Abstract
Background and Objectives
Although more and more genetic information is available, it is unclear whether this information is helpful for patients. Therefore, we assessed the positive and negative effects of informing obese people about the genetic etiology of being overweight.
Design, Participants
Two hundred ninety-four obese people were randomized to 2 interventions (a 1-session consultation for obese people on how to manage obesity either including genetic information or not); their results were compared to a control group (116). Subjects were assessed before and after consultation and 6 months later.
Measurements
Weight, scales on feeling guilty for being overweight, self-control, negative mood (primary endpoint), body acceptance, restraint eating.
Results
Both types of consultations were considered helpful by the participants, and had comparable effects on body weight. The consultation with genetic information was rated superior in terms of leading to new insights (advantage for consultation with genetic information, even 6 months later; p = 0.046). No negative effects (e.g., loss of self-efficacy/self-control, increase of body weight; all p > 0.20 for interaction consultation × time) were observed for informing obese people about the genetic etiology of being overweight. The consultation resulted in long-term improvement of negative mood if it included genetic information in the case of participants with a family history of obesity and if it included no genetic information in the case of obese people without a family history of obesity (p = 0.03 for interaction of group, intervention, and time).
Conclusions
Consultations in obesity can be helpful in general. These consultations should include genetic information if people have a family history of obesity.
KEY WORDS: obesity, consultation, genetic counseling, MC4R mutation
Evidence for high heredity of obesity is overwhelming. Family studies have shown major associations, which are higher the closer the genetic relationship is.1 Specific mutations and variations on the genome have been identified that lead to being overweight (Hinney et al., submitted for publication).2–4
Whereas these developments are exciting for scientists, the question remains whether they are of any relevance for the affected people. It could be hypothesized that knowing about the genetic basis of obesity could have positive and negative effects. This knowledge might be helpful for defining realistic goals, thus avoiding demoralization through overoptimistically started, but finally unsuccessful dietary attempts.5 Thus, knowing about the genetic origin of the person’s obesity could result in reductions of guilt for being overweight and in decreased negative mood. Such positive effects could be associated with better body acceptance, if an individual genetic background is evident after all. However, as self-control of eating behavior and physical activity is crucial for successfully avoiding further weight gains,6 it could also be postulated that knowledge about genetic etiology may have harmful effects for obese people, leading to loss of motivation to control food intake and beliefs that being overweight is unavoidable. These postulated negative effects would be associated with reductions in self-efficacy and a decrease in dietary restraint, and perhaps even weight gain. To date, only few studies have investigated the psychological impact of genetic information. For familial hypercholesterolemia, it was shown that finding a mutation had no impact on perceived control over the disease, but participants believed less strongly in the efficacy of diet in reducing their cholesterol level.7 Thus, knowing about the genetic etiology influenced the beliefs and motivation for behavior change.
The following study aims to evaluate the effects of informing obese people about the genetic determinants of obesity. This information is assumed to have positive effects in the sense of a decrease of self-blaming and feelings of guilt, but some negative effects are also possible (decrease of self-efficacy in weight control). The individual genetic evidence varies depending on whether the family history includes close relatives who are also obese or whether genetic evidence is based only on general scientific results, not on personal family data (low personal relevance). It is postulated that the effects of information on the genetic etiology of obesity will be more pronounced the more evident a personal genetic etiology is.
