Abstract
Background
“Clinical inertia” has been defined as inaction by physicians caring for patients with uncontrolled risk factors such as blood pressure. Some have proposed that it accounts for up to 80% of cardiovascular events, potentially an important quality problem. However, reasons for so-called clinical inertia are poorly understood.
Objective
To derive an empiric conceptual model of clinical inertia as a subset of all clinical inactions from the physician perspective.
Methods
We used Nominal Group panels of practicing physicians to identify reasons why they do not intensify medications when seeing an established patient with uncontrolled blood pressure.
Measurements and Main Results
We stopped at 2 groups (N = 6 and 7, respectively) because of the high degree of agreement on reasons for not intensifying, indicating saturation. A third group of clinicians (N = 9) independently sorted the reasons generated by the Nominal Groups. Using multidimensional scaling and hierarchical cluster analysis, we translated the sorting results into a cognitive map that represents an empirically derived model of clinical inaction from the physician’s perspective. The model shows that much inaction may in fact be clinically appropriate care.
Conclusions/Recommendations
Many reasons offered by physicians for not intensifying medications suggest that low rates of intensification do not necessarily reflect poor quality of care. The empirically derived model of clinical inaction can be used as a guide to construct performance measures for monitoring clinical inertia that better focus on true quality problems.
KEY WORDS: clinical inertia, primary care, conceptual model
INTRODUCTION
“Clinical inertia” is a recently described phenomenon of physicians failing to intensify medication regimens at encounters with patients who have uncontrolled risk factors. Clinical inertia, so defined, is pervasive, with reports from various health systems and for multiple risk factors, including blood pressure, cholesterol and diabetes.1–7 It has been cited to potentially account for 80% of cardiovascular events, suggesting that it may be an appropriate focus for quality improvement and possibly public reporting.5,6,8
Given its widespread prevalence, so-called “clinical inertia” is surprisingly poorly understood. Medication management is a mainstay of medical school education, and most physicians would strongly agree that this is their responsibility. Yet, studies of established, uncontrolled hypertension patients presenting to primary care visits in the early 1990s revealed that medications were intensified at only 11% of such visits,1 rising to only 21% in 1999.2 In an accompanying editorial, O’Connor9 suggested that rates should be much higher, but noted that reasons why clinicians do not intensify are unknown. The low rate of intensifying coupled with numerous reports of under-treatment of hypertension among individuals above guideline-recommended targets warrants attention.4,9
In fact, in clinical practice, intensifying medications to manage risk factors like high blood pressure is not always advisable. Many patients have other more urgent medical conditions that warrant attention. Furthermore, patients do not always take medications as directed, and few would argue that increasing a dose is ill advised if the medication is not being taken as directed. Therefore, the current broad conceptualization of “clinical inertia” includes potentially appropriate “inaction” vis-á-vis medication intensification. Quality improvement programs may be most effective if they can focus on true opportunities for improvement separated from potentially appropriate care.
To build the formative foundation for future work in this area, we empirically derived a model of clinical inaction with the goal of differentiating appropriate care from potential opportunity for improvement. Using the Nominal Group technique, a form of structured group process described below, we asked primary care clinicians why they do not intensify hypertension medications in their patients with uncontrolled high blood pressure. We used a formal sorting exercise and multidimensional scaling with hierarchical cluster analysis to organize their responses as a cognitive map. Based on this map, we propose an empiric model of clinical inaction, which identifies distinct physician-based and patient-based sources of reasons not to intensify, and provides an approach to differentiating appropriate care from true inertia. This model sheds light on why physicians do not always intensify medications; it can serve as a guide for the design of interventions and performance measures; and it can inform policy decisions regarding clinical inertia.
