Abstract
In past studies in which we mapped 2-deoxyglucose uptake evoked by systematically different odorant chemicals across the entire rat olfactory bulb, glomerular responses could be related to each odorant's particular oxygen-containing functional group. In the present study, we tested whether aliphatic odorants containing two such functional groups (esters, ketones, acids, alcohols, and ethers) would stimulate the combination of glomerular regions that are associated with each of the functional groups separately, or whether they would evoke unique responses in different regions of the bulb. We found that these very highly water-soluble molecules rarely evoked activity in the regions responding to the individual functional groups; instead, they activated posterior glomeruli located about halfway between the dorsal and ventral extremes in both the lateral and the medial aspects of the bulb. Additional highly water-soluble odorants, including very small molecules with single oxygenic groups, also strongly stimulated these posterior regions, resulting in a statistically significant correlation between posterior 2-deoxyglucose uptake and molecular properties associated with water solubility. By showing that highly water-soluble odorants stimulate a part of the bulb associated with peripheral and ventral regions of the epithelium, our results challenge a prevalent notion that such odorants would activate class I odorant receptors located in zone 1 of the olfactory epithelium, which projects to the dorsal aspect of the bulb.
Keywords: rat, 2-deoxyglucose, odors, imaging techniques, mapping
The first stage of olfactory coding involves the transduction of odorant chemical information into neural responses. This stage involves the interaction of odorant chemicals with odorant receptors present on specialized olfactory sensory neurons in the mucosa of the nose (Buck and Axel, 1991). Different odorant chemicals activate different subsets of these sensory neurons (Sicard and Holley, 1984; Sato et al., 1994; Youngentob et al., 1995; Scott et al., 2000), determined both by the specificity of the single class of odorant receptor expressed in each neuron (Malnic et al., 1999; Araneda et al., 2000) and by the physical distribution of the odorant molecules in different parts of the nose (Hornung and Mozell, 1977; Kent et al., 1996; Zhao et al., 2006). Sensory neurons that express the same odorant receptor gene are typically distributed within broad zones extending from anterior to posterior through the nose (Vassar et al., 1993; Ressler et al., 1993; Miyamichi et al., 2005). These homologous sensory neurons generally converge in their axonal projections to two glomeruli in the olfactory bulb such that the sensory neurons located medially, nearer the nasal septum, terminate in a medial glomerulus, while those located laterally in the nose terminate in a lateral glomerulus (Astic and Saucier, 1986; Clancy et al., 1994; Mombaerts et al., 1996; Levai et al., 2003). The locations of these glomeruli are largely consistent from animal to animal (Strotmann et al., 2000; Schaefer et al., 2001). Sensory neurons expressed in the central channel (zone 1) of the nose project to the dorsal half of the olfactory bulb, while sensory neurons in increasingly ventral or lateral positions within the nose project increasingly ventrally in the bulb (Mori et al., 1985; Saucier and Astic, 1986; Schwob and Gottlieb, 1986; Schoenfeld et al., 1994; Miyamichi et al., 2005).
Because of the glomerular convergence of axonal projections from homologous sensory neurons into a limited number of stereotypically located glomeruli, it is possible to monitor the activation of the full complement of odorant receptors by measuring the activation of glomeruli across the entire surface of the olfactory bulb. We have been exploiting this situation over the past several years to investigate the odorant chemical features that are most relevant to olfaction (Johnson and Leon, 2000b; Johnson et al., 2005a; 2006; 2007; Ho et al., 2006b), to determine the relative specificity of receptors for systematically different odorant chemicals (Johnson et al., 1998, 1999, 2004, 2005a,b, 2006; 2007; Johnson and Leon, 2000a,b; Farahbod et al., 2006; Ho et al., 2006a,b), and to explore relationships between odorant specificity and spatial arrangements of glomeruli within the layer (Johnson et al., 1999, 2004; Johnson and Leon, 2000b; Ho et al., 2006a). As an activity marker, we measure uptake of [14C]2-deoxyglucose (2DG) because it provides equal access to glomeruli throughout the structure (Stewart et al., 1979; Royet et al., 1987). We collect the data into anatomically standardized data matrices (Johnson et al., 1999), which allows us to evaluate responses across animals quantitatively and statistically.
As is the case with other receptor-ligand interactions, not all parts of an odorant ligand molecule are expected to be necessary for receptor binding and activation. Comparisons of odorants that differ systematically in only certain aspects of their molecular structure have shown that oxygen-containing functional groups are important determinants of activity for many glomeruli (Johnson and Leon, 2000a; Uchida et al., 2000; Johnson et al., 2002; Takahashi et al., 2004a). Simple aliphatic odorants with the same oxygenic functional groups (e.g., straight-chained carboxylic acids) tend to activate overlapping glomeruli that are not activated by corresponding aliphatic odorants possessing different functional groups (e.g., straight-chained ketones) (Johnson and Leon, 2000a; Johnson et al., 2002). Differences in hydrocarbon structure for odorants possessing the same oxygenic functional group were associated with differences in levels of glomerular activity in other parts of the bulb (Johnson et al., 1999; Johnson and Leon, 2000b). These observations led to a simple model of combinatorial odor coding wherein functional group and hydrocarbon structure were seen as separately extractable features recognized by separate groups of odorant receptors projecting to spatially segregated glomerular clusters (Johnson et al., 2002). However, more recent results have suggested that there might be interactions between certain combinations of functional groups and hydrocarbon structures that lead to patterns of activity not entirely predictable on the basis of responses to the individual chemical features of the odorants (Johnson et al., 2005a,b, 2007).
So far, systematic studies of related odorant structures have been limited to molecules possessing only a single (or no) functional group. In the present study, we have tested odorants with more than one functional group in order to test further predictions of the simple hypothesis of combinatorial coding. Will odorants with two different functional groups activate the combination of glomerular clusters previously associated with those separate functional groups, as predicted by the simple combinatorial coding hypothesis, or will the combination of the two functional groups on a single odorant molecule be treated as a new feature? If there are two of the same functional groups in an individual odorant molecule, will this odorant activate the same glomerular cluster that is activated by the corresponding odorant having only one functional group?
Some odorants may not reach equally well all of the odorant receptors that could potentially respond to them. It is predicted that more polar, water-soluble odorants would be more completely absorbed when passing across the nasal mucosa (Mozell and Jagodowicz, 1973; Hornung and Mozell, 1977; Zhao et al., 2006). In theory, a very strongly absorbed odorant could be largely removed from the air phase before it has a chance to reach the ventral or peripheral parts of the nose that project to the ventral aspect of the olfactory bulb (Zhao et al., 2006). Instead, such a strongly absorbed odorant would be predicted to interact with receptors in the central channel, or zone 1, of the nasal epithelium, thereby stimulating the dorsal half of the bulb (Schoenfeld and Cleland, 2005). Less water-soluble odorants may disperse more completely throughout the epithelium, and they therefore would be available for interactions with receptors that lead to activation of more ventral glomeruli (Hornung and Mozell, 1977; Kent et al, 1996; Zhao et al., 2006). This relationship between odorant mucosal solubility and the topography of the epithelium-to-bulb projection may explain the consistent dorsal-to-ventral chemotopic progressions of responses to odorants of increasing carbon number within various odorant homologous series (Johnson et al., 1999, 2004; Schoenfeld and Cleland, 2005; Ho et al., 2006a), given that carbon number is correlated with decreased water solubility in these series (Johnson and Leon, 2000b; Ho et al., 2006b).
