBG Users, and Intensity of SMBG Use Among Newly Treated Oral and Insulin Patients
| Initiation of SMBG | Prevalence of SMBG users | Intensity of SMBG | ||||
|---|---|---|---|---|---|---|
| Oral | Insulin | Oral | Insulin | Oral | Insulin | |
| HR [95%CI] | HR [95%CI] | OR [95%CI] | OR [95%CI] | IRR [95%CI] | IRR [95%CI] | |
| Follow-up years* | NA | NA | 0.67† [0.64, 0.71] | 0.82‡ [0.69, 0.97] | 0.84† [0.81, 0.87] | 0.93 [0.87, 1.01] |
| Black (vs white) | 1.08 [0.80, 1.46] | 1.94 [0.97, 3.87] | 0.80‡ [0.63, 0.99] | 0.85 [0.43, 1.67] | 0.75‡ [0.63, 0.90] | 0.41† [0.27, 0.62] |
| Male (vs female) | 0.79‡ [0.65, 0.96] | 1.18 [0.65, 2.14] | 0.86 [0.71, 1.03] | 1.06 [0.57, 1.97] | 0.90 [0.76, 1.07] | 0.78 [0.56, 1.09] |
| Age | 1.00 [0.99, 1.01] | 0.96‡ [0.94, 0.99] | 1.01‡ [1.00, 1.02] | 1.01 [0.98, 1.04] | 1.00 [0.99, 1.00] | 0.98‡ [0.97, 0.99] |
| ≤Poverty level (vs > poverty) | 1.48‡ [1.02, 2.15] | 1.13 [0.51, 2.49] | 0.90 [0.69, 1.18] | 0.55 [0.24, 1.25] | 0.96 [0.80, 1.16] | 0.69 [0.42, 1.15] |
| <HS degree education (vs ≥ HS degree) | 0.79 [0.62, 1.00] | 0.61 [0.29, 1.30] | 1.04 [0.83, 131] | 0.78 [0.38, 1.60] | 1.04 [0.89, 1.22] | 1.71‡ [1.01, 2.90] |
| # of MD visits§ | 0.95 [0.83, 1.08] | 0.86 [0.58, 1.29] | 1.06‡ [1.02, 1.10] | 1.13‡ [1.02, 1.24] | 1.02‡ [1.01, 1.03] | 1.00 [0.99, 1.01] |
| HbA1c value§ | 1.06‡ [1.01, 1.12] | 1.08 [0.95, 1.24] | 1.01 [0.96, 1.05] | 1.03 [0.90, 1.17] | 1.02 [0.99, 1.04] | 1.00 [0.95, 1.06] |
| Any diabetes-related hospitalization§ | 1.10† [1.04, 1.16] | 1.04 [0.91, 1.18] | 0.86 [0.67, 1.10] | 2.01 [0.74, 5.50] | 1.03 [0.90, 1.17] | 0.96‡ [0.84, 1.10] |
| Body mass index§ | 1.00 [0.98, 1.01] | 1.00 [0.96, 1.04] | 1.00 [0.98, 1.01] | 0.99 [0.96, 1.02] | 0.98 [0.97, 1.00] | 0.96 [0.94, 0.99] |
| # of Non-diabetes Rxs§ | 1.04 [0.99, 1.10] | 1.17‡ [1.02, 1.35] | 1.45† [1.32, 1.59] | 1.46‡ [1.11, 1.93] | 1.19‡ [1.15, 1.23] | 1.16† [1.10, 1.23] |
| Black-income interaction | 0.48‡ [0.27, 0.86] | – | – | – | – | – |
Interaction terms with p > 0.05 were not included in the final model; analyses adjusted for health centers with disproportionately high numbers of black patients
*Based on years 2–5 of the follow-up period
†p < 0.05
‡p < 0.001
§Time varying covariates were entered into the model with 1-month/quarter time lag for the Cox PH models. GEE models were adjusted for clinical status and utilization of services in that year.