METHODS
Information Gathering: the Nominal Group Technique
We focused first on physicians because medication intensification is directly under their purview, and they are likely to be the focus of performance measurements for accountability. We used the nominal group technique (NGT) to ask practicing physicians why they do not intensify medications. The NGT is a semi-quantitative method that lends itself to research in problem identification, with advantages over other structured group process techniques such as general focus groups. For example, NGT sessions do not allow any 1 individual to monopolize discussion; allow for group cohesiveness, which is conducive to self disclosure; are easy to implement; and results are easily interpreted.10,11
Our institution’s Institutional Review Board approved the study. Panelists were convenience samples recruited via fax invitation from lists of practicing primary care physicians, and received a $200 honorarium for participating. NGT sessions took place in a virtual meeting room, with panelists calling in by phone while logged onto an Internet web site. After introductions and explicitly stating the purpose of the meeting, panelists were asked to silently generate answers to the following: “You are seeing an established patient with known hypertension. At this visit, the blood pressure is uncontrolled (>140/90). What are some of the reasons when you might NOT adjust the blood pressure medication?”
The group facilitator then solicited each panelist’s responses in turn, generating a list visible to all panelists on the web site. Verbal exchange was limited to 1–2 sentences at a time, with the focus on understanding the meaning and logic of each item on the list. As is typical in the NGT, panelists were encouraged to “hitchhike”, or follow up on other members’ responses as a way of generating additional ideas. After panelists agreed that they had no additional items for the list, they were asked to vote anonymously online, selecting the 3 most important items on the list in their own opinion. The most important reason was given a weight of 3 points, the second most important a weight of 2, and the third most important a weight of 1. Each panelist therefore had 6 total votes to contribute. Results were summed by the facilitator and then tabulated and posted on the web site as a prioritized list reflecting the panel’s opinion about the most important reasons why they did not intensify medications.
Information Sorting: Card Sort
To organize the results of the NGT sessions, we performed a card-sorting exercise. Each of the 21 items that received any votes for being the most important reasons by the 2 NGT panels was written on an index card. We identified another convenience sample of 9 practicing physicians, none of whom had participated in the NGT sessions. Each participant worked independently. They were given the 21 index cards to sort into piles using only their own criteria to make decisions about how they thought the reasons not to intensify should group together. The only constraint that was imposed was to sort the items into at least 2 but no more than 10 groups.
Information Analysis: Cognitive Map
To analyze the results of the card-sorting exercise, we used multidimensional scaling (MDS)12 and hierarchical cluster analysis.13 The goal of MDS is to reveal the psychological dimensions in the data that can meaningfully describe the underlying cognitive constructs that are used to make sense of a phenomenon. These analyses result in a “cognitive map,” a term used to describe a spatial representation of how the items being analyzed are considered by the sorters to be similar to or different from each other. These types of data are sometimes called proximity or distance data, and this type of analysis has also been called “conceptual” or “perceptual” mapping. The empirically derived map in our study reflects how the card sorters cognitively organized the reasons not to intensify medications.
The data from the card-sorting task were first used to create a matrix for each participant. This co-occurrence matrix indicated the number of times each item was sorted with all other items (21 × 21 matrix). The individual co-occurrence matrices were then aggregated across all participants to form a group co-occurrence matrix. This matrix indicated the number of times all participants sorted each reason into similar or dissimilar piles.
The group co-occurrence matrix was then analyzed with MDS (ALSCAL algorithm) and cluster analysis to derive the cognitive map. MDS is a multivariate statistical technique intended to develop spatial structure from numerical data by estimating the observed similarities, or distances, between the units being analyzed. This iterative process is used to derive an optimal geometric solution that reflects the proximity of the data from the distances observed in the matrix (i.e., aggregated group co-occurrence matrix). The spatial configuration of the map therefore reflects the relative distance (similarity) between items, with similar reasons empirically mapped closer together, and dissimilar reasons mapped further apart, analogous to a roadmap. The map also determines the relative ordering of each attribute along the derived decisional dimensions.12
The number of dimensions in an MDS solution is determined using several strategies. Perhaps most important is the goal of a maximally interpretable solution. Kruskall and Wish recommended “as it is generally easier to work with two dimensional configurations than with those involving more dimensions, ease of use considerations are... important for decisions about dimensionality”14 MDS analysis results in two statistics that aid in selecting the number of dimensions for the solution, the R-square (RSQ) and stress statistics. The RSQ statistic reflects the extent to which the map corresponds to the actual proximity data. The closer the RSQ is to 1.0, the more closely the map represents the proximity data, with an RSQ >.90 suggesting high correspondence. The number of dimensions will increase the RSQ, but this may occur at the expense of interpretability as the number of dimensions rises above two. The stress statistic reflects the “badness of fit,” with stress >.20 indicative of poor fit, and stress <.01 suggestive of a degenerate solution. Thus, solutions with RSQ >.90 and .01< stress <.20 are acceptable. If several possible solutions meet these criteria, the one with the most interpretable number of dimensions is generally preferable.