Odorants with greater oxygen content due to the presence of multiple oxygenic functional groups are estimated to have higher water solubility than the corresponding molecules possessing only a single oxygenic functional group. The relationship between odorant water solubility and the known topography in the olfactory system therefore predicts that the odorants with two oxygenic functional groups should stimulate more dorsal aspects of the bulb than the single-functional group odorants.
Our results show that the combination of two oxygen-containing functional groups does not result in the activation of multiple glomerular modules responding to each feature. Instead, novel responses were observed that were similar for different pairs of oxygen-containing functional groups. Furthermore, instead of the predicted dorsal responses, we found responses in posterior glomeruli halfway between the dorsal and ventral extremes in both medial and lateral aspects of the bulb. We also observed similar posterior responses to other odorants that are predicted to have high water solubility.
MATERIALS AND METHODS
Odorants
This study was comprised of several systematic series of odorants exposed under similar conditions as well as isolated odorants that were part of our overall attempt to increase the breadth of odorant chemical structure and of perceived odors (Table 1). Systematic series used blocked designs involving littermates and a single subtracted blank pattern. In total, 49 patterns involving 47 odorants are illustrated in this paper. Table 1 shows the unique Chemical Abstract Service (CAS) registry number for each of the odorants as well as information about the source and purity of the chemicals, information regarding the exposure conditions, and the number of animals tested under each set of exposure conditions. In addition to the patterns illustrated in this paper, we drew on a database of patterns evoked by 311 unique odorants exposed in these and other studies to derive our conclusions regarding relationships between posterior glomerular activity and various odorant molecular properties.
Table 1.
Odorant exposures in this study1. Exposures are listed in the order illustrated.
| Odorant | CAS# | Vendor | Purity (%) |
Solvent | Solvent dilution |
Air dilution |
ppm | n |
|---|---|---|---|---|---|---|---|---|
| 2-Heptanone2 | 110-43-0 | Acros | 98 | None | None | 1/29 | 180 | 5 |
| Ethyl butyrate2 | 105-54-4 | Acros | 99 | None | None | 1/220 | 75 | 5 |
| Ethyl acetoacetate | 141-97-9 | Acros | > 99 | None | None | 1/8 | 100 | 4 |
| Methyl valerate3 | 624-24-8 | Acros | 99 | None | None | 1/50 | 260 | 3 |
| Methyl levulinate | 624-45-3 | Fluka | > 98 | None | None | 1/8 | 170 | 4 |
| 2-Hexanone3 | 591-78-6 | Acros | 98 | None | None | 1/60 | 170 | 3 |
| Propyl acetate2 | 109-60-4 | Acros | 97 | None | None | 1/600 | 75 | 5 |
| Acetoxyacetone | 592-20-1 | TCI | > 97 | None | None | 1/8 | 160 | 4 |
| 2-Butanone | 78-93-3 | Acros | > 99 | None | None | 1/48 | 2500 | 3 |
| Butanedione | 431-03-8 | Acros | 99 | None | None | 1/30 | 2500 | 4 |
| 2-Hexanone | 591-78-6 | Aldrich | 98 | None | None | 1/43 | 250 | 4 |
| 2,5-Hexanedione | 110-13-4 | Acros | 97 | None | None | 1/15 | 250 | 4 |
| 3-Hexanone | 589-38-8 | Acros | 98 | None | None | 1/58 | 250 | 4 |
| 3,4-Hexanedione | 4437-51-8 | Acros | 96 | None | None | 1/15 | 250 | 4 |
| 2,3-Hexanedione | 3848-24-6 | Aldrich | 90 | None | None | 1/15 | 250 | 4 |
| Ethyl caproate | 123-66-0 | Acros | > 99 | None | None | 1/21 | 75 | 3 |
| Diethyl malonate | 105-53-3 | Acros | > 99 | None | None | 1/12 | 16 | 3 |
| Ethyl heptanoate | 106-30-9 | Acros | 99 | None | None | 1/12 | 75 | 3 |
| Diethyl succinate | 123-25-1 | Acros | 99 | None | None | 1/12 | 3 | 3 |
| Ethyl valerate | 539-82-2 | Acros | 99 | None | None | 1/84 | 75 | 3 |
| Diethyl oxalate | 95-92-1 | Acros | 99 | None | None | 1/12 | 26 | 3 |
| 1-Butanol | 71-36-3 | Acros | > 99 | None | None | 1/8 | 1100 | 3 |
| Methyl acetate | 79-20-9 | Acros | 99 | None | None | 1/1100 | 250 | 3 |
| Methyl glycolate | 96-35-5 | Acros | 98 | None | None | 1/33 | 12 | 3 |
| Valeric acid | 109-52-4 | Acros | 99 | Mineral oil | 1/20 | 1/10 | nd | 5 |
| Ethyl propionate | 105-37-3 | Aldrich | 99 | Mineral oil | 1/20 | 1/10 | nd | 5 |
| Monoethyl malonate | 1071-46-1 | Aldrich | 90 | Mineral oil | 1/20 | 1/10 | nd | 5 |
| 2-Butanol | 78-92-2 | Aldrich | > 99 | None | None | 1/96 | 200 | 5 |
| 2-Butanone | 78-93-3 | Aldrich | > 99 | None | None | 1/600 | 200 | 5 |
| Acetoin | 513-86-0 | Aldrich | > 92 | None | None | 1/9 | 200 | 5 |
| Butyric acid | 107-92-6 | Acros | > 99 | Mineral oil | 1/20 | 1/10 | nd | 5 |
| 2-Ketobutyric acid | 600-18-0 | Acros | 99 | Mineral oil | 1/20 | 1/10 | nd | 4 |
| Dimethyl malonate | 108-59-8 | Acros | > 99 | None | None | 1/130 | 75 | 3 |
| Diethyl suberate | 2050-23-9 | Aldrich | 97 | None | None | 1/12 | 0.8 | 3 |
| 3-Acetyl-1-propanol | 1071-73-4 | Aldrich | 95 | None | None | 1/8 | 20 | 5 |
| 2-Butoxyethanolacetate | 112-07-2 | Alfa Aesar | 98 | None | None | 1/8 | 49 | 3 |
| Pinacol | 76-09-5 | Acros | 99 | Mineral oil | 1/20 | 1/10 | nd | 3 |
| 2-Acetylcyclopentanone | 1670-46-8 | Acros | 99 | None | None | 1/8 | 37 | 3 |
| Diethylacetal | 105-57-7 | Acros | 99 | None | None | 1/140 | 250 | 3 |
| Acetone4 | 67-64-1 | Acros | > 99 | None | None | 1/110 | 2800 | 6 |
| Ethyl acetate5 | 141-78-6 | Acros | > 99 | None | None | 1/1600 | 70 | 3 |
| 1-Propanol4 | 71-23-8 | Acros | > 99 | None | None | 1/10 | 2600 | 3 |
| 2-Propanol | 67-63-0 | Aldrich | > 99 | None | None | 1/21 | 2700 | 5 |
| Isobutanol | 78-83-1 | Acros | 99 | Mineral oil | 1/4 | 1/45 | nd | 4 |
| Isobutyric acid | 79-31-2 | Acros | > 99 | None | None | 1/130 | 15 | 3 |
| 2-Methylcyclopropane- carboxylic acid |
29555-02-0 | Acros | 98 | None | None | 1/68 | 15 | 3 |
| Iodoform | 75-47-8 | Aldrich | > 99 | Mineral oil | 1/10 | 1/10 | nd | 4 |
| Dipropylamine | 142-84-7 | Acros | 99 | Water | 1/100 | 1/8 | nd | 3 |
| Isopentyl formate | 110-45-2 | Fluka | > 95 | None | None | 1/270 | 75 | 4 |
CAS#, Chemical Abstract Services registry number; ppm, parts per million in air; nd, not determined.