METHODS
Participants
Participants were part of a larger genetics study on obesity. For the present purpose, the study was designed to detect small effects with a power of 0.90 and an alpha-error level of p < 0.05 (minimum sample size is 300). We were able to include 410 obese persons in total, and 294 of them were randomized to 2 treatment conditions (see Table 1), which allowed us to detect differences in the direct comparison of the 2 intervention groups. The sample was a randomly selected subsample of obese people who were mostly encouraged by their GPs to participate in this study. Inclusion criterion was a BMI ≥30 and a willingness to participate if selected for a consultation session. One hundred sixteen participants were recruited far away from the study center (>200 miles) and served as the untreated control group; this control group was recruited the same way as the intervention group (mostly from their GPs). From the intervention participants, 294 obese people were randomly assigned to 1 of 2 consultations, either with or without genetic information (Fig. 1). The results of the 2 intervention groups were compared to the control group of obese people receiving no specific consultation. The 3 groups did not differ in terms of obesity duration (22 years in all groups; F = 0.2, p > 0.50), education (χ2 = 11.8; p = 0.07), and experiences with former diets (between 82 % in the genetic consultation group and 86% in the nongenetic consultation group; χ2 = 1.1, p > 0.20). The 2 intervention groups were also divided into 2 subgroups depending on a family history of obesity (criteria: at least 1 parent or 1 brother/sister was also obese).
Table 1.
Sample Characteristics
| Sample | N | Age (mean, SD) | % Female | BMI (mean, SD) | % Lower education |
|---|---|---|---|---|---|
| Intervention group 1 (with genetic information) | 147 | 44.7 (13.2) | 69.3 | 35.5 (5.3) | 29.4 |
| Intervention group 2 (without genetic information) | 147 | 45.2 (12.8) | 62.0 | 35.6 (6.0) | 29.9 |
| Control group | 116 | 47.2 (12.3) | 71.5 | 36.7 (5.1) | 44.6 |
Fig. 1.
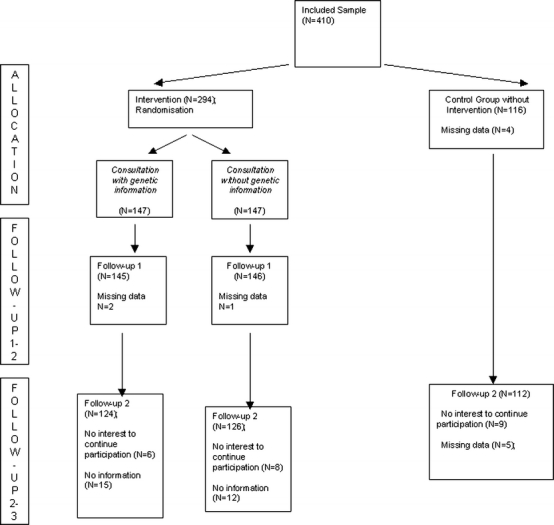
Sampling procedure
Measurements
According to the hypotheses, the following variables were relevant: BMI, restraint eating, body acceptance, feeling guilty, self-efficacy, and negative affectivity. Since we did not expect substantial weight changes after a 1-session consultation, primary outcome variables were feeling guilty and negative affectivity. To assess family history of obesity, Stunkard’s standard silhouettes were used to rate the body shape of parents and brothers/sisters.8 A different set of silhouettes for men and women was available; this procedure previously showed satisfactory reliability and validity.9
Restraint eating The restraint scale of the Dutch Eating Behavior Questionnaire (DEBQ-R) was included.10 The scale comprises 10 items describing intentions to restrict food intake for weight reasons. Items were rated on a 5-point scale from ‘never’ to ‘sometimes’ to ‘always’. Higher scores indicate more restraint eating.
Body acceptance We administered the subscale body self-acceptance from the Frankfurt Body Image Scale.11 It comprises 6 items (e.g., “I am pleased with my appearance”), which were administered with a 6-point scale (strongly disagree to strongly agree). The subscale discriminated well between obese and normal subjects.11 Higher scores indicate more body acceptance.
Feeling guilty Obesity-associated feelings of guilt were assessed by the subscale of the Weight-and-Body-Related Shame and Guilt Scale (WEB-SG).12 Subscale scores for the 6 guilt items range from 0 to 24 with higher scores indicating more frequent feelings of guilt.
Self-efficacy An adaptation of Bandura’s 10-item scale to assess self-efficacy was used.13 Participants answered on a 5-point Likert scale ranging from 0 (strongly disagree) to 4 (strongly agree).