Cluster analysis is another multivariate statistical technique which “divides a set of objects into a smaller number of homogeneous groups on the basis of their similarity,”13 facilitating the interpretation of the MDS map. The coordinates defining each item’s position in multidimensional space resulting from the MDS analysis are used as input data in the cluster analysis, which also iteratively seeks an optimal solution. The agglomeration schedule is examined to select the optimal number of clusters. The cluster analysis results can be designated on the map as groups of like reasons, often by enclosing the clusters within lines. The cluster analysis can create multiple levels of such groupings, essentially grouping highly similar areas into “families” within more broadly similar “extended families.”
It is worth noting that although MDS and cluster analysis are computationally sophisticated, they are not based on parametric statistics. Consequently, the validity of an MDS and cluster analysis solution is not sample size dependent, but a function of the representational adequacy of the sample participating in the sorting task.15 Validity in this context is a function of the map’s ability to accurately portray the data in a way that makes sense to the audience. While the location of items on the map is analytically determined, the meaning of the dimensions (i.e., axis labels) must be interpreted. A similar interpretation is required to present the results of a factor analysis, in which the meaning of the mathematically derived factors must be proposed. We used a standard approach to interpreting the derived dimensions by engaging the entire research team and examining the substantive differences between items at the extremes of the dimensions.14,16 An ideal interpretation makes sense to the audience.
RESULTS
Information Gathering
The initial 2 NGT panels generated remarkably similar lists of reasons not to intensify (Table 1), suggesting that additional panels would not be likely to add new reasons. Therefore, we only conducted 2 panels. The 2 independent NGT panels had 7 and 6 participants, respectively. Two of the 13 participants were female, age ranged from 42 to 63; 5 identified themselves as general internists and 8 as family practitioners. Three graduated from non-US medical schools (one did not specify), with mean 23 years since graduation (range 10–37 years).
Table 1.
Results* from 2 Nominal Group Technique Panels in Response to the Question: “When Would You NOT Intensify Medications when the Blood Pressure is High?”
| Panel 1 | Panel 2 |
|---|---|
| 1. Patients currently are not taking medications correctly | 1. Patient demonstrated poor compliance as a result of misunderstanding of medication instructions |
| 2. Patient demonstrates “white coat” or reactive hypertension and their home BP is lower than their office BP | 2. If a patient was upset before coming into the office |
| 3. If it has been difficult to bring a patient’s hypertension under control and several medication adjustments have been tried previously | 3. Cost ...if a patient cannot afford medications |
| 4. If I just changed a patient’s medications and there had not been sufficient time to observe an adequate effect | 4. If a patient’s BP was trending downward already |
| 5. If patients were symptomatic with light headedness/dizziness at intervals | 5. If a patient’s intercurrent illness (asthma, chronic bronchitis, COPD) supersedes hypertension |
| 6. If a patient was reluctant to take another medication | 6. If a patient’s medications have not had sufficient time to show an effect |
| 7. If a patient was under psychological or physical stress for another reason | 7. If a patient skips doses or ran out of medications |
| 8. If a patient’s BP was significantly better than the last time their medications were adjusted | 8. If a patient was intolerant of medication side effects |
| 9. If a patient had taken an OTC medication known to increase BP before arrival | 9. If a patient had good ambulatory BP (BP was elevated just in the office) |
| 10. If a patient had multiple allergies and chemical sensitivities | 10. If a patient showed confusion regarding dosage |
| 11. If a patient was already at their best possible level of control | 11. If a patient was not adhering to concurrent factors...such as weight control, diet, smoking, and alcohol use |