Odorant Exposures
Odorants either were used neat or were dissolved or suspended in mineral oil or water (Table 1). Neat odorants or odorants dissolved in water were placed in a gas-washing bottle fitted with a coarse diffuser stopper assembly (50 or 100 mL in a 125-mL bottle), followed by volatilization using high-purity nitrogen at a flow rate of 250 mL/min. For odorants suspended or dissolved in mineral oil, we used 200 mL in a 500-mL gas-washing bottle and a flow rate of 100 mL/min. The volatilized odorant then was mixed with ultra-zero, high-purity air before entering the exposure chamber. For odorants within a systematic series that were adjusted to achieve equal vapor phase concentrations, part of the volatilized odorant stream was diverted to a vent. Final flow rates were 2 L/min for neat odorants and odorants dissolved in water and 1 L/min for odorants suspended or dissolved in mineral oil.
All surfaces in contact with the odorant stream were made of Teflon, brass, or glass to minimize interactions with odorants. Each odorant was used with a dedicated set of tubing to prevent cross-contamination. The exposure chamber was constructed from a 2-L glass Mason jar with holes bored in the lid for odorant entry and exhaust. All components except for the exposure chamber were equilibrated at the final flow rates for at least fifteen minutes. Odorant entry into the exposure chamber began after the rat was introduced so that the odorant concentration would rise steadily as 2DG became available to the olfactory bulb.
The University of California, Irvine Institutional Animal Care and Use Committee approved all procedures using rats. Wistar rats between 18 and 21 days of age (about 30-50 g) were transferred with the dam into a cage containing clean bedding at least one hour prior to the first exposure in order to reduce carryover of odors from soiled home cages into the exposure apparatus. Each rat was given a subcutaneous injection of [14C]-2DG (Sigma, 54 mCi/mmol, 0.1 mCi/ml in 0.9% saline, 0.08 mL for a 50-g rat) at the back of the neck immediately prior to being placed in the odorized chamber. Exposure then continued for 45 minutes, after which the rat was decapitated, and the brain was removed and frozen in 2-methylbutane at about −45° C.
Brains were stored at −80° C, after which they were warmed to a temperature of −20° C and were sectioned at 20-μm thickness in a cryostat. Every sixth section was taken for autoradiography, and adjacent sections were used for staining with cresyl violet.
Data Analysis
Mapping of 2DG uptake in the glomerular layer of autoradiographic images was performed as described in detail previously (Johnson et al., 1999; 2004). Discrete measurements were taken as dictated by a set of polar grids, the particular grid being chosen on the basis of the section's anterior-posterior position relative to landmarks detected in the cresyl violet-stained sections. Values of film density were acquired in units of grayscale and then were transformed into nCi/g tissue by comparison to radioactivity standards. The data from separate sections were collapsed into matrices, the anterior-posterior dimensions of the matrices were adjusted relative to anatomical landmarks, and the matrices from left and right bulbs of the same rat were averaged. The units then were transformed into ratios of glomerular uptake to uptake occurring in a defined span of the subependymal zone in order to control for different amounts and specific activities of radiolabel reaching the bulb.
On each experimental day, one rat was exposed to a vehicle blank condition while each littermate was exposed to a different odorant. For a given experiment, the matrices for the blank conditions were averaged and this average blank was subtracted from each matrix from the experimental rats. This blank-subtracted matrix then was converted into a matrix of z scores relative to the mean and standard deviation of values across the original matrix. The z-score matrices from rats exposed to the same odorant within an experiment then were averaged and visualized as a color-coded contour chart. We prefer a ventral-centered orientation for displaying these charts to minimize the impact of missing tissue from the dorsal edge during sectioning. Alternative orientations, including dorsal-centered charts and rotatable 3-D maps of these glomerular responses, are available at http://leonserver.bio.uci.edu and at [URL to be supplied by publisher].
To explore the molecular determinants of activity in the posterior region of the glomerular layer, we used the boundaries for a set of previously defined posterior glomerular modules, “h” and “H” (Johnson et al., 2002). While it is possible that these boundaries do not correspond precisely to the boundaries of responses to the new odorants in the present study, we prefer to analyze the spatial distribution of responses in a consistent manner to facilitate comparisons across studies. Statistically significant results in the present analysis do not necessarily validate the borders of these previously defined modules.
Before correlating molecular properties with uptake in posterior modules, we first eliminated a number of patterns that resembled blank patterns due to excessive odorant dilution, low odorant volatility, or other unknown reasons. We also eliminated certain patterns from earlier studies for which no blank condition was available for subtraction. When our database contained multiple patterns representing different concentrations of the same odorant, we used the pattern for the highest concentration, and when there was more than one pattern available at the highest concentration, we used the average of values for that concentration. A total of 311 odorant-evoked activity patterns were used in these correlations. The names of the odorants, their CAS numbers, and their dilutions are provided in Supplemental Table 1.
Values for hydrogen bond acceptor strength, hydrogen bond donor strength, hydrophilic-lipophilic balance, percent hydrophilic surface, Hansen polarity, surface area, molecular length, and dipole moment were estimates from Molecular Modeling Pro chemistry modeling software (version 3.14) from ChemSW (Fairfield, CA) performed after minimizing the energy of the odorant molecules with the MOPAC method using Chem3D Pro software (version 5.0, CambridgeSoft, Cambridge, MA). Values for Log P, water solubility, and vapor pressure were the modes of all unique experimental and estimated values obtained from the Molecular Modeling Pro software package, from ChemDraw Ultra v.6.0 modeling software (CambridgeSoft, Cambridge, MA), and from two Internet databases (Interactive PhysProp Database from Syracuse Research Corporation: http://www.syrres.com/esc/physdemo.htm and the Chemical and Physical Properties Database from the Pennsylvania Department of Environmental Protection: http://www.dep.state.pa.us/physicalproperties/CPP_Search.htm).
