Abstract
Background
The care of patients with complex illnesses requires careful management, but systems of care management (CM) vary in their structure and effectiveness.
Objective
To create a framework identifying components of broad-based CM interventions and validate the framework, including using this framework to evaluate the contribution of varying components on outcomes of patients with chronic illness.
Design
We create the framework using retrospective information about CM activities and services over 12 months and categorize it using cluster and factor analysis. We then validate this framework through content and criterion techniques. Content validity is assessed through a Delphi study and criterion validity through relationship of the dosage measures and patterns of care to process and outcomes measures.
Participants
Patients with diabetes and/or cardiovascular disease receiving CM services in a model known as Care Management Plus implemented in primary care.
Results
Six factors of CM activity were identified, including a single dosage summary measure and 5 separate patterns of care. Of these, the overall dosage summary measure, face-to-face time, duration of follow-up, and breadth of services were all related to improved processes for hemoglobin A1c and LDL testing and control. Brief intense patterns of care and high face-to-face care manager time were also related to improved outcomes.
Conclusions
Using this framework, we isolate components of a CM intervention directly related to improved process of care or patient outcomes. Current efforts to structure CM to include face-to-face time and multiple diseases are discussed.
KEY WORDS: care management, management interventions, process of care, patient outcomes, chronic disease, disease management
INTRODUCTION
Because of the increasing evidence and recognition of the import of chronic diseases in the health system1 and current deficiencies in their treatment,2 improved chronic disease management has become a significant health policy issue in the United States. Various processes and programs to structure health care to improve chronic disease management and meet the needs of patients and other stakeholders have been recommended as potential solutions to the challenges of chronic disease care. Terms such as care coordination, disease management, case management, and care management (CM) are frequently used to describe these programs, but are used broadly and variably. For instance, disease management has been described as redesigning care within clinical settings for specific diseases3,4 or by third party companies.5,6 Care management may be used as a broad term to describe all processes where care is carefully structured to assure quality,7 or may refer only to the use of a care manager.8,9
Even within a narrowly defined example, such as telephonic CM, actual activities within programs vary. Calls may range from reminders (“Did you have the test?”), education (“Here is why it is important to have this test”), motivation (“Congratulations! You had the test!”), and many others. In addition, duration of the programs vary as do costs.3,10–16 Finally, similar services may be used independently, creating inefficiencies.1 These differences in programs may produce different outcomes.17 For example, impact on hospitalizations has ranged from an increase of 25% to a decrease of 80%.18,19
Given this variation in definitions, activities, and outcomes of CM, it is difficult to design an implementation of such a program, as no simple rules or a useful framework for success exists.19 Very general frameworks, such as that of Weingarten et al.,20 use categories such as physician profiling or patient education and find different success rates between categories. Intensity (resources consumed or time spent per patient) can be used, but neglects different types of services.21 A final option is to combine categories with measures of intensity, as Huber et al.22 did in their medication therapy model for CM. In prescribing a medication-based treatment for illness, one designates the breadth (e.g., isoniazid, rifampin, pyrazinamide, and ethambutol for tuberculosis), amount (e.g., 100 mg), duration (for 6 months), and frequency (four times per week). For addiction CM, Huber defined amount as minutes spent by a care manager, duration as time followed, frequency as number of visits or time between visits, and the number of different types of services as breadth. This model demonstrated some improved outcomes for certain “dosages” or regimens of CM.
At Intermountain Healthcare in Salt Lake City, United States, we have been involved in a large team-based chronic disease and CM program since 1997. The program provides a combination of technology in the form of care plans, best practices, and electronic communication, as well as people in the form of care managers and additional primary care team training, which we collectively refer to as Care Management Plus (CMP). The CMP model has proven strongly effective, improving control of disease and limiting hospitalizations.23–26 However, persons referred to CMP may have any of a variety of conditions and needs, leading to variation in processes and outcomes.23 We wondered whether we could better define effective and efficient CMP services based on this variation. In this paper, we expand Huber’s dosage framework to create a much broader framework for CM that is predictive of patient outcomes. We assessed both content validity (could we describe all variations in services?) and criterion validity (could variations predict differences in outcomes?). Our hypothesis was that the components and patterns of care would accurately describe services received and predict differences in outcomes.