As shown in Figure 2, the prevalence over time of continuing SMBG among insulin- and oral-hypoglycemic-treated patients was consistently lower in blacks than whites. Substantial drops in SMBG prevalence occurred by the end of the initial year. Among insulin patients, 70% of white and 65% of blacks continued self-monitoring in the second year; however, after adjusting for covariates, this black–white difference was not significant (Table 2). Only 62% of white and 56% of black oral-hypoglycemic-treated patients continued SMBG after the first year (p < 0.0001). Prevalence of SMBG steadily decreased thereafter for both drug and race groups, although blacks had consistently lower SMBG rates than whites among oral users.
Figure 2.
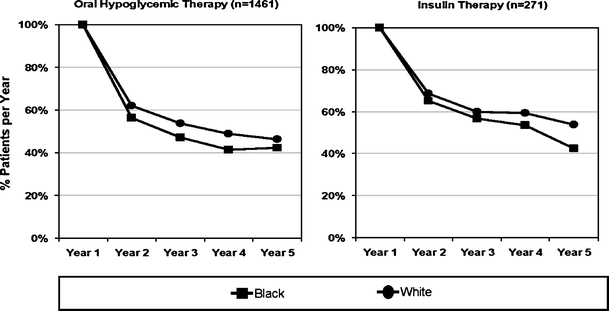
Race differences in prevalence of self-monitoring from first SMBG. Year 1 represents start of SMBG for each initiator of SMBG. Analytical sample is restricted to patients with >12 follow-up months.
Effects of Race on Adherence to ADA Recommendations for SMBG
As expected, intensity of SMBG was greater for patients on insulin therapy compared to patients on oral hypoglycemic therapy over time (Fig. 3). Intensity of SMBG remained consistently lower among blacks than whites during 5 years of follow-up; both blacks and whites performed well below ADA standards. Intensity in both race groups fell sharply in the first year of monitoring, then tended to remain stable over the remaining years for both insulin- and oral-hypoglycemic-treated patients.
Figure 3.
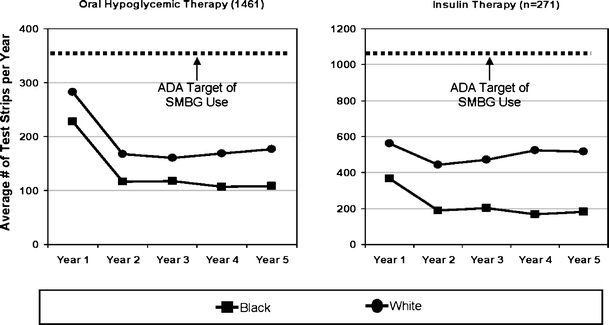
Race differences in long-term intensity of SMBG since first SMBG. Year 1 represents start of SMBG for each initiator of SMBG. Patient assignment to drug therapy group based on drug use in 12 months of first drug therapy. ADA target of SMBG use per year is approximately 365 strips for oral-hypoglycemic-treated patients and 1,100 strips for insulin-treated patients.
In Table 2, after adjusting for covariates, blacks compared with whites were self-monitoring at a significantly lower intensity, irrespective of drug treatment, [IRR for insulin-treated = 0.41 (0.27–0.62); IRR for oral-hypoglycemic-treated = 0.75 (0.63, 0.90)]. Furthermore, the relative difference between blacks and whites did not increase over time (p > 0.05).
Figure 4 compares race differences in rates of adherence to standards of SMBG among newly SMBG-initiated patients on insulin therapy, a group for whom there is well-established clinical benefits of frequent SMBG.17–20 Less than 1% of blacks and <10% of whites were self-monitoring at the ADA standard of 3 or more strips per day, and only 36% of white and 10% of black insulin-treated patients met the less stringent standard of 1 strip per day. After adjusting for covariates, the prevalence of achieving either recommendation standards of SMBG levels differed between blacks and whites (Table 3). Blacks were less likely than whites to adhere to a once-a-day recommendation of SMBG across follow-up [OR = 0.30 (0.13, 0.70)]. This black–white difference was even greater under the ADA recommendation [OR = 0.03 (0.01–0.55)].