Negative affectivity The ‘Positive and Negative Affect Scales’ contains 10 mood descriptors for negative effect using a 7-point Likert scale ranging from 1 (“strongly disagree”) to 7 (“strongly agree”).14 Higher scores indicate more negative affect.
As ad hoc screening for participant’s satisfaction, a self-rating scale was included after the consultation asking about satisfaction with the consultation, new insights, recommendation for others, etc. (Range from 1 [not at all] to 5 [yes, exactly]; see Table 2). One additional variable asked for acceptance of a genetic etiology before and after the intervention, and 6 months later.
Table 2.
Participants’ Satisfaction with Consultation
| Variable | Consultation without genetic information | Consultation with genetic information | Statistics (ANOVA F; p scores) |
|---|---|---|---|
| Today a lot of new insights about overweight became clear (after intervention) | 3.38 (1.1) | 3.60 (1.0) | F = 2.9 (p = 0.09) |
| 51.6% agreed | 65.4% agreed | ||
| 6 months later | 3.05 (1.2) | 3.33 (1.0) | F = 4.0 (p = 0.046) |
| 40.0% agreed | 52.8% agreed | ||
| I could benefit from this consultation (agreement after intervention) | 4.09 (0.7) | 4.11 (0.7) | F = 0.9 (p > 0.50) |
| 89.3% agreed | 90.0% agreed | ||
| 6 months later | 3.59 (0.9) | 3.65 (0.9) | F = 0.2 (p > 0.50) |
| 62.3% agreed | 63.8% agreed | ||
| I think every obese person should receive this consultation (agreement after intervention) | 4.45 (0.7) | 4.45 (0.7) | F = 0.0 (p > 0.50) |
| 92.3% agreed | 91.5% agreed | ||
| 6 months later | 4.15 (0.9) | 4.10 (0.9) | F = 0.2 (p > 0.50) |
| 80.8% agreed | 80.8% agreed | ||
| I think genetic factors are a major cause of obesity (baseline) | 3.67 (1.1) | 3.53 (1.1) | F = 0.6 (p > 0.50) |
| After consultation | 3.44 (1.1) | 3.91 (1.0) | F = 12.1 (p = 0.001) |
| 6 months later | 3.54 (1.1) | 3.63 (1.1) | F = 0.2 (p > 0.50) |
Range from 1 (not at all) to 5 (yes, exactly); only completers’ data.
Agreement was defined as a rating 4 or 5 of the 5-point Likert scale (5 = yes, I completely agree).
Procedure
The study center was jointly located at the University of Marburg, Department of Childhood and Adult Psychiatry, and Department of Clinical Psychology. The study protocol was approved by the local ethics committee of the medical school. A group of primary care physicians and occupational health physicians motivated many of their patients to participate. Further participants contacted the study center because of press releases. People interested to participate were informed that this study serves to analyze determinants and risk factors of obesity, and that some of the interested people will receive a consultation including helpful management strategies for obesity. As this study was part of a larger genetic study, all participants gave written informed consent before blood samples for genetic tests were collected. Of the Marburg sample, 294 participants were randomly selected and randomized to the 2 consultation conditions. Randomization was based on a list of the principal investigator who was not a consultant and, therefore, independent (WR); the sequence was generated by a random-number table. Consultants made the diagnostic interview with the participants and checked the randomization sequence to decide about the consultation condition. The 116 participants from more distant areas served as the control group not receiving a specific intervention (Fig. 1). The obese people of the intervention groups were assessed before the consultation (T1), immediately after the consultation (T2), and 6 months later (T3). Follow-up assessments (T3) were done using mailings (self-rating scales) and telephone interviews. If people did not respond, at least 3 further attempts were made to motivate for participation. The control group was only assessed at T1 and T3. Dropout rates were 15%, confirming satisfactory data quality. At T1, patients were weighed in light clothes in the laboratory of the university department, and body height was assessed. At T1 and T3, participants filled in the self-rating scales mentioned above. At T2, some of the self-ratings and an additional rating for consumer satisfaction was used. At T3, participants also reported their current body weight.