METHODS
Setting and Intervention
The intervention setting has been described previously.23 Briefly, 7 care managers were installed in 7 clinics with 54 physicians at Intermountain Healthcare, a large, multipayer integrated delivery system in Utah. Training of physicians for appropriate referral was done formally and informally. The program was introduced to physicians at a formal training session; the physicians were encouraged to refer persons (especially older ones) with complex chronic diseases and social needs. Informally, the care managers gave feedback about the services and help they could provide for patients to the physicians.
Once a patient was referred, the CMP program had 2 major components. First, care managers assessed the patients’ needs, formulated (with the patient and caregivers) an individual care plan, then acted as a catalyst to help enact the plan. The plans themselves involved multiple members of the local clinic team (for example, physicians, pharmacists, medical assistants, and clerical staff) as well as off-site specialists, such as psychiatrists and endocrinologists. Care managers provided education, coached the patients, and identified barriers and solutions. They had the freedom to schedule in-house or home appointments with the patients, converse with physicians, contact outside agencies and companies to advocate on behalf of the patients, call care conferences, or arrange other services important for patient care. Care managers focused on several specific chronic conditions, including depression, diabetes, cardiovascular risk modification, and dementia. They received specific protocols in each condition, but had the flexibility to work outside the protocols.
The second component was information and communication technology. The CMP team had access to patient information and care plans in a variety of forms, including an electronic health record (EHR), a CMP-specific module for documentation and reminders, and a comprehensive summary sheet for chronic illness.24 Best practices in the form of general guidelines and specific protocols were taught to the entire team, and instituted in the summary sheet and as alerts within the EHR. Reminder (or “tickler”) lists for phone calls and unfinished components of longitudinal care plans entered into the EHR could be easily generated. Communication was facilitated through an electronic messaging system attached to patients’ charts; most referrals were generated through this system as were updates from clinicians on the team.
Data Collection Process
In addition to a robust EHR, care managers helped develop a separate care manager tracking database. This database captured structured care plans and instruments (such as the Patient Health Questionnaire 9-symptom checklist), in addition to reasons for each care manager activity. All dosage data were captured from this database.
Design
The study was a retrospective cohort, where all care-managed patients seen in 7 clinics from 2002 to 2004 were enrolled into the cohort. Baseline data (such as a patient’s previous adherence to protocols and disease burden) were calculated from the year before first visit, dosage data for the first year of CM, and follow-up data (such as adherence and disease control) from 13 months after the patient was last seen by the care manager.
Metrics for dosage were defined as amount (time spent on a patient’s care), duration (days followed), frequency (number of CMP services completed), and breadth (number of types of services completed). For greater clarity on time spent, amount of time was divided into direct (interacting with the patient) and indirect (away from the patient) categories. Direct time was further divided into face-to-face time and phone calls. Standard office visit times were excluded from the dosage calculations except when the care manager also attended. The care managers created the breadth categories through a 2-stage Delphi method; initial categories and definitions were distributed, commented on individually by care managers, and adjusted by the facilitator in an iterative process.
Dosage components were expected to be correlated, so principal components factor analysis with rotation was used to generate one factor for each major component. A summary dosage variable was created as the sum of the factors.