| 12. If a patient is experiencing side effects, e.g., edema or orthostasis | 12. If a patient was resistant to adding more medications or changing medications |
| 13. If a patient had just smoked a cigarette or had coffee/cola, I would be more likely to address their health habits | 13. If medication adjustment had been tried previously and patient’s BP was intractable/unresponsive |
| 14. If a patient cannot afford their medications | 14. If a patient had eaten salty food recently—the day of visit or the day before the visit |
| 15. If a patient was a particularly heavy salt eater | 15. If a patient was intolerant of normal BP...complains of fatigue and orthostasis at normal BP |
| 16. If a patient was coming to me for another problem...and their cardiologist is the one who manages their hypertension | 16. If I were concerned about medication interactions |
| 17. If there was a possibility of a medication interacting with other medication taken...comorbid conditions | 17. If a patient had a history of ischemia events |
| 18. If a patient was already on several medications for BP | 18. If medication adjustment would increase the risk of falling |
| 19. If a patient was in acute pain | 19. If a patient was seeing a specialist I would let the other doctor make medication adjustments |
| 20. If a patient had recent ischemia...and you want to maintain their BP at a higher level | 20. If this was the first time I had observed a patient’s BP at this level I might not adjust it |
| 21. If a patient was between dialysis treatments when their BP will be higher than usual | 21. If a patient had taken other medications, e.g., decongestants, that could increase the BP |
| 22. If a patient was having focal deficits | |
| 23. If a patient was developing acute renal failure or possible renal artery stenosis | |
| 24. If a patient was starting to show signs of MI/angina—ischemia seems to be worsening and I was worried about lowering BP |
*Reasons are presented in the order they were generated by the Panel. Reasons in bold were those that received any votes for being most important.
Panel 1 generated 24 distinct reasons why they did not intensify medications, and Panel 2 generated 21 (Table 1). Twenty of the 24 (83%) items generated by Panel 1 had direct corollaries in the list generated by Panel 2, sometimes with remarkably similar wording. Likewise, 19 of the 21 (90%) items generated by Panel 2 had corollaries on the list generated by Panel 1.
Panel 1 identified 11 items as being most important, and Panel 2 identified 10 (Fig. 1). While there were differences in which reasons each panel rated as most important, the same reason was rated most important by both panels. For Panel 1, the most important reason was that “patients were currently not taking medications as directed,” which received 26% of available votes, and similarly, for Panel 2, the most important reason was “patient had skipped doses or run out of medications,” with 25% of available votes. The Panels diverged on next most important reason, with Panel 1 rating “white coat hypertension” as the second most important, with 17% of available votes. White coat hypertension is a widely recognized phenomenon in which out of office blood pressures are in the normal range, and elevations are recorded in the office only.17 For Panel 2, “poor compliance due to misunderstood instructions” was the second most important, with 19% of available votes. The remainder of the reasons rated as important by each Panel are shown in Figure 1.
Figure 1.
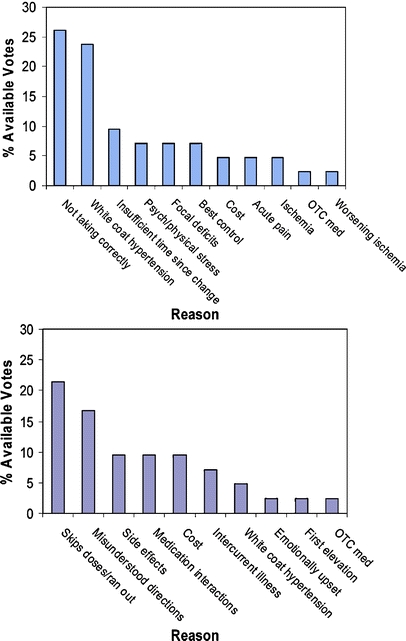
Most important* reasons why primary care doctors do not intensify medications for uncontrolled hypertension according to 2 NGT panels. *“Three Most important” was determined as follows. The Panel first generated the list in the Table, then each Panelist was asked to select their 3 most important reasons from this list: 3 = most important; 2 = second most important; 1 = third most important. Ratings for each reason were summed to arrive at a score reflecting the Panel’s most important reasons. Note that items from the Table not rated among the top 3 for any panelist received no score. Panel 1, with 7 members, had 42 available votes, and Panel 2, with 6 members, had 36. Psych=Psychological; OTC=Over-the-counter. See also text.
