Abstract
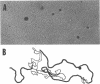
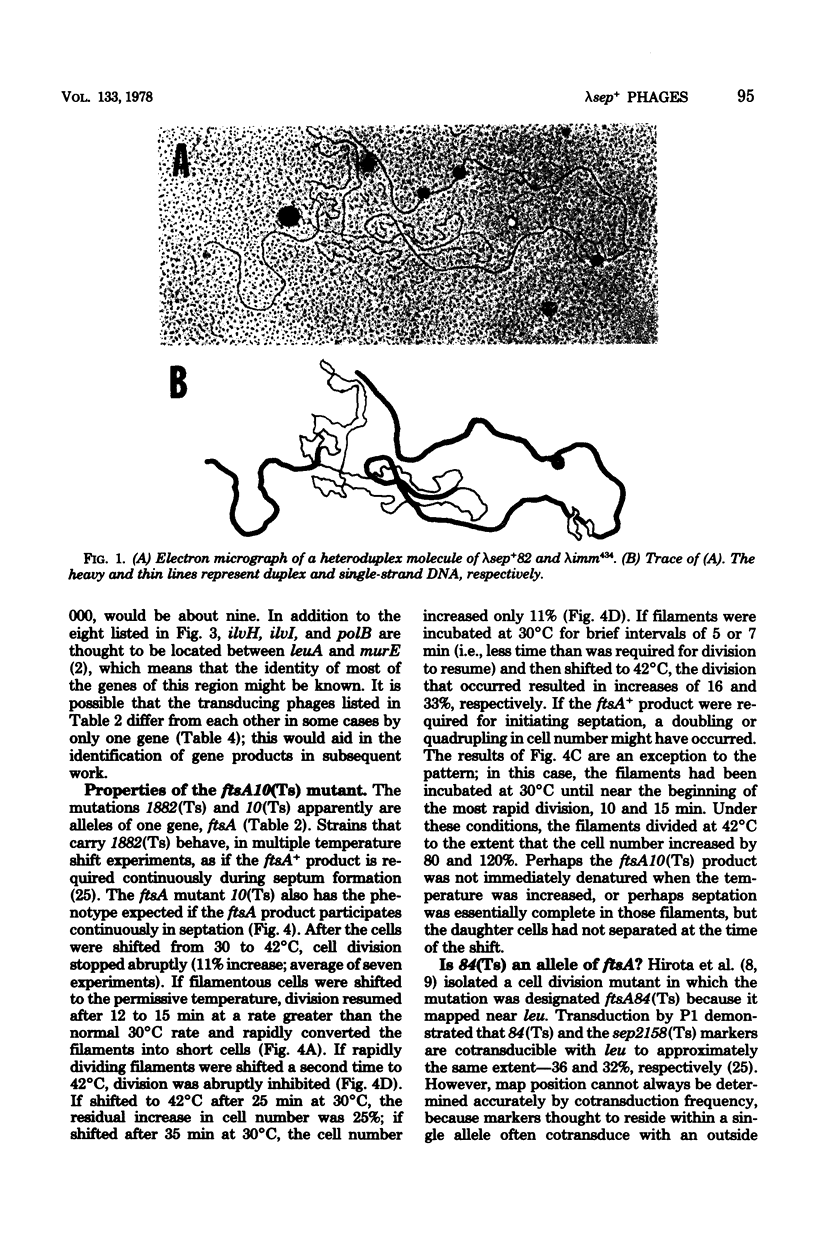
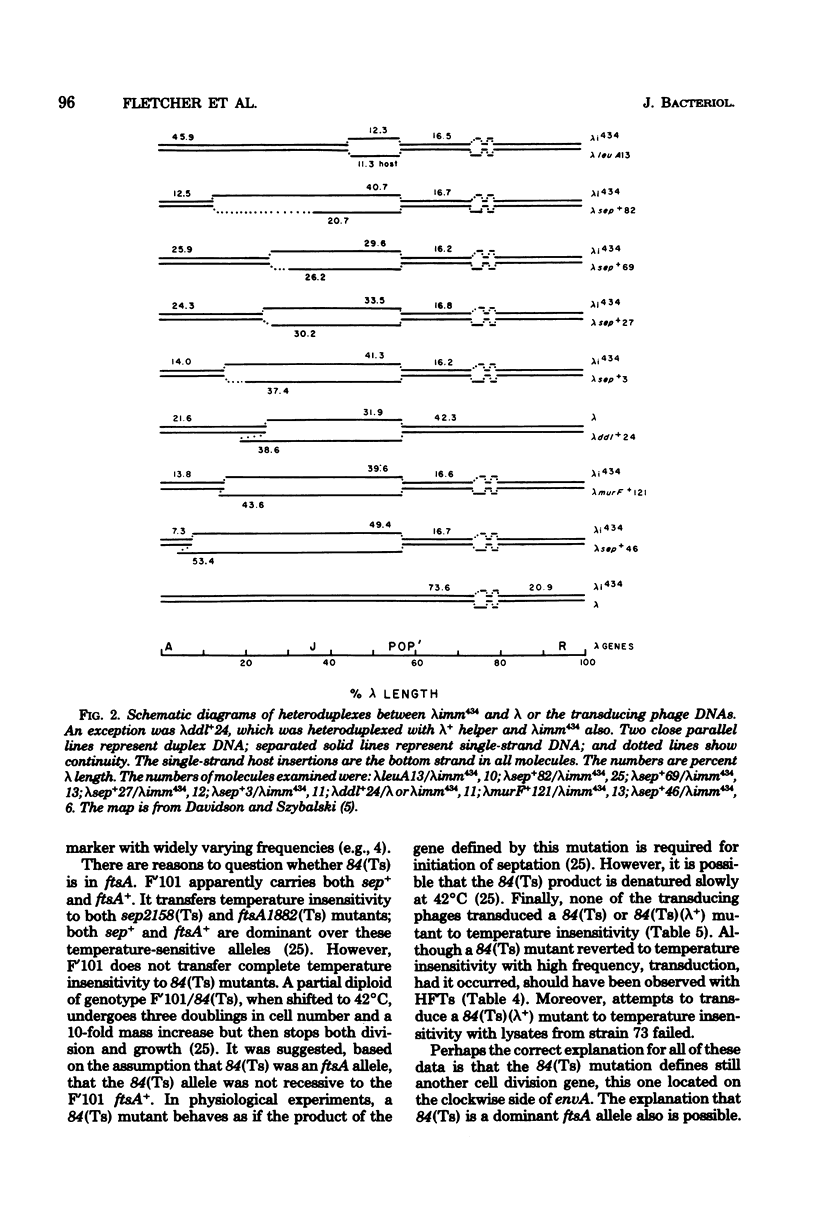
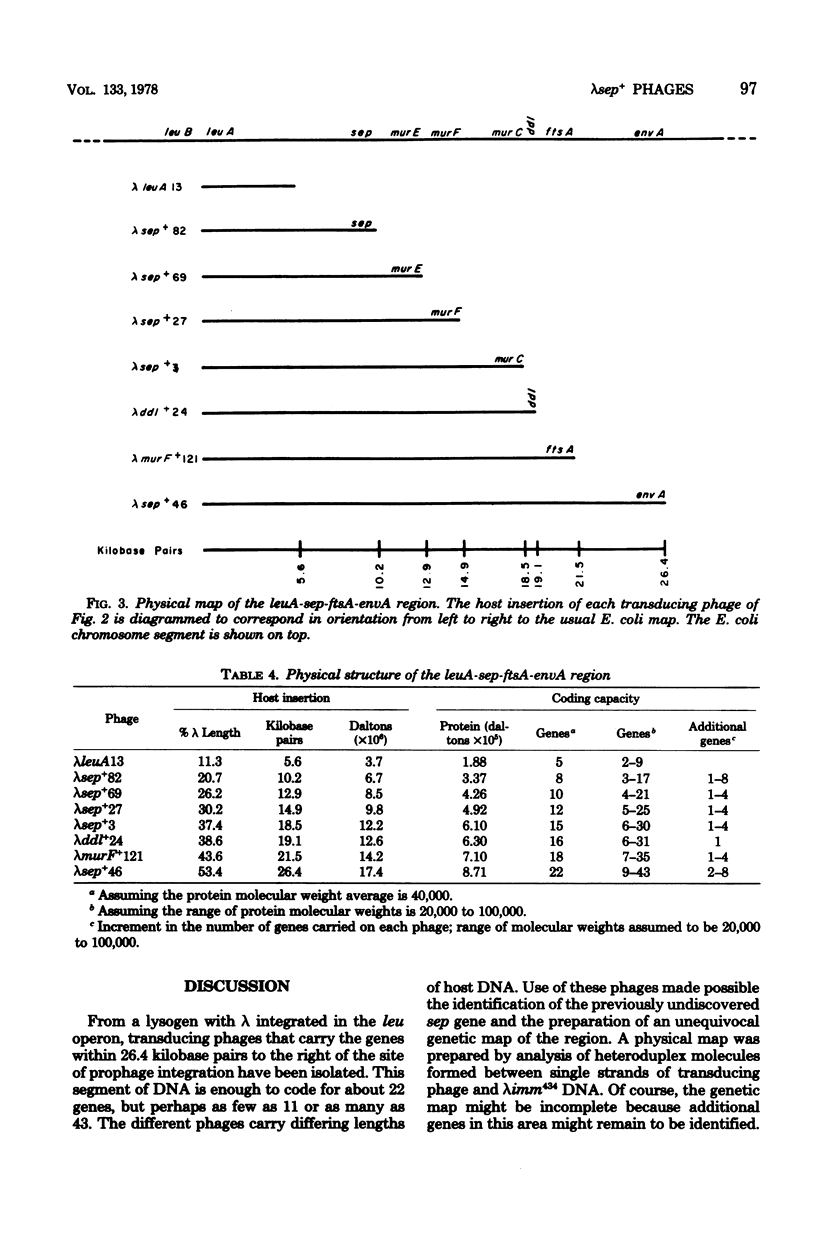
From a lysogen with lambda integrated in the leu operon, specialized transducing phages that carry the cell division, murein biosynthesis, and envelope permeability genes located about 0.5 min to the right of leu were isolated. These phages were used to identify the previously undiscovered cell division gene sep. A genetic map proves that sep is located in the sequence leuA sep murE murF murC ddl ftsA envA. A physical map of this region was prepared by heteroduplex analysis of the phage DNAs. Overlapping segments of host DNA extended rightward for as much as 26.4 kilobase pairs from the prophage insertion point (thought to be in leuA) to include all the genes through envA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. S., Filip C. C., Gustafson R. A., Allen R. G., Walker J. R. Regulation of bacterial cell division: genetic and phenotypic analysis of temperature-sensitive, multinucleate, filament-forming mutants of Escherichia. J Bacteriol. 1974 Mar;117(3):978–986. doi: 10.1128/jb.117.3.978-986.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L. R., Dowell C. E. Replication of coliphage M-13. II. Intracellular deoxyribonucleic acid forms associated with M-13 infection of mitomycin C-treated cells. J Virol. 1968 Nov;2(11):1296–1307. doi: 10.1128/jvi.2.11.1296-1307.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Godson G. N. Mutants of Escherichia coli with temperature-sensitive lesions in membrane phospholipid synthesis: genetic analysis of glycerol-3-phosphate acyltransferase mutants. Mol Gen Genet. 1972;116(3):199–210. doi: 10.1007/BF00269765. [DOI] [PubMed] [Google Scholar]