Patterns of care were separated using seeded k-means clustering techniques with 6 seeds, 1 for each uncorrelated dosage factor (minutes spent face-to-face, minutes spent calling, indirect minutes spent, duration, frequency, and breadth). The k-means clustering technique attempts to minimize variability of the factors within clusters and maximize variability between clusters. Each distinct cluster or pattern of care that emerged was given a name to highlight the approach taken (for example, the cluster with high face-to-face time and duration was called “active disease management” whereas the cluster with high indirect time and duration was called “active coordination”).27
For process and outcomes measures, appropriate patients were selected who would be eligible for hemoglobin A1c (HbA1c; patients with diabetes) and LDL (primary and secondary cardiovascular prevention) monitoring, and their adherence was compared to standard guidelines. For HbA1c testing, adherence was a test of at least every 6 months and for LDL testing, adherence was a yearly test. Baseline testing rates were calculated from 13 months to 2 weeks before first care manager contact to allow for previsit testing after referral. Changes in LDL and HbA1c were calculated from the first test immediately at or before care manager contact to the first test after care manager dosage calculation ended (at least 1 year after start date).
Statistical Analysis
For factor and cluster analyses, measures of discrimination were used to assess the validity of the factors and groupings. For the effect of doses and patterns on process, a 2-stage approach was used. First, variability in physician- and clinic-level adherence at referral was assessed using Mantel–Haenszel (MH) χ2. These helped us to understand the differences between physicians and clinics in both care manager dosage and process and outcomes. Then, patient-level multivariable logistic regression was used to compare dose with process measures, with variables to account for confounders of comorbidities (using a scale similar to Charlson, but with new weights for our population),28,29 age, sex, race, physician visits, and previous guideline adherence status. Conditional logistic regression models were used to further test assumptions of clinic-level effect to see if benefits were limited to one clinic/care manager.30 Model goodness of fit was assessed using the Hosmer–Lemeshow test. For the effect on outcomes, multivariable general linear modeling was used to predict changes in LDL and HbA1c with the same confounders, excluding previous process adherence measures but including previous LDL and HbA1c levels. We tested for clinic-level effects by using a fixed-effect-clustered model.30 SAS® version 9.1 was used for all statistical analyses.
RESULTS
Overview
During the study period, 7 care managers contacted 2,356 patients, as shown in Figure 1. Of these, 2,168 had calculable dosage; the remaining 8% moved or died during the dosage calculation period. Of those initially eligible for HbA1c (n = 866) and LDL guidelines (n = 1356), 92 (10.6%) and 133 (10.0%) moved or died during the study period, respectively, leaving 768 and 1,223 eligible for outcome measures.
Figure 1.
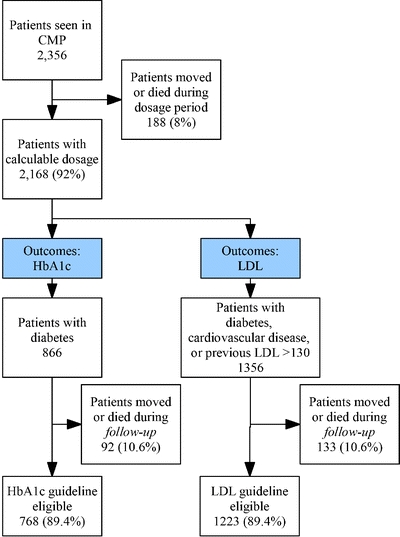
Process and outcome population selection for HbA1c and LDL guidelines.
As shown in Table 1, 10,194 (66.7%) of 15,290 attempted encounters or services by the CMP team were completed, or an average of 4.7 ± 6.4 per patient. Care managers entered 3,198 treated diagnoses for 2,132 (98.3%) patients, or 1.5 ± 1.2 diagnoses treated per patient. Of these patients, 463 (21.7%) had 2 or more diagnoses treated by the care manager. The most frequent diagnosis treated was diabetes in 866 (39.9% of 2,168) patients. Mental health (774 patients, 35.7%) and pure social referrals (438, 20.2%) were the next most frequent diagnoses (Table 2). Social needs included resource assistance, such as facilitation of medication or financial aid programs for patients, and other issues such as caregiver burnout. The primary diagnosis for referral was similar in frequency, with diabetes and mental health the most common, followed by social needs.
Table 1.