Information Sorting and the Cognitive Map
Nine practicing physicians performed the card-sorting exercise; none had participated in either NGT panel. Eight were general internists and 1 was an endocrinologist, ranging in age from 31 to 46. Five were women and 2 graduated from a non-US medical school, with a mean of 12.7 years elapsed since graduation from medical school (range 5–20).
The MDS revealed 3 possible solutions. The unidimensional solution had stress = 0.28 and RSQ = 0.76, indicative of a poor solution. The 2-dimensional solution, also the most interpretable, had stress = 0.09 and RSQ = 0.95. A 3-dimensional solution had stress = 0.04 and RSQ = 0.99, providing only modest gain over the 2-dimensional solution. We therefore present the 2-dimensional solution. The agglomeration schedule from the cluster analysis revealed 4 groups of cognitively most similar items, each within 2 larger groups of cognitive similarity. The cognitive map resulting from the MDS and cluster analysis is shown in Figure 2.
Figure 2.
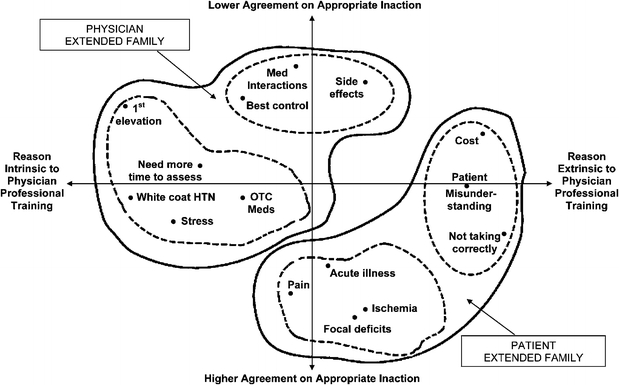
Failure to intensify blood pressure medications: Empirically derived* cognitive map from the physician’s perspective. *Cognitive map of 9 physicians’ sorting preferences analyzed using multidimensional scaling and hierarchical cluster analysis. We conceptualize reasons mapping toward the lower right of the map as falling into a “patient extended family,” and reasons mapping toward the upper left into a “physician extended family” (shapes defined by the solid line). Within each extended family are 2 distinct family groups of reasons (shapes defined by dashed lines). In the patient extended family, these family groups of cognitively most similar reasons relate to psychosocial and physical reasons; and in the physician extended family, they relate to potential transient elevations and limitations of medications. We propose that the y-axis may represent a domain of physician decision making relating to the degree of agreement on whether the reason is clinically appropriate for foregoing medication intensification. The x-axis may represent a domain of decision making relating to whether identifying the reason relies on, or is intrinsic to, the physician’s professional training. HTN=Hypertension; OTC=Over-the-counter; Med=Medication. See also text.
We propose that the upper left 2 family groups (dashed lines in Fig. 2) can be thought of as part of a larger “physician extended family” (solid line), within which the upper family group relates to the limitations of medications, and the left family group relates to possible transient elevations of blood pressure. The 2 lower right family groups can be thought of as part of a larger “patient extended family,” within which the bottom family group relates to the presence of physical symptoms, and the rightmost family group relates to psychosocial reasons for not intensifying medications.
We conceptualize the x-axis as a dimension of physician decision making with a neighborhood of reasons more extrinsic to the physician’s professional training on the right, and a neighborhood of reasons more intrinsic to the physician’s professional training on the left. The reasons on the left are ones that result primarily from the physician’s internal thinking process that relies on her professional training. Those on the right result from processes that these physicians viewed as more external to their professional training, and more involved with the patient’s psychosocial situation, understanding or motivation. The y-axis may represent a dimension of physician decision making that has a neighborhood of high agreement among physicians on the reasons to forego medication intensification at the bottom, and a neighborhood of less agreement among the reasons nearer the top. For example, most physicians would agree that hypertension medication management is unwarranted when the patient is in acute pain, a clinical situation known to elevate blood pressure transiently. The lower half of the map therefore represents an area enriched for “appropriate inaction.” On the other hand, a patient’s blood pressure judged by one physician to be “at the best level of control” may be judged by another physician to leave room for improvement. The top half of the map is likely to be enriched for true clinical inertia (Fig. 3).