Consultation
The consultation without genetic information lasted 30 minutes, and included general information on body weight, information on the failure of most dietary approaches, encouragement of normal and regular food intake (to avoid yoyo weight cycles and binge eating), and regular activity. It was emphasized that weight change is only possible in a limited range (realistic goal setting). The consultation with genetic information included the same content, plus specific information on heredity, twin studies, and genetic transmission. If patients had a family history of obesity, this was integrated in the consultation. Because of the addition of genetic information, this consultation lasted about 10 minutes longer than the other one. Participants from both intervention groups received information leaflets summarizing the central information. Both consultations were manualized consisting of a 19-page (without genetic information) and a 28-page (with genetic information) manuscript (main topics see Table 5). The interventions were provided by 6 trained consultants (3 MDs, 3 clinical psychologists) doing both types of consultation to a comparable amount.
Table 5.
Consultation and Recommendations from GPs for Obese Patients
| Consultation and recommendations |
|---|
| Information on weight regulation, energy intake, and output balance. |
| Weight reduction is difficult; 95% of people fail to benefit from weight reduction programs. |
| Define realistic goals (e.g., maximally 5% loss of body weight) because overoptimistic goals are discouraging. For many people, it is a better option to accept being overweight than to fail permanently in dietary programs. |
| Define behavior changes (eating behavior, physical activity) that can be maintained for months or even years. |
| Avoid cycles of restraint eating and overeating; try to adopt a stable eating behavior pattern. |
| Do plan more physical activity as precise as possible (When? What? Who can support you? What are possible motivational barriers? How can they be overcome?). |
| In case of increased genetic risk (e.g., ≥50% of parents and siblings are overweight): |
| Inform patient about the heredity of obesity (twin studies). |
| Inform patient about molecular transmission of obesity-relevant genes. |
| If obesity is inherited, patients should not feel guilty because of being overweight. |
| Realistic goals (see above) are more important. |
Statistical Methods
The effect of the 2 interventions was analyzed using ANOVAS with repeated-measure designs with a group factor (3; 2 interventions plus 1 control group), and the repeated-measure factor assessment point (2; T1 versus T3; 3 if the instrument was also used at T2). The participant’s satisfaction was analyzed item-wise using one-way ANOVA. To analyze the possible moderating effect of personal relevance, the intervention groups were separated into those with a family history of obesity and those without. Afterwards, participants receiving an intervention were subjected to an ANOVA with 2 group factors (consultation type [2]; family history [2]), and again 1 repeated-measure factor (2, respectively 3 assessment points).
RESULTS
Participants’ Satisfaction with the Consultation
Table 2 shows the mean answers to items asking for participants’ satisfaction after the consultation and 6 months later. Of the participants, 80% to 92% rated the consultation as something every obese person should receive. Immediately after the consultation, most patients reported some benefits (thus, group differences could not be shown with this item because of a ceiling effect). For the item “a lot of new insights about obesity became clear”, those in the group receiving counseling and genetic information were significantly higher than those in the counseling group that did not receive genetic information. This group difference was stable at the 6-month follow-up. If the consultation included genetic information, a significant increase in genetic attributions could be observed, leading to significant group differences after the consultation (Table 2, last 3 rows). This effect decreased by the 6-month follow-up. However, if only the first assessment (before consultation) and the follow-up consultation (6 months later) were included into a repeated-measures ANOVA, the group × time interaction was almost significant (F = 3.6; p = 0.059). Thus, the inclusion of genetic information resulted in a change of causal attributions in participants, which was highly significant after the consultation and could be found as a trend at follow-up examination.