RESULTS
Combinations of ketone and ester functional groups
To determine how the olfactory system processes information carried in odorants containing multiple functional group-related molecular features, we first compared odorants containing both a ketone and an ester group to related odorants possessing only the ketone or the ester group (Figure 1). Specifically, we compared a seven-carbon odorant containing a ketone and an ester group, ethyl acetoacetate, to both the seven-carbon ketone, 2-heptanone, and the seven-carbon ethyl ester, ethyl butyrate (Fig. 1A). We made similar comparisons of methyl levulinate to 2-heptanone and methyl valerate (Fig. 1B), and acetoxyacetone to 2-hexanone and propyl acetate (Fig. 1C).
Fig.1.
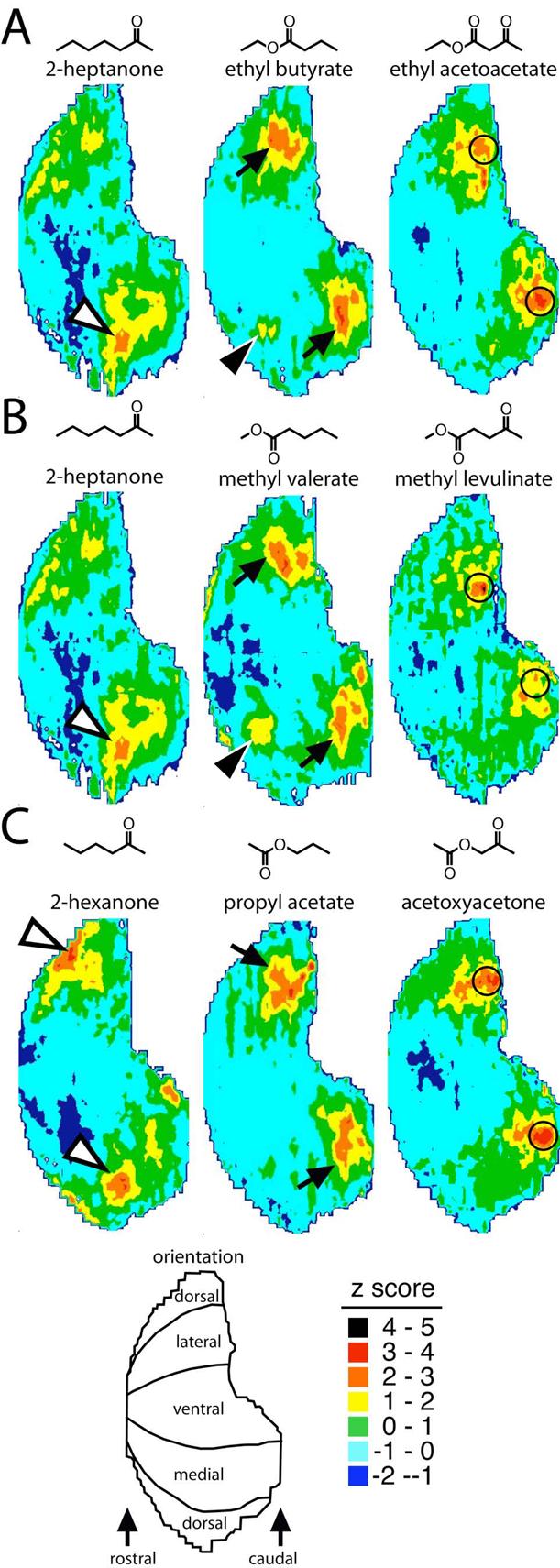
Anatomically standardized contour charts illustrating the distribution of 2DG uptake across the entire glomerular layer in rats exposed to odorants possessing ketone groups, ester groups, or combinations of ketone and ester groups. A: Ethyl acetoacetate and comparator odorants that contain only one functional group. B: Methyl levulinate and comparator odorants. C: Acetoxyacetone and comparator odorants. Each chart represents the average of both bulbs of several rats exposed to the same odorant (Table 1). The charts are in a ventral-centered orientation as shown at bottom left. Warmer colors indicate higher uptake and cooler colors lower uptake in color steps corresponding to the number of standard deviations above or below the mean uptake across the layer as shown at bottom right. Open arrowheads indicate a glomerular module that responds to ketone odorants, black arrowheads indicate glomerular modules responding to methyl and ethyl esters, and black arrows indicate glomerular modules responding to aliphatic esters in general. Circled areas denote posterior regions responding optimally to the odorants possessing both a ketone and an ester group.
Whereas the ketones 2-heptanone and 2-hexanone both stimulated the dorsal modules commonly activated by ketones (Fig. 1, white arrowheads; Johnson and Leon, 2000a; Johnson et al., 2002, 2004, 2005a), these dorsal responses were either diminished or lacking altogether from the patterns evoked by ethyl acetoacetate, methyl levulinate, and acetoxyacetone, all of which possess an ester group in addition to the ketone group (Fig. 1). The anterior, dorsal glomerular modules that reliably respond to aliphatic odorants with methyl and ethyl ester functional groups (Fig. 1A,B, black arrowheads; Johnson et al., 1998, 2002, 2004, 2005b; Johnson and Leon, 2000a) also were lacking from the response patterns for the odorants combining these features with a ketone functional group. Finally, the midlateral and midmedial response areas that predominate for most ester odorants (Fig. 1, black arrows; Johnson et al., 1998, 2002, 2004, 2005b; Johnson and Leon, 2000a) appeared to be shifted more caudally for the odorants possessing both ketone and ester functional groups (outlined regions in Fig. 1).
Diketones
We next tested whether the loss of the dorsal ketone response for odorants possessing both ester and ketone functional groups was caused by a specific interaction between the ester functional group and the receptors recognizing ketones, or whether it was a more nonspecific effect of the presence of two oxygenic functional groups. To this end, we compared responses of odorants containing two copies of the ketone functional group with related odorants containing only one ketone group (Figure 2).
Fig. 2.