Baseline Description of Care Management (CM): Categories
| Category | Number of patients | Percent | Mean | SD | Number of services (%) | Frequency |
|---|---|---|---|---|---|---|
| Patients seen in CM | 2,356 | 100 | 15,290 (100)* | 7.1 ± 8.0 | ||
| Patients eligible for dosage calculation | 2,168 | 92 | 10,194 (66.7)** | 4.7 ± 6.4 | ||
| Total conditions | 3,198 | |||||
| Average conditions treated per patient | 1.5 | (1.2) | ||||
| Patients with more than 2 diagnoses | 463 | 21.7 |
SD = Standard deviation
*attempted services
**completed services
Table 2.
Baseline Description of Care Management (CM): Treated Conditions
| Conditions | Any condition | Primary condition | ||
|---|---|---|---|---|
| N | %* | N | % | |
| Diabetes | 866 | 39.9 | 768 | 35.4 |
| Mental health | 774 | 35.7 | 493 | 22.7 |
| Social/Organizational needs | 438 | 20.2 | 356 | 16.4 |
| Cardiovascular/Hypertension | 206 | 9.5 | 34 | 1.6 |
| Preventive needs | 117 | 5.4 | 27 | 1.2 |
| Asthma/COPD | 93 | 4.3 | 66 | 3.0 |
| Other endocrine | 84 | 3.9 | 47 | 2.2 |
| Addiction | 79 | 3.6 | 45 | 2.1 |
| Pain | 74 | 3.4 | 52 | 2.4 |
| Cognitive issues† | 27 | 1.2 | 18 | 0.8 |
| Fragile | 9 | 0.4 | 8 | 0.4 |
| Others | 431 | 19.9 | 213 | 9.8 |
*Percent of dosage eligible (N = 2,168).
†Includes dementia, poststroke confusion, and similar diagnoses.
Dosages and Components of Care
Table 3 displays the initial dosage estimates by patient. On average, the care team spent an amount of time equal to 203.5 ± 268.2 recorded minutes on CMP activities, 97.1 of which were direct and 106.4 were indirect. The majority of time with the patient was spent in CMP office or home visits. The frequency of services was 4.3 ± 6.4 per patient, and the average duration of CMP was under 4 months (112.1 days). Out of the 6 types of services, or breadth, the average patient received 2.6 ± 1.2. Correlation between dosage components ranged from Pearson’s r = .21 to 0.51. Factor analysis yielded 6 factors; all eigenvalues were >1.
Table 3.
Dosage, Process, and Outcomes Measures
| Dosage components* | Mean (SD) per patient per year | Number of patients | Baseline | Study | Change |
|---|---|---|---|---|---|
| Amount, minutes | 203.5 (268.2) | ||||
| Direct time | 97.1 (146.1) | ||||
| Face to face | 71.7 (130.5) | ||||
| Calls | 25.3 (41.8) | ||||
| Indirect time | 106.4 (142.1) | ||||
| Frequency (number of services) | 4.3 (6.4) | ||||
| Duration (days of follow-up) | 112.1 (104.4) | ||||
| Breadth† | 2.6 (1.2) | ||||
| Dosage summary (factors) | 0.0 (1) | ||||
| Process (%) | |||||
| HbA1c testing adherence | 768 | 50.0 | 70.2 | +20.2 | |
| LDL testing adherence | 1,223 | 65.0 | 76.0 | +11 | |
| Outcomes, mean (SD) | |||||
| HbA1c levels (%) | 539 | 8.2 (2.1) | 7.1 (1.6) | −1.1 (2.0) | |
| LDL levels (mg/dL) | 693 | 187 (50) | 155 (56) | −32.5 (63) |
*Calculated from 10,194 completed encounters
†Breadth was the count of the following service categories: education, motivation, following protocols, communication/collaboration, community advocacy, and coordinating activities.