Figure 4.
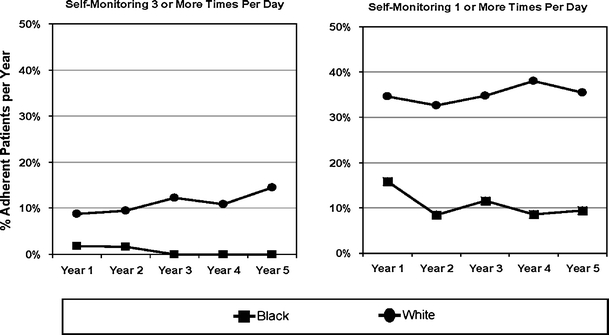
Race differences in rates of insulin-treated patients adherent to recommended standards of SMBG (N = 271). Year 1 represents start of SMBG for each initiator of SMBG. The ADA-recommended standard of SMBG is 3 or more times per day.
Table 3.
Multivariate Models for Prevalence of Insulin-Treated Patients Adherent to Recommended Standards of SMBG
| ADA standard for SMBG (≥3 times/day) | Relaxed standard for SMBG (≥1 time/day) | |
|---|---|---|
| OR [95%CI] | OR [95%CI] | |
| Follow-up years* | 0.90† [0.62, 1.31] | 0.85† [0.73, 0.98] |
| Black (vs white) | 0.03† [0.01, 0.55] | 0.30† [0.13, 0.70] |
| Male (vs female) | 1.58 [0.55, 4.55] | 0.79 [0.41, 1.54] |
| Age | 0.06 [0.02, 0.10] | 0.98 [0.95, 1.00] |
| ≤Poverty level (vs > poverty) | 3.41 [0.43, 27.1] | 0.56 [0.17, 1.80] |
| <High school degree education (vs ≥ high school degree) | 0.23† [0.08, 0.69] | 1.22 [0.52, 2.84] |
| # of MD visits | 1.00 [0.93, 1.06] | 1.02 [0.98, 1.06] |
| HbA1c value‡ | 0.95 [0.81, 1.12] | 1.03 [0.92, 1.14] |
| Any diabetes-related hospitalization‡ | 10.69† [1.51, 75.85] | 0.95 [0.61, 1.47] |
| Body mass index‡ | 1.13† [1.02, 1.25] | 0.98 [0.93, 1.03] |
| # of Non-diabetes drug dispensings | 0.65§ [0.51, 0.83] | 1.32† [1.10, 1.58] |
Interaction terms with p > 0.05 were not included in the final model; analyses adjusted for health centers with disproportionately high numbers of black patients
*Based on years 2–5 of the follow-up period
†p < 0.05
‡Time varying clinical status and utilization of services covariates based in that year
§p < 0.001
CONCLUSIONS
In this longitudinal investigation, we followed a cohort of 1,732 newly treated diabetes patients for 5 years to determine whether patterns of long-term SMBG practice differed by race. After adjusting for key covariates, black and white patients treated by insulin initiated and continued SMBG at the same rates. However, the lower intensity of SMBG over time by black insulin-treated patients suggests that there may be race-related differences in affordability, competing demands, understanding, or valuation of regular self-monitoring.
In contrast, among oral hypoglycemic patients, both the intent and inclination to adhere to SMBG appeared to differ between blacks and whites. Race differences were evident across all SMBG outcome measures. Blacks living in poorer neighborhoods were less likely to initiate SMBG than whites. Blacks were also slightly less likely to continue monitoring after 1 year, and these differences in continued use remained small throughout follow-up. Larger differences in intensity of SMBG were apparent in the first year, and these also remained constant over time.