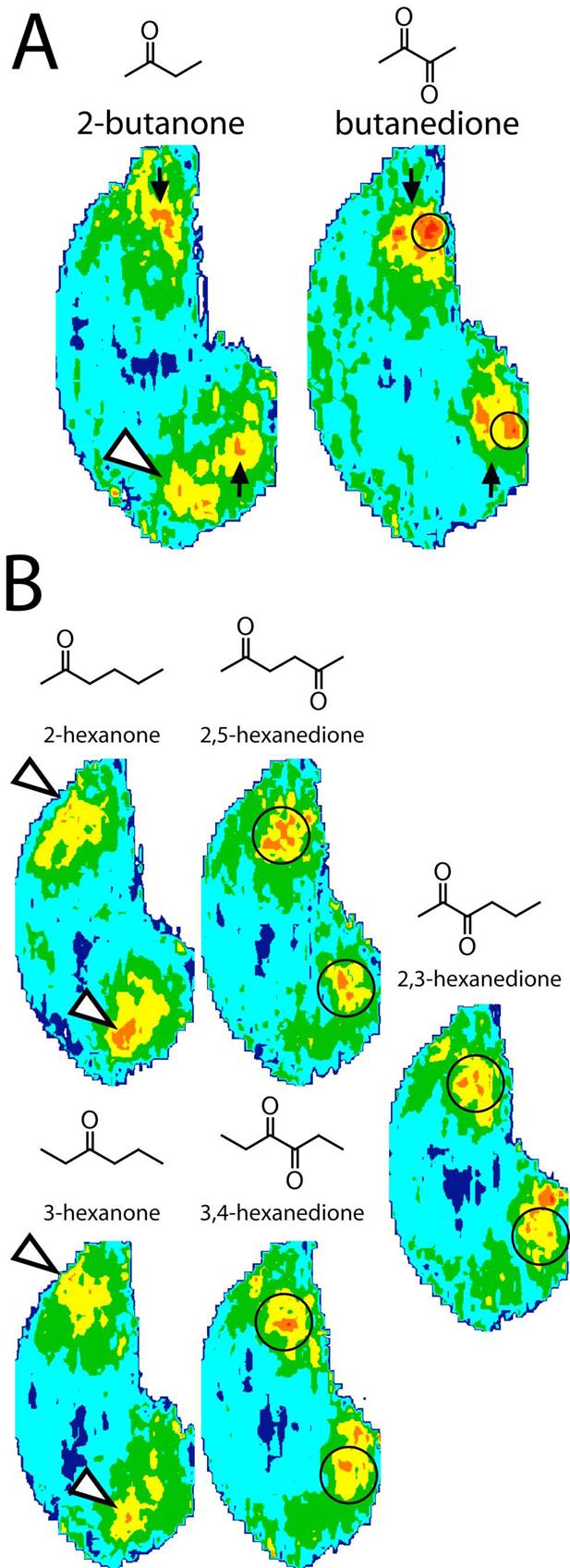
Anatomically standardized contour charts illustrating the average distribution of 2DG uptake across the entire glomerular layer in rats exposed to (A) four-carbon or (B) six-carbon ketones or diketones. Orientation and color scale are the same as in Figure 1. Open arrowheads indicate glomerular modules that respond to ketone odorants. Black arrows in A show a region of uptake evoked by 2-butanone for comparison to the more caudal area activated by the corresponding diketone, butanedione (circled). Circled areas in B indicate posterior regions stimulated by six-carbon diketones but not by corresponding odorants with only one ketone group.
First, we compared the four-carbon ketone 2-butanone to the symmetrical molecule butanedione, also known as diacetyl (Fig. 2A). Whereas 2-butanone stimulated the dorsal, ketone-responsive region (Fig. 2A, white arrowhead), butanedione did not. Therefore, the presence of a second ketone functional group seems to prevent the dorsal responses as effectively as the presence of an ester functional group. The midlateral and midmedial regions responding to 2-butanone also were not activated by butanedione, which instead stimulated more posterior regions (outlined in Fig. 2A) that overlapped in location with the posterior glomeruli activated by the two odorants possessing both ketone and ester groups, ethyl acetoacetate and acetoxyacetone (Figure 1).
We also compared responses evoked by the six-carbon ketone isomers, 2-hexanone and 3-hexanone, to those evoked by three different hexanedione isomers (Fig. 2B). Consistent with the findings involving butanedione, the dorsal, ketone-responsive glomeruli that were activated strongly by the hexanone isomers (white arrowheads in Fig. 2B) were activated only weakly by the hexanedione isomers. Instead, the hexanediones activated glomeruli located more caudally than those that responded to 2- or 3-hexanone (outlined areas in Fig. 2B). The areas activated by the hexanedione isomers were larger and somewhat more anterior than the areas that were strongly activated by butanedione, ethyl acetoacetate, and acetoxyacetone.
Diesters
We further tested the effect of having two copies of the same functional group by comparing a set of ethyl esters to a set of related, symmetrical odorants containing two ethyl ester moieties (Fig. 3). We first compared ethyl caproate to diethyl malonate (Fig. 3A). Whereas ethyl caproate stimulated glomeruli in the anterior region that is generally responsive to ethyl and methyl esters (arrowhead in Fig. 3A), diethyl malonate did not stimulate this region. The other major responses to ethyl caproate occurred in the medial and lateral paired regions of the bulb that are generally responsive to a wide variety of ester odorants (arrows in Fig. 3A). These responses also appeared to be missing for diethyl malonate. Instead of these typical ester and ethyl ester-related responses, the bulk of the 2DG uptake evoked by diethyl malonate was located in more posterior and somewhat more ventral regions than were activated by ethyl caproate (circled in Fig. 3A).
Fig. 3.
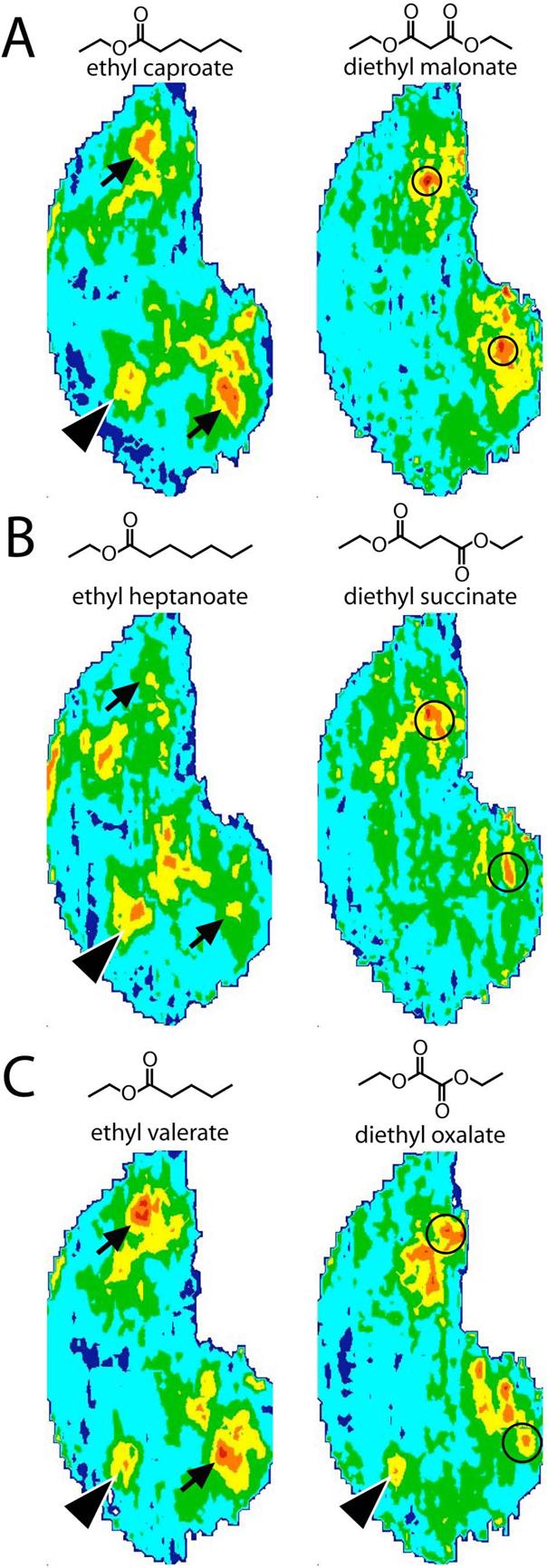
Anatomically standardized contour charts illustrating the average distribution of 2DG uptake across the entire glomerular layer in rats exposed to (A) eight-carbon, (B) nine-carbon, or (C) seven-carbon ethyl esters or corresponding diesters. Orientation and color scale are the same as in Figure 1. Black arrowheads indicate glomerular modules responding to ethyl esters, and arrows indicate modules responding to esters in general. Circled areas indicate posterior regions activated by the diesters.