Five CMP patterns of care were identified through the cluster analysis (Cubic Clustering Criterion ranged from 3 to 6). First, active coordination was dominated by many indirect sessions over most of the year and covered a wide variety of activities (4.4 out of 6 breadth categories). The second, active disease treatment, had high face-to-face minutes. Patients receiving ongoing maintenance had a low amount of CMP over the entire year. Brief intense had moderate breadth and high amount over a short duration, and resource aid had very short duration and amount. Patients who received resource aid were most likely to be referred solely for social and financial needs rather than specifically for the chronic conditions they possessed. More about the dosage elements and patterns of care are included in an appendix hosted at http://www.caremanagementplus.org.
Adherence to Testing Guidelines: Process Measures
Values for process and outcomes revealed significant changes. HbA1c testing adherence started at 50% and increased to 70.2% during the study, whereas LDL testing increased from 65 to 76%. There were some significant physician- and clinic-level effects; baseline rates of testing adherence by clinic varied for HbA1c (MH χ2 = 20.2; P value = .001) and LDL (MH χ2 = 21.1; P = .0003). Within-clinic physician-level effects did not show significant differences, but sample sizes per physician were small (average of 14.2 and 22.1 patients per physician for HbA1c and LDL analyses, respectively).
The results of the multivariable logistic regression, shown in Figure 2, found that some dosage components were significantly related to the increase in process adherence (overall models all P < .0001; c = 0.65–0.72). For every standard deviation (SD) increase in the dosage summary score, the odds ratio (OR) of receiving appropriate HbA1c testing was 1.42 (95% confidence intervals (CI) 1.26, 1.59). A 3 SD increase in dosage score (for example, the difference between a 30-minute visit once with only education and three 30-minute visits over a 6-month duration with multiple different services) was associated with a 2.8 times increase in the odds of compliance. In addition, higher quantities of face time (OR 1.48; 95% CI 1.16, 1.89), duration (OR 1.43; 95% CI 1.23, 1.90), and breadth (OR 1.39; 95% CI 1.11, 1.73) were associated with higher HbA1c adherence. Adherence to LDL guidelines was associated with higher dosage quantity received; each increase in SD of dosage summary score was associated with an 8% higher odds of receiving LDL on time, as was increased face time (14% higher odds) and breadth (13% higher odds; all P < .05). Conditional logistic regressions (data not shown) with adjustment for clinics did not significantly alter these results.
Figure 2.
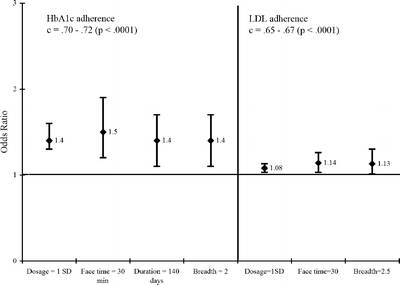
Dosage prediction of process measures for HbA1c and LDL adherence. HbA1c = Hemoglobin A1c, LDL = low density lipoprotein, SD = standard deviation, c = c statistic from logistic regression, min = minutes.
Patterns of care were also related to HbA1c and LDL guideline adherence, as shown in Figure 3. Patients receiving CMP patterns in the form of active coordination, ongoing maintenance, and active disease treatment categories were all significantly more likely to be adherent to the protocols than those receiving resource aid or brief intense (P < .01).
Figure 3.
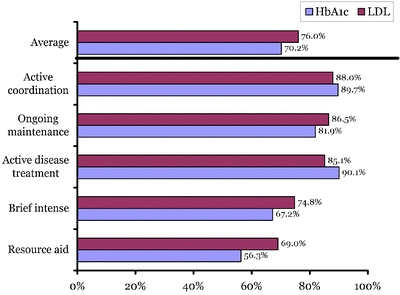
Percent adherent to guideline by pattern of care. HbA1c = Hemoglobin A1c, LDL = low density lipoprotein.