Long-term Effects of Interventions
Long-term positive or harmful effects of a consultation with genetic information would be seen by a significant interaction between group and assessment point in the repeated-measure ANOVA. Table 3 shows that this interaction was not significant for any of the measures. For some variables, however, a strong assessment time effect can be seen reflecting significant changes from baseline to the 6-month follow-up assessment. The body mass index was significantly lower at follow-up compared to baseline, a fact that was mainly because of the 2 intervention groups, although the group × time interaction did not reach significance. There was also a slight increase in restraint eating. Participants in both interventions had lower feelings of guilt because of their weight at follow-up compared to baseline. The consultation led to a slight increase in body acceptance and to a substantial decrease in negative affectivity, although both effects disappeared at follow-up. The highly positive short-term effects about negative affectivity underline that participants appreciated the consultation.
Table 3.
Long-term Effects (6 Months; Only Completers’ Data)
| Variable | Consultation without genetic information (126) | Consultation with genetic information (121) | No consultation (97) | Statistics (repeated-measure ANOVA) |
|---|---|---|---|---|
| BMI | ||||
| Baseline | 35.2 (5.0) | 35.8 (7.2) | 36.6 (5.1) | G: F = 2.5 (p = 0.08) |
| 6 months later | 34.6 (5.2) | 35.0 (5.5) | 36.5 (5.3) | T: F = 6.2 (p = 0.01) |
| G × T: F = 0.9 (p = 0.40) | ||||
| Restraint eating | ||||
| Baseline | 26.47 (7.7) | 28.76 (7.0) | 27.91 (6.4) | G: F = 1.9 (p = 0.15) |
| 6 months later | 28.12 (8.1) | 29.04 (6.7) | 29.17 (7.3) | T: F = 12.2 (p = 0.001) |
| G × T F = 1.9 (p = 0.15) | ||||
| Self-efficacy | ||||
| Baseline | 29.52 (5.0) | 29.29 (4.6) | 29.32 (5.3) | G: F = 0.0 (p > 0.5). |
| 6 months later | 29.24 (4.6) | 29.76 (4.1) | 29.78 (5.6) | T: F = 1.1 (p = 0.29) |
| G × T: F = 1.6 (p = 0.20) | ||||
| Feeling guilty | ||||
| Baseline | 19.2 (5.1) | 20.4 (5.5) | 20.4 (5.0) | G: F = 2.8 (p = 0.06); |
| 6 months later | 18.1 (5.4) | 19.5 (5.4) | 19.4 (5.5) | T: F = 22.4 (p = 0.0001) |
| G × T: F = 0.1 (p > 0.5) | ||||
| Body acceptance* | ||||
| Baseline | 19.67 (6.3) | 19.24 (5.4) | 18.71 (5.6) | G: F = 1.3 (p = 0.27) |
| After consultation | 21.38 (6.2) | 21.13 (5.3) | T: F = 9.9 (p = 0.002) | |
| G × T: F = 1.8 (p = 0.16) | ||||
| 6 months later | 20.78 (6.1) | 19.37 (5.1) | 19.71 (5.6) | |
| Negative affectivity* | ||||
| Baseline | 21.09 (7.3) | 21.06 (7.0) | 22.12 (8.2) | G: F = 0.6 (p > 0.5) |
| After consultation | 13.90 (13.9) | 13.02 (4.3) | T: F = 11.1 (p = 0.001) | |
| 6 months later | 20.31 (7.7) | 19.72 (7.0) | 20.61 (7.1) | G × T: F = 0.4 (p > 0.5) |
G: consultation group (3), T: assessment time point (2 or 3), G × T: interaction of diagnostic group and assessment time point in ANOVA.
*As these variables included post intervention assessments; only data from the 2 intervention groups were included into the ANOVA.
Differential Effects in Obese with Increased Genetic Risk Factors
To analyze whether the interventions led to differential effects depending on the personal relevance of genetic burden, the data were reanalyzed including a group factor “family history of obesity”. Persons from this group might be more influenced by the genetic information, as at least one of their parents or siblings were obese.