- Davis M. G., Calvo J. M. Isolation and characterization of lambda pleu bacteriophages. J Bacteriol. 1977 Feb;129(2):1078–1090. doi: 10.1128/jb.129.2.1078-1090.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Klingmüller W., Krell K., Shimada K. Genetic analysis of bacteriophage lambda strains which transduce the genes for leucine biosynthesis. Mol Gen Genet. 1973 Oct 16;126(1):1–6. doi: 10.1007/BF00333476. [DOI] [PubMed] [Google Scholar]
- Lugtenberg E. J., v Schijndel-van Dam A. Temperature-sensitive mutant of Escherichia coli K-12 with an impaired D-alanine:D-alanine ligase. J Bacteriol. 1973 Jan;113(1):96–104. doi: 10.1128/jb.113.1.96-104.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg E. J., v Schijndel-van Dam A. Temperature-sensitive mutants of Escherichia coli K-12 with low activities of the L-alanine adding enzyme and the D-alanyl-D-alanine adding enzyme. J Bacteriol. 1972 Apr;110(1):35–40. doi: 10.1128/jb.110.1.35-40.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg E. J., v Schijndel-van Dam A. Temperature-sensitive mutants of Escherichia coli K-12 with low activity of the diaminopimelic acid adding enzyme. J Bacteriol. 1972 Apr;110(1):41–46. doi: 10.1128/jb.110.1.41-46.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S., Boman H. G., Bloom G. D. Cell division in a chain-forming envA mutant of Escherichia coli K12. Fine structure of division sites and effects of EDTA, lysozyme and ampicillin. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(5):651–664. doi: 10.1111/j.1699-0463.1971.tb00093.x. [DOI] [PubMed] [Google Scholar]