Change in HbA1c and LDL: Outcomes Measures
HbA1c values dropped from an average of 8.2 ± 2.1 to 7.1 ± 1.6%, whereas LDL levels dropped from 187 ± 50 to 155 ± 56 mg/dL (−32 mg/dL difference). Overall regression models were strongly positive (R2 = .48–0.50; F = 33.2–95.9; P < .0001). Both overall dosage measures and patterns of care were associated with changes in HbA1c, whereas only specific patterns of care were associated with changes in LDL (Table 4). Increased face time (where the CMP team and the patient and their caregivers met in person) was related to greater negative (or desirable) changes in HbA1c levels. Both active disease treatment and brief intense patterns of care were associated with decreases in HbA1c, and the brief intense pattern was the only significant component related to decreased LDL levels. However, active coordination was related to a clinically significant downward trend in LDL levels. Neither clinic level nor physician level were significant in a multilevel fixed-effect version of the model.
Table 4.
Multivariate Dosage Prediction of Changes in HbA1c and LDL Levels
| R2 | Significant predictor variables (direction) | P value | Other variables | |
|---|---|---|---|---|
| Change in HbA1c | ||||
| Dosage factors | 0.48 | Face time (−) | .005 | Baseline HbA1c (−) |
| Frequency (+) | .004 | Risk Score (+) | ||
| Dosage clusters | 0.50 | Active disease treatment (−) | .02 | Baseline HbA1c (−) |
| Brief intense (−) | .01 | Risk Score (+) | ||
| Change in LDL | ||||
| Dosage factors | 0.49 | None | Baseline LDL (−) | |
| Risk Score (−) | ||||
| Dosage clusters | 0.49 | Brief intense (−) | .04 | Baseline LDL (−) |
| Active coordination (−) | .07 | Risk Score (−) | ||
HbA1c = Hemoglobin A1c; LDL = low density lipoprotein
DISCUSSION
We created and tested a framework to describe CMP efforts in terms of dosage and patterns of care. Using the expertise of CM practitioners, new definitions of amount, duration, frequency, and breadth of CMP were created and quantified. Time spent face-to-face with patients, the number of different services provided, and the duration of time affected process and outcome measures for patients. The most persistent factor, face-to-face time, influenced all but the change in LDL. With the current rise of telephonic CM programs, the importance of face-to-face time should be carefully considered when designing programs. Five distinct patterns of care were revealed and were related to improved process and outcome measures. Content validity was met through the breadth of categories, whereas the relationship of the variation of the dosage components and patterns to the subsequent changes in process and outcomes constituted criterion validity.
These patterns of care can and are being used to define and prescribe CM services. We found that a broad set of services were frequently required to care for the patients; the care managers typically prioritize global issues first, such as social needs and mental health needs, and then work on the complex physical illnesses. In our location, we are adapting referral forms to reflect specific patterns such as these. We are also testing the ability to prescribe CM through a randomized controlled trial, where a physician randomized to treatment receives structured alerts on patients eligible for CMP and the care manager receives a general prescription. For others interested in adopting these kind of CM programs, the patterns seen here may be translated into general workflow for the care manager or clinic staff, with allowances made to deviate.
Testing the framework retrospectively limits the study. First, patterns and dosages of care received may be dependent on patient characteristics related to adherence, such as willingness to participate or to engage in care, rather than the obverse. Second, referral bias may occur. Low HbA1c and LDL testing adherence in the population may have triggered the referral and the suggested treatment; this supposition does not alter the benefit from CM. Rather, it demonstrates that an appropriate population was referred and was likely to improve with CM. Third, we selected process and outcomes measures specifically addressed by care managers; other process and outcomes measures not addressed by care managers could have worsened in the process. The care managers addressed general issues (such as social needs and mental health issues) that affected many chronic illnesses; for instance, we found that 35% of patients had barriers to adherence, and that 68% of them were amenable to intervention.25 Disparities between patients and settings may affect the results and conclusions and, besides caring for patients with financial and social barriers, we did not specifically measure socioeconomic status. Finally, the framework does not test causality, but rather detects associations between services rendered and improvement in the processes and outcomes.
In all, a quantifiable framework for dosage was created and partially validated against process and outcomes measures. By drilling down to the core components of a CM program, we attempt to describe patterns and components of CM, which can be easily extended and modified elsewhere, aiding those interested in gaining the benefits of such programs.