Table 4 shows that family history and time of assessment showed a significant interaction for BMI with no change in participants with other family members being overweight, whereas those people without family members with obesity reported a small weight reduction. The participants with a family history of obesity reported more improvements in “feelings of guilt” from T1 to follow-up, although this was not significantly different between the intervention groups. An interesting triple interaction was found for family risk group, type of intervention, and assessment time for the variable “negative affectivity”. All groups have reduced scores for negative affectivity after the intervention, but 2 groups relapsed until 6 months later. However, the groups with a match between family history and consultation type (no family history of obesity and no genetic information in consultation [1st column, Table 4]; family history of obesity and receiving consultation with genetic information [4th column, Table 4]) showed substantial improvements in negative affectivity compared to the other 2 groups. This is shown by a subsequent test including only assessment points 1 and 3, confirming a significant interaction between group and consultation type (F(1,233) = 4.6; p < 0.04).
Table 4.
Family History (Obese Family Members) and Effects of Consultation (Only Completers’ Data)
| Variable | Group without obese family members | Group with obese family members | Statistics (interaction effects with family history “FH”) | ||
|---|---|---|---|---|---|
| Consultation without genetic information (60) | Consultation with genetic information (46) | Consultation without genetic information (66) | Consultation with genetic information (75) | ||
| BMI (T1) | 35.4 (4.6) | 36.6 (10.1) | 35.1 (5.4) | 35.4 (4.7) | |
| BMI (6 months later) | 34.6 (4.9) | 34.5 (6.1) | 34.7 (5.6) | 35.3 (5.2) | FH × time: F = 4.8, p = 0.03; FH × IV × time: F = 2.3, p = 0.13 |
| Restraint eating (T1) | 26.7 (7.9) | 27.8 (8.1) | 26.3 (7.5) | 29.4 (6.2) | |
| Restraint eating (6 months later) | 28.1 (7.9) | 28.9 (7.7) | 28.1 (8.2) | 29.1 (6.0) | FH × time: F = 0.4, p > 0.5; FH × IV × time: F = 1.6, p = 0.21 |
| Self efficacy (T1) | 29.0 (5.1) | 29.1 (4.5) | 30.0 (4.9) | 29.4 (4.6) | |
| Self efficacy (6 months later) | 29.2 (4.5) | 29.9 (3.5) | 29.3 (4.8) | 29.7 (4.4) | FH × time: F = 2.1, p = 0.15; FH × IV × time: F = 0.2, p > 0.5 |
| Feeling guilty (T1) | 19.2 (5.7) | 19.5 (6.2) | 19.2 (4.5) | 20.9 (5.0) | |
| Feeling guilty (6 months later) | 18.8 (5.8) | 19.3 (5.3) | 17.5 (5.1) | 19.7 (5.5) | FH × time: F = 5.0, p = 0.03; FH × IV × time: F = 0.0, p > 0.5 |
| Body acceptance (T1) | 19.2 (6.4) | 19.7 (6.1) | 20.2 (6.2) | 18.9 (5.0) | |
| Body acceptance (after consultation) | 21.3 (5.7) | 21.6 (6.0) | 21.5 (6.6) | 21.0 (4.7) | |
| Body acceptance (6 months later) | 20.3 (6.3) | 19.8 (5.4) | 21.2 (6.0) | 19.1 (5.0) | FH × time: F = 0.0, p > 0.5; FH × IV × time: F = 0.0, p > 0.5 |
| Negative affectivity (T1) | 20.8 (7.3) | 19.5 (6.7) | 21.3 (7.4) | 22.0 (8.1) | |
| Negative affectivity (after intervention) | 13.1 (4.9) | 13.2 (4.2) | 14.6 (5.9) | 12.8 (3.8) | FH × time: F = 0.0, p > 0.5; FH × IV × time: F = 4.6, p = 0.03 |
| Negative affectivity (6 months later) | 19.1 (6.5) | 19.3 (6.2) | 21.3 (8.5) | 20.0 (7.4) | |
The column statistics includes only significant interactions with the factor “family history (FH) of obesity”; other statistical factors see the other tables and the “Results” section.
IV: intervention group (with versus without genetic consultation).