These findings are consistent with our earlier investigation33 in which we found that coverage of self-monitoring equipment increased glucose self-monitoring, particularly among blacks, but that sustainability was low. Nearly all patients who initiated SMBG as the result of the coverage increase discontinued within 18 months, with blacks discontinuing at higher rates than whites. In the current study, we assessed race differences in frequency of SMBG over a longer follow-up period and adjusted for covariates. Furthermore, unlike prior studies,9–12,32,33 our longitudinal analyses compared rates of adherence based on well-established standards of SMBG for both insulin and oral hypoglycemic treatment groups.
The ADA continues to recommend frequent SMBG as a necessary part of self-care. However, intensity of SMBG was surprisingly low in the current study, particularly among black insulin-treated patients. While black insulin-treated patients were at <1% of the recommended target, their white counterparts only performed slightly better, with 10% meeting the ADA target. While we anticipated significant race differences in SMBG performance, we did not expect that overall adherence would be so low. Additional research is needed to explain these low rates, particularly among insulin-treated patients for whom frequent SMBG reduces the risk of serious and costly diabetes-related complications.17–20,34
The longitudinal follow-up of a large cohort of patients within an HMO where essentially all utilization can be captured and the use of ADA-recommended standards of SMBG as a base to measure compliance make this study unique. Furthermore, the ability to identify time points when black and white patients diverge with respect to self-monitoring is important for future interventions. However, the study has several potential limitations. First, because race was determined from clinician reports, misclassification may have occurred, although previous work has shown that assessments of white and black race in this setting are highly valid.35 In addition, race may serve as a proxy for a myriad of cultural and psychosocial factors that can influence patient self-monitoring.36 Although we did not have individual SES measures, we were able to adjust for neighborhood educational attainment and income. Unfortunately, we did not have measures of ethnicity (Hispanic/non-Hispanic) or other cultural and psychosocial factors that may be closely correlated with race. For this reason, the observed racial differences may in part represent cultural or biological constructs.37
We recognize that the way we constructed our samples for longitudinal analyses may underestimate utilization of SMBG among black patients relative to whites. Our longitudinal results are based on patients who initiated SMBG, excluding those who never initiated. Racial differences in SMBG performance, particularly in intensity and compliance with ADA recommended guidelines, would be more prominent if we included all non-initiators of SMBG in these analyses. Thus, our findings represent the lower bound of the racial gap that exists and persists in this setting.
Finally, while we are not able to generalize our findings to non-managed care settings, the large proportion of black patients enrolled at HVMA allows for comparison to other studies of racial differences in diabetes self-management in similar settings.
In conclusion, race differences exist and persist over time within a managed care system that provides equal access to services and quality care for diabetes patients. Race differences are not apparent in rates of initiation of SMBG among insulin patients, but large gaps appear in sustainability and intensity of SMBG over time. Among oral hypoglycemic users, race differences are prominent in all 3 outcome measures. In the presence of an established, evidence-based standard of self-care for patients on insulin therapy, intensity of SMBG may be too low to have clinically meaningful impacts. Our findings suggest that early and continued emphasis on adherence may be necessary to improve and maintain optimal levels of SMBG. Furthermore, integration of interventions may be needed to reduce persistent racial differences in long-term SMBG practice and clinical outcomes.
Acknowledgments
The authors wish to thank Dr. Richard Grant of the General Medicine Unit, Department of Medicine, Massachusetts General Hospital, and Harvard Medical School for his thoughtful comments on the analyses and Ms. Irina Miroshnik of the Department of Ambulatory Care and Prevention, Harvard Medical School and Harvard Pilgrim Health Care for her assistance in data extraction. The authors also wish to thank the reviewers for their insightful comments and suggestions made on an earlier submission of this paper. An abstract of this work was presented at the SGIM 29th Annual Meeting on April 29, 2006, Los Angeles, CA, and on May 2, 2006 at the HMO Research Network Annual Meeting, Cambridge, MA. This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases and by the Harvard Pilgrim Health Care Foundation. Dr. Meigs is supported by an American Diabetes Association Career Development Award. Dr. Piette is a VA Career scientist and supported by the Michigan Diabetes Research and Training Center.