Acknowledgements
This study was supported by grant no. 2001-0465 from the John A. Hartford Foundation and David A. Dorr was supported through a grant from the National Library of Medicine (K22 LM 8427-01). We would like to thank Paul D. Clayton, PhD, for his tireless efforts with the CM program and his mentorship to our team, as well as the Intermountain management team for their financial support and encouragement. We would also like to thank Diane Huber, PhD, for her pioneering work in this area. Additional information about the program is available at http://www.caremanagementplus.org.
Conflict of Interest None disclosed.
References
- 1.Wolff JL, Boult C, Boyd C, Anderson G. Newly reported chronic conditions and onset of functional dependency. J Am Geriatr Soc. 2005;53(5):851–5 (May). [DOI] [PubMed]
- 2.Institute of Medicine. Crossing the Quality Chasm. A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001. [PubMed]
- 3.Keyserling TC, Ammerman AS, Samuel-Hodge CD, Ingram AF, Skelly AH, Elasy TA, et al. A diabetes management program for African American women with type 2 diabetes. Diabetes Educ. 2000;26(5):796–805 (Sep–Oct). [DOI] [PubMed]
- 4.Wagner EH. The role of patient care teams in chronic disease management. BMJ. 2000;320(7234):569–72 (Feb 26). [DOI] [PMC free article] [PubMed]
- 5.Nobel JJ, Norman GK. Emerging information management technologies and the future of disease management. Dis Manag. 2003;6(4):219–31 (Winter). [DOI] [PubMed]
- 6.Kwon HS, Cho JH, Kim HS, Lee JH, Song BR, Oh JA, et al. Development of web-based diabetic patient management system using short message service (SMS). Diabetes Res Clin Pract. 2004;66(suppl)1:S133–7 (Dec). [DOI] [PubMed]
- 7.Casalino L, Gillies RR, Shortell SM, Schmittdiel JA, Bodenheimer T, Robinson JC, et al. External incentives, information technology, and organized processes to improve health care quality for patients with chronic diseases. JAMA. 2003;289(4):434–41 (Jan 22–29). [DOI] [PubMed]
- 8.Huston CJ. The role of the case manager in a disease management program. Lippincotts Case Manag. 2001;6(5):222–7 (Sep–Oct). [DOI] [PubMed]
- 9.Overhage JM, Perkins S, Tierney WM, McDonald CJ. Controlled trial of direct physician order entry: effects on physicians’ time utilization in ambulatory primary care internal medicine practices. J Am Med Inform Assoc. 2001;8(4):361–71 (Jul–Aug). [DOI] [PMC free article] [PubMed]
- 10.Maljanian R, Grey N, Staff I, Conroy L. Intensive telephone follow-up to a hospital-based disease management model for patients with diabetes mellitus. Dis Manag. 2005;8(1):15–25 (Feb). [DOI] [PubMed]
- 11.Ferrer-Roca O, Franco Burbano K, Cardenas A, Pulido P, Diaz-Cardama A. Web-based diabetes control. J Telemed Telecare. 2004;10(5):277–81. [DOI] [PubMed]
- 12.Noel HC, Vogel DC, Erdos JJ, Cornwall D, Levin F. Home telehealth reduces healthcare costs. Telemed J E Health. 2004;10(2):170–83 (Summer). [DOI] [PubMed]
- 13.Montori VM, Helgemoe PK, Guyatt GH, Dean DS, Leung TW, Smith SA, et al. Telecare for patients with type 1 diabetes and inadequate glycemic control: a randomized controlled trial and meta-analysis. Diabetes Care. 2004;27(5):1088–94 (May). [DOI] [PubMed]
- 14.Krishna S, Balas EA, Spencer DC, Griffin JZ, Boren SA. Clinical trials of interactive computerized patient education: implications for family practice. J Fam Pract. 1997;45(1):25–33. [PubMed]