- Normark S. Genetics of a chain-forming mutant of Escherichia coli. Transduction and dominance of the envA gene mediating increased penetration to some antibacterial agents. Genet Res. 1970 Aug;16(1):63–78. doi: 10.1017/s0016672300002287. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Vinograd J. Small polydisperse circular DNA of HeLa cells. J Mol Biol. 1972 Aug 21;69(2):163–178. doi: 10.1016/0022-2836(72)90222-7. [DOI] [PubMed] [Google Scholar]
- Taylor K., Hradecna Z., Szybalski W. Asymmetric distribution of the transcribing regions on the complementary strands of coliphage lambda DNA. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1618–1625. doi: 10.1073/pnas.57.6.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- VAN DE PUTTE P., VAN DILLEWIJN, ROERSCH A. THE SELECTION OF MUTANTS OF ESCHERICHIA COLI WITH IMPAIRED CELL DIVISION AT ELEVATED TEMPERATURE. Mutat Res. 1964 Jul;106:121–128. doi: 10.1016/0027-5107(64)90014-4. [DOI] [PubMed] [Google Scholar]
- Walker J. R., Kovarik A., Allen J. S., Gustafson R. A. Regulation of bacterial cell division: temperature-sensitive mutants of Escherichia coli that are defective in septum formation. J Bacteriol. 1975 Aug;123(2):693–703. doi: 10.1128/jb.123.2.693-703.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijsman H. J., Koopman C. R. The relation of the genes envA and ftsA in Escherichia coli. Mol Gen Genet. 1976 Aug 10;147(1):99–102. doi: 10.1007/BF00337942. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]
- Zuccarelli A. L., Benbow R. M., Sinsheimer R. L. Formation of the parental replicative form of bacteriophage phiX174. J Mol Biol. 1976 Sep 15;106(2):375–402. doi: 10.1016/0022-2836(76)90092-9. [DOI] [PubMed] [Google Scholar]