Conflict of Interest None disclosed.
References
- 1.Litzelman DK, Slemenda CW, Langefeld CD, et al. Reduction of lower extremity clinical abnormalities in patients with non-insulin dependent diabetes mellitus. Ann Intern Med. 1993;119:36–41. [DOI] [PubMed]
- 2.Agrawal L, Emanuele NV, Abraira C, et al. Ethnic differences in the glycemic response to exogenous insulin treatment in the Veterans Affairs Cooperative Study in Type 2 diabetes mellitus (VA CSDM). Diabetes Care. 1998;21(4):510–15. [DOI] [PubMed]
- 3.Agurs-Collins TD, Kumanyika SK, Ten Have TR, Adams-Campbell LL. A randomized controlled trial of weight reduction and exercise for diabetes management in older African-American subjects. Diabetes Care. 1997;20(10):1503–11. [DOI] [PubMed]
- 4.Mazzuca SA, Moorman NH, Wheeler ML, et al. The diabetes education study: a controlled trial of the effect of diabetes patient education. Diabetes Care. 1986;9:1–10. [DOI] [PubMed]
- 5.Jaber LA, Halapy H, Fernet M, Tummalapalli S, Diwakaran H. Evaluation of a pharmaceutical care model on diabetes management. Ann Pharmacother. 1996;30:238–43. [DOI] [PubMed]
- 6.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002;287:2519–27. [DOI] [PubMed]
- 7.Cowie CC, Harris MI. Ambulatory medical care for non-Hispanic whites, African-Americans, and Mexican-Americans with NIDDM in the U.S. Diabetes Care. 1997;20(2):142–47. [DOI] [PubMed]
- 8.Spangler JG, Konen JC. Predicting exercise and smoking behaviors in diabetic and hypertensive patients. Arch Fam Med. 1993;2:149–55. [DOI] [PubMed]
- 9.Auslander WF, Thompson S, Dreitzer D, White NH, Santiago JV. Disparity in glycemic control and adherence between African-American and Caucasian youths with diabetes. Diabetes Care. 1997;20(10):1569–75. [DOI] [PubMed]
- 10.Harris MI, Cowie CC, Howie LJ. Self-Monitoring of blood glucose by adults with diabetes in the United States population. Diabetes Care. 1993;16(8):1116–23. [DOI] [PubMed]
- 11.Glasgow RE, Hampson SE, Strycker LA, Ruggiero L. Personal-model beliefs and social-environmental barriers related to diabetes self-management. Diabetes Care. 1997;20(4):556–62. [DOI] [PubMed]
- 12.U.S. Department of Health and Human Services. Care knowledge and practices among persons with diabetes mellitus-North Carolina, 1994–1995. MMWR Morb Mortal Wkly Rep. 1997;46:1023–27. [PubMed]
- 13.Oki JC, Flora DL, Isley WL. Frequency and impact of SMBG on glycemic control in patients with NIDDM in an urban teaching hospital clinic. Diabetes Educ. 1997;23(4):419–24. [DOI] [PubMed]
- 14.Ruggiero L, Glasgow RE, Dryfoos JM, et al. Diabetes self-management: Self-reported recommendations and patterns in a large population. Diabetes Care. 1997;20(4):568–76. [DOI] [PubMed]
- 15.Langer O, Langer N, Piper JM, Elliott B, Anyaegbunam A. Cultural diversity as a factor in self-monitoring blood glucose in gestational diabetes. J Assoc Acad Minor Physicians. 1995;6:73–77. [PubMed]
- 16.Karter AJ, Ferrara A, Darbinian JA, Ackerson LM, Selby JV. Self-monitoring of blood glucose: Language and financial barriers in a managed care population with diabetes. Diabetes Care. 2000;23(4):477–83. [DOI] [PubMed]
- 17.The United Kingdom Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulfonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53. [DOI] [PubMed]