- 15.Edmonds M, Bauer M, Osborn S, Lutfiyya H, Mahon J, Doig G, et al. Using the Vista 350 telephone to communicate the results of home monitoring of diabetes mellitus to a central database and to provide feedback. Int J Med Inform. 1998;51(2–3):117–25 (Aug–Sep). [DOI] [PubMed]
- 16.Leggett-Frazier N, Swanson MS, Vincent PA, Pokorny ME, Engelke MK. Telephone communications between diabetes clients and nurse educators. Diabetes Educ. 1997;23(3):287–93 (May–Jun). [DOI] [PubMed]
- 17.Ferguson JA, Weinberger M. Case management programs in primary care. J Gen Intern Med. 1998;13(2):123–6 (Feb). [DOI] [PMC free article] [PubMed]
- 18.D’Ercole A, Struening E, Curtis JL, Millman EJ, Morris A. Effects of diagnosis, demographic characteristics, and case management on rehospitalization. Psychiatr Serv. 1997;48(5):682–8 (May). [DOI] [PubMed]
- 19.Hillestad R, Bigelow J, Bower A, Girosi F, Meili R, Scoville R, et al. Can electronic medical record systems transform health care? Potential health benefits, savings, and costs. The adoption of interoperable EMR systems could produce efficiency and safety savings of $142–$371 billion. Health Aff (Millwood). 2005;24(5):1103–17 (Sep–Oct). [DOI] [PubMed]
- 20.Weingarten SR, Henning JM, Badamgarav E, Knight K, Hasselblad V, Gano A, Jr., et al. Interventions used in disease management programmes for patients with chronic illness—which ones work? Meta-analysis of published reports. BMJ. 2002;325(7370):925. [DOI] [PMC free article] [PubMed]
- 21.Philbin EF. Comprehensive multidisciplinary programs for the management of patients with congestive heart failure. J Gen Intern Med. 1999;14(2):130–5 (Feb). [DOI] [PubMed]
- 22.Huber DL, Sarrazin MV, Vaughn T, Hall JA. Evaluating the impact of case management dosage. Nurs Res. 2003;52(5):276–88 (Sep–Oct). [DOI] [PubMed]
- 23.Dorr DA, Wilcox A, Donnelly SM, Burns L, Clayton PD. Impact of generalist care managers on patients with diabetes. Health Serv Res. 2005;40(5 Pt 1):1400–21 (Oct). [DOI] [PMC free article] [PubMed]
- 24.Wilcox A, Jones S, Dorr D, Cannon W, Burns L, Radican K, et al. Usage and impact of a computer-generated patient summary worksheet for primary care. Proc AMIA Symp. 2005:8240828. [PMC free article] [PubMed]
- 25.Dorr D, Brunker C, Wilcox A, Burns L. Implementing protocols is not enough: the need for flexible, broad based care management in primary care. TRIPP conference; 2006 July 10–12. Washington, DC: AHRQ; 2006.
- 26.Dorr DA, Wilcox A, Burns L, Brunker CP, Narus SP, Clayton PD. Implementing a multidisease chronic care model in primary care using people and technology. Dis Manag. 2006;9(1):1–15 (Feb). [DOI] [PubMed]
- 27.Kaufman L, Rousseeuw P. Finding Groups in Data: An Introduction to Cluster Analysis. New York, NY: Wiley; 1990.
- 28.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9 (Jun). [DOI] [PubMed]
- 29.Dorr DA, Jones SS, Burns L, Donnelly SM, Brunker CP, Wilcox A, et al. Use of health-related, quality-of-life metrics to predict mortality and hospitalizations in community-dwelling seniors. J Am Geriatr Soc. 2006;54(4):667–73 (Apr). [DOI] [PubMed]
- 30.Localio AR, Berlin JA, Ten Have TR, Kimmel SE. Adjustments for center in multicenter studies: an overview. Ann Intern Med. 2001;135(2):112–23 (July 17). [DOI] [PubMed]