Figure 3.
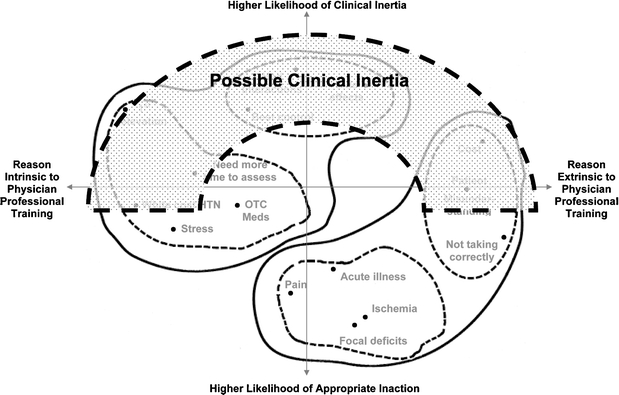
The empirically derived model of clinical inaction: area of cognitive map that may be enriched for clinical inertia. The Empirical Model of Clinical Inaction within all possible reasons for failure to intensify is based directly on the analytically derived cognitive map presented in Figure 2. In this Model, clinical inertia is a subset of all clinical inactions. It is more likely to be present when physicians disagree most about appropriateness of reasons offered for not intensifying (upper half of map). All reasons within possible clinical inertia (gray shaded shape) are not necessarily true clinical inertia. See also text.
It is worth noting that 61.5% of the total available votes for important reasons reside in the lower, “appropriate inaction” half of the cognitive map, suggesting that many of the reasons cited by physicians may reflect high quality care. The two reasons receiving the most votes for importance overall (not taking medications as directed (25.6%) and white coat hypertension (14.7%) are both within the “appropriate inaction” area.
DISCUSSION
The term “clinical inertia” has been used to describe “lack of treatment intensification in a patient not at evidence-based goals for care”.5 Our findings suggest that many such apparent “failures” to intensify medication regimens reflect potentially appropriate decisions in many cases. These findings suggest that part of the explanation for previously reported low intensification rates is appropriate inaction. Our empirically derived model of clinical inaction presents a framework that narrows the focus from all encounters at which medications were not intensified, to only a subset with more potential for improvement. Distinguishing potential clinical inertia from appropriate inaction is an important initial step for interventionists seeking to identify strategies to improve care and for policy makers seeking to measure quality of health care.
Although some have proposed that intensification should occur at 80% of encounters where risk factors are not controlled,6 this study suggests that appropriate intensification rates may be substantially lower. Indeed, 61.5% of the total available votes for important reasons not to intensify reside in the “appropriate inaction” neighborhood of the cognitive map. Importance as rated by physicians may not reflect actual prevalence of these reasons in practice, and our study was not able to determine this prevalence. However, in a study of lipid management, medical record review revealed that apparent failure to intensify lipid medications actually reflected acceptable quality of care in 24% of cases.18 Additional study of actual prevalence rates of appropriate inaction would help to quantify the size of the problem of true inertia.
It is worth noting that each reason even in the neighborhood of potential clinical inertia could in some cases represent clinically appropriate care. Consider the reason “at best level of control.” If the patient has had a trial of 1–2 antihypertensive medications and is currently on only 1 agent, the patient might in fact be able to achieve better control if additional medications were tried. On the other hand, in the Antihypertensive and Lipid Lowering to Prevent Heart Attack Trial, only 68% of trial participants achieved the target blood pressure level after an average of over 4 years in the aggressive medication management environment of a clinical trial.7 The 32% of trial participants who remained uncontrolled were likely to have received appropriate care.7 In addition, “best level of control” may appropriately differ from patient to patient as patients increase in complexity, especially in the geriatric population.19–21
The fact that none of the reasons generated by the physicians in our study stands out as clear instances of clinical inertia is noteworthy. We note that we purposely focused on physicians in this study, and it is possible that social undesirability may have prevented “forgetting to intensify” from emerging as a reason cited, leaving only more socially acceptable reasons. It is possible that observing actual practice could uncover reasons not generated by the physicians.