- 18.The United Kingdom Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–65. [DOI] [PubMed]
- 19.Turner R, Cull C, Holman R. United Kingdom Prospective Diabetes Study 17: A 19-year update of a randomized controlled trial on the effect of improved metabolic control on complications in non-insulin dependent diabetes mellitus. Ann Intern Med. 1996;124:136–45. [DOI] [PubMed]
- 20.Karter AJ, Moffett HH, Liu J, et al. Achieving good glycemic control: Initiation of new antihyperglycemic therapies in patients with type 2 diabetes from the Kaiser Permanente Northern California Diabetes Registry. Am J Manag Care. 2005; 11:262–70. [PMC free article] [PubMed]
- 21.Welcshen L, Bloemendal E, Nijpels G, et al. Self-monitoring of blood glucose in patients with type 2 diabetes who are not using insulin. Diabetes Care. 2005;28(6):1510–17. [DOI] [PubMed]
- 22.Welcshen L, Bloemendal E, Nijpels G, et al. Self-monitoring of blood glucose in patients with type 2 diabetes who are not using insulin (review). The Cochrane Library. 2005;2:1–23. [DOI] [PubMed]
- 23.Gulliford M, Latinovic R. Variations in glucose self-monitoring during oral hypoglycaemic therapy in primary care. Diabet Med. 2004;21:685–90. [DOI] [PubMed]
- 24.Franciosi M, Pellegrini F, De Berardis G, et al. The impact of blood glucose self-monitoring on metabolic control and quality of life in type 2 diabetic patients: an urgent need for better educational strategies. Diabetes Care. 2001;24:1870–77. [DOI] [PubMed]
- 25.Coster S, Gulliford M, Seed PT, Powrie JK, Swaminathan R. Self-monitoring in Type 2 diabetes mellitus: a meta-analysis. Diabet Med. 2000;17:755–61. [DOI] [PubMed]
- 26.American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2005;28(1):S4–S36. [DOI] [PubMed]
- 27.Adams AS, Zhang F, Mah C, et al. Race differences in long-term diabetes management in an HMO. Diabetes Care. 2005;28(12):2844–49. [DOI] [PubMed]
- 28.Choo PW, Rand CS, Inui TS, et al. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med Care. 1999;846–57. [DOI] [PubMed]
- 29.Chan KA, Platt R. Harvard Pilgrim Health Care / Harvard Vanguard Medical Associates. In: Strom B, ed. Pharmacoepidemiology. Chichester: Wiley, 2000.
- 30.Schneeweiss S, Seeger JD, Maclure M, Wang PS, Avorn J, Glynn RJ. Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol. 2001;154:854–64. [DOI] [PubMed]
- 31.Allison PD. Survival Analysis Using the SAS System: A Practical Guide. Cary, NC: SAS Institute Inc., 1995.
- 32.Zgbor JC, Simmons D. Barriers to blood glucose monitoring in a multiethnic community. Diabetes Care. 2002;25(10):1772–77. [DOI] [PubMed]
- 33.Mah C, Soumerai S, Adams A, Ross-Degnan D. Racial differences in response to coverage of glucose monitors in an HMO. Med Care. 2006;44(5):392–97. [DOI] [PubMed]
- 34.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. [DOI] [PubMed]
- 35.Adams AS, Foley JW, Lobb R, et al. Addressing health disparities in primary care: Meeting health plan and provider data needs, Unpublished data.
- 36.Meier JL, Swislocki AL, Lopez JR, et al. Reduction in self-monitoring of blood glucose in persons with type 2 diabetes results in cost savings and no change in glycemic control. Am J Manag Care. 2002;8(6):557–65. [PubMed]
- 37.Williams DR. The concept of race in health services research: 1966–1990. Health Serv Res. 1994:29(3):261–74. [PMC free article] [PubMed]