A comparison of activity patterns for ethyl heptanoate and diethyl succinate gave results similar to those for ethyl caproate versus diethyl malonate (Fig. 3B). The regions responding to ethyl esters (arrowhead) and esters in general (arrows) were activated by ethyl heptanoate but not by diethyl succinate, which instead activated more posterior glomeruli (circled in Fig. 3B). Finally, diethyl oxalate preferentially stimulated more posterior glomeruli (for example, the region circled in Fig. 3C), but did not stimulate the typical midlateral and midmedial ester response regions that were activated by the related odorant ethyl valerate (arrows). However, diethyl oxalate did appear to stimulate the anterior ethyl ester response region that was activated by ethyl valerate (arrowheads in Fig. 3C).
Other combinations of oxygen-containing functional groups
The studies with esters and ketones suggested that the response to molecular features is not additive when functional groups are combined in a single odorant molecule. To test the generality of this observation, we compared a number of other odorants with combinations of oxygenic functional groups to molecules of similar chain length that contain only a single functional group (Fig. 4).
Fig. 4.
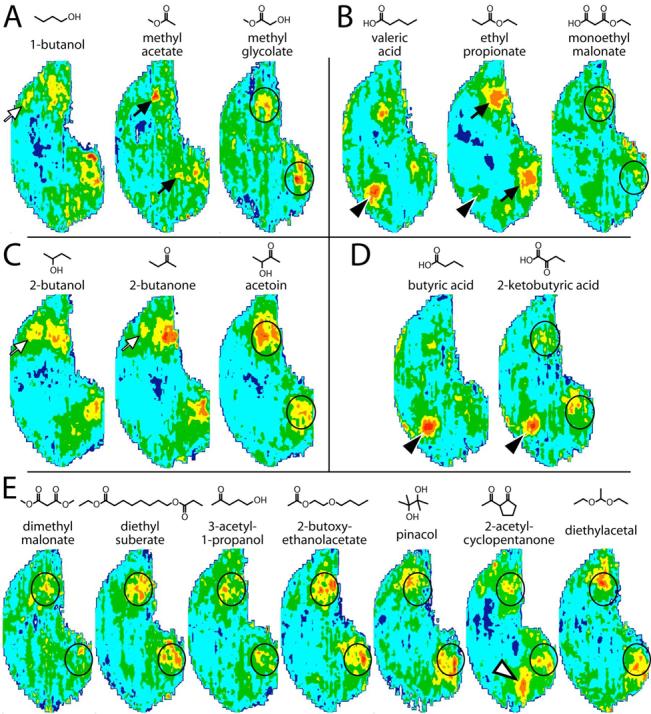
Anatomically standardized contour charts illustrating the average distribution of 2DG uptake across the entire glomerular layer in rats exposed to odorants. Orientation and color scale are the same as in Figure 1. A: Odorants possessing a primary alcohol group, an ester group, or both. B: Odorants possessing a carboxylic acid group, an ester group, or both. C: Odorants possessing a secondary alcohol group, a ketone group, or both. D: Odorants possessing a carboxylic acid group or both a carboxylic acid and a ketone functional group. E: Odorants possessing various combinations of oxygenic functional groups. Open arrows indicate a glomerular module activated by alcohols and by 2-butanone. Black arrows indicate modules activated by esters in general. Black arrowheads indicate a module that responds to carboxylic acids and ethyl esters. The open arrowhead indicates a module that responds to ketone odorants. The outlined regions show the similarity in location of posterior glomeruli activated by various odorants having two oxygenic functional groups.
We compared the response to methyl glycolate, which has both an alcohol and an ester functional group, to the related alcohol 1-butanol and the related ester methyl acetate (Fig. 4A). Methyl glycolate neither stimulated a glomerular region typically responding to primary alcohols (Fig. 4A, white arrow; Johnson and Leon, 2000a; Johnson et al., 2002, 2004, 2005a), nor did it stimulate the area responsive to aliphatic esters in general (Fig. 4A, black arrows). Instead, methyl glycolate stimulated posterior glomeruli (circled region in Fig. 4A), thus resembling the previous odorants containing two oxygenic functional groups.
We compared the pattern of response to monoethyl malonate, which has both a carboxylic acid and an ester functional group, to the related carboxylic acid, valeric acid, and the related ester, ethyl propionate (Fig. 4B). As we have reported many times previously (Johnson et al., 1999, 2004; Johnson and Leon, 2000a,b), valeric acid activated four clusters of glomeruli in the olfactory bulb, including a strongly stimulated anterior, dorsomedial module (black arrowhead in Fig. 4B) that responds to a wide variety of odorants containing a carboxylic acid feature. As we also reported previously (Johnson et al., 2004), ethyl propionate weakly stimulated the same anterior region (black arrowhead). In contrast to each of these odorants with only one functional group, the odorant monoethyl malonate did not activate glomeruli in this anterior area. Rather, it yielded weak activation of posterior glomeruli in the same general region as was activated by other odorants possessing two oxygenic functional groups (outlined in Fig. 4B). Ethyl propionate strongly activated glomeruli in this same general region (black arrows in Fig. 4B).
We compared the pattern of 2DG uptake evoked by acetoin, which has both an alcohol and a ketone functional group, to the pattern evoked by the related secondary alcohol, 2-butanol, and the related ketone 2-butanone (Fig. 4C). Both 2-butanol and 2-butanone evoked 2-DG uptake in an anterior, slightly dorsal, glomerular domain (white arrows) that is commonly stimulated by alcohols (Johnson and Leon, 2000a; Johnson et al., 2002, 2004, 2005a) and by some ketones (Johnson et al., 2005a). Acetoin did not stimulate this region. Instead, acetoin evoked activity in a broad, posterior area (outlined in Fig. 4C). Both 2-butanol and 2-butanone also activated glomeruli in this same general region.