In contrast to the ambiguity in the potential clinical inertia neighborhood, many of the reasons grouped by the analyses into the appropriate inaction neighborhood are more clear-cut and potentially detectable. For example, acute complaints that warrant attention may be documented in the medical record. Medication nonadherence may not be consistently documented in medical records, but it may be detected in pharmacy data. Our empiric model can be used to define detectable exclusion criteria for intervention studies and in performance measures, focusing attention on where improvement is needed. Simplistic performance measures that do not separate appropriate inaction from opportunity for improvement may alienate American doctors, many of whom are disenchanted with aspects of the accountability movement.22,23
Our model of clinical inaction differs from previous models of clinical inertia such as O’Connor’s et al.,5 which defines clinical inertia broadly to include all patients with uncontrolled risk factors without evidence of intensification. O’Connor’s model was not empirically derived, but includes 3 major domains that are proposed to contribute to clinical inertia, namely, physicians, patients, and the health system. Our empirically derived model is from only the physician perspective, and mapped reasons into 2 extended families that were also proposed in O’Connor’s model, namely, physicians and patients. O’Connor proposed that physicians are responsible for 50% of clinical inertia, patients 30%, and the health system 20%; these proportions were not empirically derived. While it is possible that physicians may not focus on health system factors when generating reasons for not intensifying, the reasons offered by O’Connor as lying in the health system domain were not generated by either of our panels. These reasons included lack of guidelines or disease registries, no visit planning, no outreach, no decision support, lack of team approach to care or poor communication between physician and staff. A strength of the empiric approach is that it sheds some light on whether these theoretical reasons are actually considered important by practicing physicians in their day-to-day decision making. In fact, most reasons in the ‘potential opportunity’ area of the map are in the physician extended family, suggesting that physicians themselves view the greatest opportunity to overcome clinical inertia as lying within their own power.
Some limitations to this study are worth noting. We developed the empiric model based on a qualitative study of a convenience sample of 22 practicing primary care physicians, therefore it is possible that our results may not be representative. Nevertheless, the remarkable agreement between the 2 NGT panels on reasons why they do not intensify medications suggests that their opinions might reflect those of similar physicians. The empirically derived model is limited to the primary care physician’s perspective, and it is possible that subspecialists, nurses, health administrators, and patients could generate different lists of reasons, rate very different specific reasons as most important, and group them differently. While these other perspectives are important, hypertension management is performed mostly by primary care physicians in the US, therefore understanding their unique perspective is an important initial step in better understanding clinical inertia. Lastly, our interpretation of the axes and underlying cognitive similarities of the empiric model is not the only interpretation possible.
CONCLUSIONS
In summary, the model of clinical inaction that we empirically derived suggests that, from the physician perspective, appropriate inaction is an important component of the previous broad conceptualization of clinical inertia. Our model presents an alternative framework that can be used to differentiate appropriate inaction from potential true clinical inertia. In contrast to appropriate inaction, clinical inertia may influence clinical decisions with the most variation from physician to physician. Before performance measures for public accountability can be implemented, further studies to better define true clinical inertia, quantify its prevalence, and demonstrate variability suggestive of quality problems should be completed.
Acknowledgment
We thank Nelda Wray, MD, MPH for her helpful comments on an early draft of the manuscript. This work was made possible by support from NIDDK R18DK65001-01A2 (supported all authors, Allison, PI) and VA HSR&D IIR04-266 (supported Safford and Allison, Safford, PI).
Conflict of Interest Disclosure None disclosed.
References
- 1.Berlowitz DR, Ash AS, Hickey EC, et al. Inadequate management of blood pressure in a hypertensive population. N Engl J Med. 1998;339(27):1957–63. [DOI] [PubMed]
- 2.Borzecki AM, Wong AT, Hickey EC, Ash AS, Berlowitz DR. Hypertension control: how well are we doing? Arch Intern Med. 2003;163(22):2705–11. [DOI] [PubMed]
- 3.Grant RW, Cagliero E, Dubey AK, et al. Clinical inertia in the management of Type 2 diabetes metabolic risk factors. Diabet Med. 2004;21(2):150–55. [DOI] [PubMed]
- 4.National Institutes of Health N. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. http://www.nhlbi.nih.gov/guidelines/hypertension/jncintro.htm. [DOI] [PubMed]
- 5.O’Connor P, Sperl-Hillen J, Johnson P, Rush W, Biltz G. Clinical inertia and outpatient medical errors. In: Advances in Patient Safety: From Research to Implementation, Volume 2: Concepts and Methodology. Vol 2 (of 4). Rockville, MD: Agency for Healthcare Research and Quality; 2005:293–308.