DISCUSSION
To our knowledge, this is one of the first studies evaluating the effects of informing obese people about the possible genetic origin of their being overweight, and it was unclear whether this information is helpful or harmful. In familial hypercholesterolemia, it was found that information about genetic backgrounds can reduce the beliefs that diets might be helpful.7 The results of our study show that obese people highly appreciate a general consultation, and the additional inclusion of genetic information can lead to new insights about weight problem. This suggests that genetic information about obesity is well-received. It was previously hypothesized that genetic testing and information about obesity may motivate healthier behavior,15 although others reject this idea.16
The results do not show any evidence for negative effects of genetic information on the eating behavior or self-control of obese people. Self-efficacy was comparable between different intervention groups and remained stable at the 6-month follow-up. This is very important, as self-motivation is one of the few predictors of weight control in obesity.17 Restraint eating increased at follow-up, but did not differ between groups. Weight was not substantially influenced by the interventions.
Another hypothesis was that personal relevance of genetic information might moderate the effects of the intervention. This was confirmed for the variable “negative affect”. The consultations led to substantial short-term decreases in negative affect, which relapsed partially at follow-up. However, this effect was moderated by family history of obesity (parents, brothers or sisters are also obese) and intervention type. If participants had a family history of obesity and received consultations including genetic information, this resulted in less negative mood at follow-up than before the intervention; the same was true for those participants without a family history of obesity who received a consultation without genetic information. Thus, depending on the personal relevance, the consultation should include genetic information or not. If the consultation matches the needs of the participants, it results in small, but positive long-term effects in terms of reduced negative affectivity.
There were no negative effects of the interventions on body weight. The only effect on body weight was for family history of obesity. Participants without a family history reported somewhat lower body weights at 6 months follow-up. Although this might be related to easier weight reduction in participants without family determinants of obesity, these data should not be overinterpreted. The weight data at follow-up were self-reported and might be subject to different distortions.
A criticism of these results could be that the effects are weak and that a correction of alpha inflation would abolish most described effects. However, with a 1-session consultation lasting 30 to 40 minutes, stronger effects cannot be expected and alpha correction would lead to a nondetection of possible weak or moderate effects. As possible negative effects were also tested, it would be more harmful not to detect them than accepting some alpha error inflation. We also did not report the intend-to-treat analyses, as they confirm the significance of results whereas reducing effect sizes; thus, not changing the pattern of results. Furthermore, the sample was informed that genetic tests would be done because of an associated study, which might have led to a selection bias, favoring the inclusion of people with positive attitudes toward genetics. This information had to be provided for ethical reasons; and therefore, this selection bias was unavoidable. To know more about generalizability, the study should be replicated without genetic tests and with other samples than GP patients. Finally, the interventions differed in duration with the genetic consultation lasting about 10 minutes longer; however, as the whole assessment took about 1 1/2 hours, it is unlikely that this time difference explains the effects. These shortcomings point to the fact that further replication is warranted.
These results have direct implications for clinical work. Considering the failure or weak effects of nonsurgical interventions in obesity,18–20 providing realistic information to affected people is crucial. Responding to the global epidemic of obesity, consultation is the major ingredient of a stepped care decision.19 Many obese people appreciate receiving information about realistic goals for weight change, about normalizing eating behavior, and increasing physical activity. If obese people have signs of genetic risk (e.g., MC4R mutations or family history of obesity), the consultation should include information about the genetic transmission of obesity. A summary of topics for consultation, which were appreciated from our obese patients is presented in Table 5.
Acknowledgement
This study was supported as part of the national genome research network NGFN by a grant from the German Ministry of Education and Research BMBF to Dr. Rief et al.
Conflict of Interest None disclosed.