2-Butanone was used at a lower vapor phase concentration in the experiment shown in Figure 4C (200 ppm) than in the experiment illustrated in Figure 2A (2500 ppm), where uptake was prominent in very dorsal glomeruli (Fig. 2A, white arrowhead). In the second experiment, we used the lower concentration to match that of acetoin, whereas in the first experiment we used a higher concentration to match that of butanedione. We have found previously that aliphatic ketones used at high concentrations activate glomeruli on the dorsal surface of the bulb, while lower concentrations of the same odorants do not stimulate these dorsal glomeruli (Johnson and Leon, 2000a; Johnson et al., 2004).
Our final systematic comparison contrasted the response evoked by 2-ketobutyric acid, which has both a ketone and a carboxylic acid functional group, with the response to the related carboxylic acid, butyric acid (Fig. 4D). Unlike our findings with the previous odorant sets, the acid-responsive region (black arrowhead) that was strongly activated by butyric acid was activated to almost the same level by 2-ketobutyric acid despite the presence of a second oxygenic functional group. In addition to activating the acid-preferring module, 2-ketobutyric acid stimulated a separate group of glomeruli located in somewhat more posterior regions than those activated by butyric acid (outlined area in Fig. 4D).
As a complement to the systematic comparisons of odorants with one or two oxygenic functional groups, we also investigated a range of other odorous compounds with two functional groups, most of which were part of a general survey of odorants with different structures and perceived odors (Fig. 4E). In most cases, we did not map responses to corresponding odorants possessing only one of the functional groups. The odorants included examples of two ester bonds (dimethyl malonate and diethyl suberate), one ketone and one alcohol functional group (3-acetyl-1-propanol), one ester and one ether functional group (2-butoxyethanolacetate), two alcohol groups (pinacol), two ketone groups (2-acetylcyclopentanone), and two ether groups (diethylacetal).
These odorants with two oxygenic groups all activated glomeruli in the posterior part of the olfactory bulb (outlined areas in Fig. 4E), the same area that had been activated in our other studies of odorants with two oxygenic functional groups. For all of the odorants shown in Figure 4E except for 2-acetylcyclopentanone, most of the 2DG uptake was confined to the posterior region, and we did not observe appreciable activation of modules previously identified as responding to isolated functional group features. The alicyclic diketone, 2-acetylcyclopentanone, however, clearly activated the dorsomedial region that responds to many ketones (white arrowhead in Fig. 4E).
Correlations between water solubility and activation of posterior glomeruli
Most of the odorants containing two oxygenic functional groups, independent of the exact nature of those groups, stimulated posterior glomeruli, while related odorants possessing only one of the functional groups usually did not (Figs. 1-4). This observation led us to ask if the odorants with two functional groups shared some molecular property that might be the critical determinant for activating these posterior glomeruli. We therefore compared thirteen molecular properties to see if they differed between the odorants with two functional groups and their single-functional-group counterparts. The properties we investigated were the logarithm of water solubility, hydrogen bond acceptor strength, hydrogen bond donor strength, log P, the logarithm of vapor pressure, hydrophilic-lipophilic balance, percent hydrophilic surface, Hansen polarity, molecular weight, surface area, the number of freely rotatable bonds, molecular length, and dipole moment.
The most robust difference we encountered involved the water solubilities of the compounds. The aliphatic odorants with two oxygenic functional groups had an average log water solubility of 5.3 (water solubility of 200,000 mg/L), whereas the comparison odorants with only one oxygenic functional group had an average log water solubility of 0.7 (5 mg/L). When we compared the odorants with two oxygenic functional groups to all of the other odorants for which we have obtained evoked activity patterns, the aliphatic odorants with two oxygenic functional groups also were significantly more soluble in water. This larger set of odorants had an average log water solubility of 2.7 (500 mg/L).
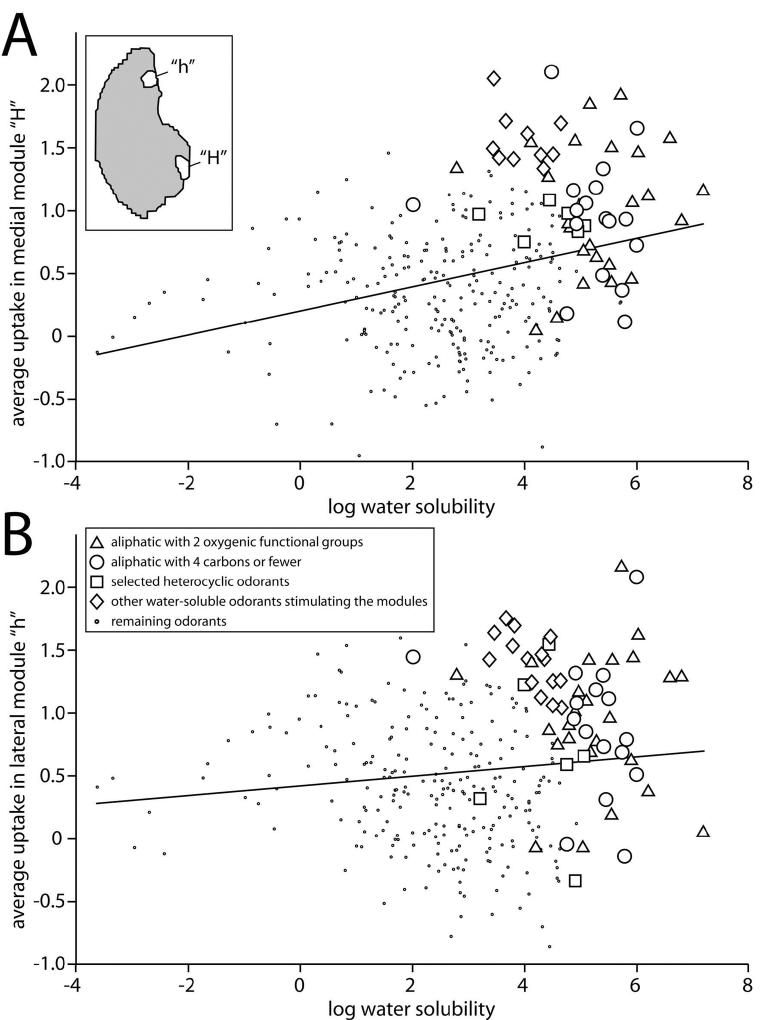
Given that the odorants with two oxygenic functional groups were more water-soluble than the corresponding single-oxygen odorants, we wanted to determine if odorant water solubility in general would explain the activation of posterior glomeruli. We therefore correlated the water solubility of 311 distinct odorants with average levels of 2DG uptake they evoked in previously defined posterior glomerular modules. The modules that we analyzed are labeled module “h” (lateral) and “H” (medial), and they are located at about one-half the distance between the dorsal and ventral extremes of the bulb (Fig. 5A, inset).
Fig. 5.