- 6.O’Connor PJ. Overcome clinical inertia to control systolic blood pressure. Arch Intern Med. 2003;163(22):2677–78. [DOI] [PubMed]
- 7.Wright JT, Jr., Dunn JK, Cutler JA, et al. Outcomes in hypertensive black and nonblack patients treated with chlorthalidone, amlodipine, and lisinopril. JAMA. 2005;293(13):1595–608. [DOI] [PubMed]
- 8.Rodondi N, Peng T, Karter AJ, et al. Therapy modifications in response to poorly controlled hypertension, dyslipidemia, and diabetes mellitus. Ann Intern Med. 2006;144(7):475–84. [DOI] [PMC free article] [PubMed]
- 9.O’Connor P. Commentary—improving diabetes care by combating clinical inertia. Health Serv Res. 2005;40(6 Pt 1):1854–61. [DOI] [PMC free article] [PubMed]
- 10.Shewchuk R, O’Connor SJ. Using cognitive concept mapping to understand what health care means to the elderly: an illustrative approach for planning and marketing. Health Market Q. 2002;20(2):69–88. [DOI] [PubMed]
- 11.Levine DA, Saag KG, Casebeer LL, Colon-Emeric C, Lyles KW, Shewchuk RM. Using a modified nominal group technique to elicit director of nursing input for an osteoporosis intervention. Journal of the American Medical Directors Association 2006;7(7):420–5. [DOI] [PMC free article] [PubMed]
- 12.Schiffman S, Reynolds M, Young F. Introduction to Multidimensional Scaling. New York: Academic Press; 1981.
- 13.Aldenderfer M, Blashfield R. Cluster Analysis. Beverly Hills, CA: Sage Publications; 1984.
- 14.Kruskall J, Wish M. Multi-Dimensional Scaling. Newbury Park, NJ: Sage Publications; 1990.
- 15.Speece D. Methodological issues in cluster analysis: how clusters become real. In: Learning disabilities: Theoretical research issues. Hillsdale, NJ: Erlbaum; 1990:210–213.
- 16.Joseph F, Hair J, Anderson RE, Tatham RL, Black WC. Multivariate Data Analysis. 5th ed. Upper Saddle River, NJ: Prentice-Hall, Inc.; 1998.
- 17.Pickering TG. White coat hypertension: time for action. Circulation. 1998;98(18):1834–36. [DOI] [PubMed]
- 18.Kerr EA, Smith DM, Hogan MM, et al. Building a better quality measure: are some patients with ‘poor quality’ actually getting good care? Med Care. 2003;41(10):1173–82. [DOI] [PubMed]
- 19.Goodwin JS. Embracing complexity: a consideration of hypertension in the very old. J Gerontol Ser A Biol Sci Med Sci. 2003;58(7):653–8. [DOI] [PubMed]
- 20.Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294(6):716–24. [DOI] [PubMed]
- 21.Safford MM, Allison JJ, Kiefe CI. Patient complexity: more than comorbidity. The vector model of complexity. J Gen Intern Med. 2007;22(s9). [DOI] [PMC free article] [PubMed]
- 22.Bodenheimer T, May JH, Berenson RA, Coughlan J. Can Money Buy Quality? Physician Response to Pay for Performance. Center for Studying Health System Change; 2005. Available at http://www.hschange.org/CONTENT/807/. Accessed August 8, 2007. [PubMed]
- 23.Casalino LP, Alexander GC, Jin L, Konetzka RT. General internists’ views on pay-for-performance and public reporting of quality scores: a national survey. Health Aff (Millwood). 2007;26(2):492–9. [DOI] [PubMed]