References
- 1.Stunkard AJ, Harris JR, Pedersen NL, McClearn GE. The body mass index of twins who have been reared apart. N Engl J Med. 1990;222:1483–7. [DOI] [PubMed]
- 2.Farooqi IS, Keogh JM, Yeo GSH, Lank EJ, Cheetham T, O’Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348:1085–95. [DOI] [PubMed]
- 3.Hinney A, Bettecken T, Brumm H, et al. Prevalence, spectrum and functional characterization of Melanocortin-4 receptor gene variations in a representative population-based sample and obese adults from Germany. J Clin Endocrinol Metab. 2006;91(5):1761–9. [DOI] [PubMed]
- 4.Dempfle A, Hinney A, Heinzel-Gutenbrunner M, et al. Large quantitative effect of melanocortin-4 receptor gene mutations on body mass index. J Med Genet. 2004;41(10):795–800. [DOI] [PMC free article] [PubMed]
- 5.Byrne SM. Psychological aspects of weight maintenance and relapse in obesity. J Psychosom Res. 2002;53:1029–36. [DOI] [PubMed]
- 6.Goodrick GK. Inability to control eating: Addiction to food or normal response to abnormal environment? Drugs Soc. 1999;15:123–40. [DOI]
- 7.Marteau T, Senior V, Humphries SE, et al. Psychological impact of genetic testing for familial hypercholesterolemia within a previously aware population: A randomized controlled trial. Am J Med Genet. 2004;128A:285–93. [DOI] [PubMed]
- 8.Stunkard A, Sörensen T, Schulsinger F. Use of the Danish Adoption Register for the study of obesity and thinness. In: Kety S, Roland L, Sidman R, Matthysse S, eds. The Genetics of Neurological and Psychiatric Disorders. New York: Raven Press; 1983. [PubMed]
- 9.Bulik CM, Wade TD, Heath AC, Martin NG, Stunkard AJ, Eaves LJ. Relating body mass index to figural stimuli: Population-based normative data for Caucasians. Int J Obes. 2001;25:1517–24. [DOI] [PubMed]
- 10.Van Strien T, Frijters JER, Bergers GPA, Defares PB. The Dutch Eanting Behavior Questionnaire (DEBQ) for assessment of restrained, emotional and external eating behavior. Int J Eat Disord. 1986;5:295–315. [DOI]
- 11.Deusinger IM. Die Frankfurter Körperkonzeptskalen (FKKS) [The Frankfurt self-concept body scales]. Göttingen: Hogrefe; 1998.
- 12.Conradt M, Dierk JM, Schlumberger P, Rauh E, Hebebrand J, Rief W. Development of the weight- and body-related shame and guilt scale (WEG-SG) in a nonclinical sample of obese subjects. J Pers Assess. 2007;88:317–27. [DOI] [PubMed]
- 13.Schwarzer R, Jerusalem M. Generalized self-efficacy scale. In: Weinman J, Wright S, Johnston M, eds. Measures in Health Psychology: A User’s Portfolio. Windsor, UK: NFER-NELSON; 1995:35–7.
- 14.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1988;54:1063–70. [DOI] [PubMed]
- 15.Frosch DL, Mello P, Lerman C. Behavioral consequences of testing for obesity risk. Cancer Epidemiol Biomark Prev. 2005;14:1485–9. [DOI] [PubMed]
- 16.Lerman C, Croyle RT, Tercyak KP, Hamann H. Genetic testing: Psychological aspects and implications. J Consult Clin Psychol. 2002;70:784–97. [DOI] [PubMed]
- 17.Teixeira PJ, Going SB, Sardinha LB, Lohman TG. A review of psychological pre-treatment predictors of weight control. Obes Rev. 2005;6:43–65. [DOI] [PubMed]
- 18.Carels RA, Darby L, Cacciapaglia HM, et al. Applying a stepped-care approach to the treatment of obesity. J Psychosom Res. 2005;59:375–83. [DOI] [PubMed]
- 19.Wadden TA, Brownell KD, Foster GD. Obesity: Responding to the global epidemic. J Consult Clin Psychol. 2002;70:510–25. [DOI] [PubMed]
- 20.Dansinger M, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, weight watchers, and zone diets for weight loss and heart disease risk reduction. JAMA. 2005;293:43–53. [DOI] [PubMed]