Scatter plots showing the relationship between water solubility and 2DG uptake in posterior regions of both the medial (A) and the lateral (B) aspects of the glomerular layer. For every odorant-evoked activity pattern in our database (311 total patterns), uptake was averaged within the two regions shown as an inset in A. The value of uptake then was plotted as a function of the logarithm of water solubility, which was originally obtained in units of mg/L. Each point represents an odorant, and larger symbols are used to show the contribution made by particular classes of odorants as indicated in the key. The lines are the result of least squares, linear regressions of the data, which showed a statistically significant correlation between water solubility and uptake in both aspects of the bulb. Odorants of various classes that were highly water-soluble evoked high levels of uptake in the posterior modules.
We found that water solubility was indeed correlated with uptake in the posterior modules on both lateral and medial aspects of the bulb, with the correlation being strongest for the medial aspect (medial module: r = 0.32, p < 0.0001; lateral module: r = 0.12, p = 0.03). The average z-score values of uptake in the medial and lateral posterior modules are plotted as a function of log water solubility in Figures 5A and 5B, respectively. The values for the aliphatic odorants with two oxygenic functional groups are shown as triangles, and their participation in the overall correlation is evident from their being clustered in the upper right quadrant of the plots (Fig. 5). Other classes of odorant appeared to contribute similarly to the relationship between water solubility and uptake in these posterior modules. For example, aliphatic odorants that possessed four carbons or fewer (larger circles in Fig. 5) also tended to be both soluble in water and good stimuli for the posterior modules.
In a recent study, we reported that several heterocyclic odorants with low hydrocarbon content stimulated posterior modules (Johnson et al., 2006). The values for five of these odorants (furaneol, abhexone, delta-valerolactone, gamma-caprolactone, pyrrole, and thiazole) are plotted as square symbols in Figure 5. Finally, we have indicated a mixed class of other odorants that both have high water solubility and stimulate the posterior modules (Fig. 5, diamonds). In the case of the medial module “H,” these odorants included a number of small esters (ethyl butyrate, ethyl propionate, isopentyl formate, methyl valerate, and propyl acetate), three alcohols (3-methyl-2-buten-1-ol, 4-heptanol, and cyclohexylmethanol), 2-pentanone, 2-methylcyclopropanecarboxylic acid, and cyclohexanecarboxaldehyde. In the case of the lateral module “h,” these odorants included many of the same ones most effective for the medial module (ethyl butyrate, ethyl propionate, methyl valerate, propyl acetate, 3-methyl-2-buten-1-ol, 2-pentanone, 4-heptanol, 2-methylcyclopropanecarboxylic acid, and cyclohexylmethanol), as well as additional secondary alcohols (2-heptanol, 2-hexanol, and 2-pentanol), 3-methyl-2-butenal, mesityl oxide, dipropylamine, and isopropyl propionate.
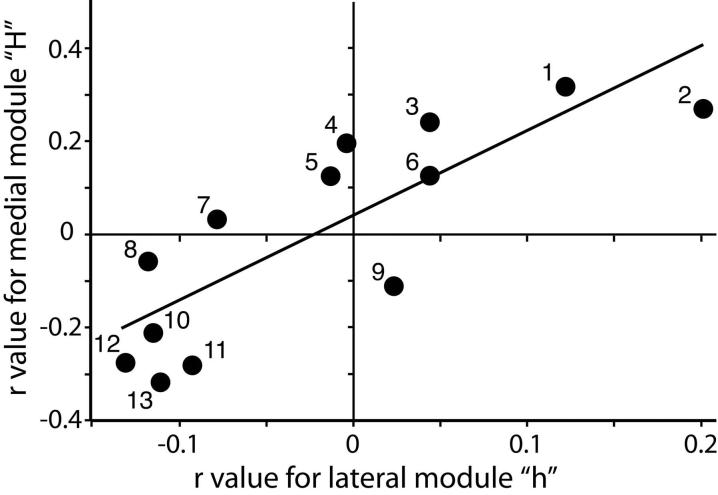
The activation of lateral and medial modules, “h” and “H,” were generally alike in terms of their correlations with various molecular properties. Figure 6 shows this similarity in a plot of the correlation coefficients for the medial module “H” versus the coefficients for the lateral module “h”. Values in the two aspects were highly correlated with each other (r = 0.82; F1,11 = 23; P = 0.0006). The similarity between lateral and medial modules extended to the relative positions of particular odorants in plots of water solubility and uptake. This close relationship between the modules is an expected functional consequence of the paired projections to lateral and medial glomeruli that are made by sensory neurons expressing the same odorant receptor gene (Mombaerts et al., 1996). However, the correlations involving the medial module were much higher than those involving the lateral module, and there were particular molecular properties (e.g., hydrophilic-lipophilic balance and dipole moment) that were well correlated with uptake in one aspect but that were not as well correlated in the other aspect (Fig. 6).
Fig. 6.
Scatter plot showing that molecular properties correlated with uptake in posterior glomeruli tend to be the same ones on both the medial and lateral aspects of the bulb. We determined the degree to which each of 13 molecular properties was correlated with average uptake in posterior modules on each aspect of the bulb, and then we plotted the correlation coefficient for the lateral module (h) relative to the correlation coefficient obtained for the medial module (H). Each point represents a single molecular property (1, log water solubility; 2, log vapor pressure; 3, hydrophilic-lipophilic balance; 4, percent hydrophilic surface; 5, hydrogen bond acceptor strength; 6, Hansen polarity; 7, hydrogen bond donor strength; 8, dipole moment; 9, number of freely rotatable bonds; 10, molecular length; 11, surface area; 12, molecular weight; 13, log P). The line is a result of least squares linear regression, the correlation coefficient for which was 0.82. Molecular properties related to water solubility and volatility were positively correlated with uptake in posterior modules both medially and laterally, while molecular properties related to size and hydrophobicity were negatively correlated with uptake in both aspects.
Individual odorants activating posterior glomerular modules
Many of the small odorants activating the posterior modules have been published in previous studies (Johnson et al., 1998, 1999, 2002), and the reader is referred to these other papers and to our web site (http://leonserver.bio.uci.edu) to view the majority of these published patterns. Several of the aliphatic odorants with four carbons or fewer (1-butanol, 2-butanol, 2-butanone, and methyl acetate) were used as comparison odorants in this study, and examples of their patterns can be seen in Figure 4. Ethyl propionate, a water-soluble ester that stimulated the posterior modules, was also used as a comparator odorant in the present study, and its evoked pattern can be viewed in Figure 4.
We show in Figure 7 previously published patterns evoked by acetone, ethyl acetate, and 1-propanol, which stimulate the posterior modules to a particularly remarkable degree. Also shown in Figure 8 are heretofore unpublished patterns evoked by aliphatic odorants with less than four carbons as well as patterns evoked by other odorants that are quite soluble in water and that stimulate posterior modules on the lateral and/or medial aspect of the bulb. The areas previously defined as modules “h” and “H” (outlined regions in Fig. 7) are unambiguously stimulated by odorants possessing a wide range of functional groups (ketones, esters, alcohols, and carboxylic acids), but sharing the property of water solubility due to their low hydrocarbon content and their ability to accept hydrogen bonds.
Fig. 7.
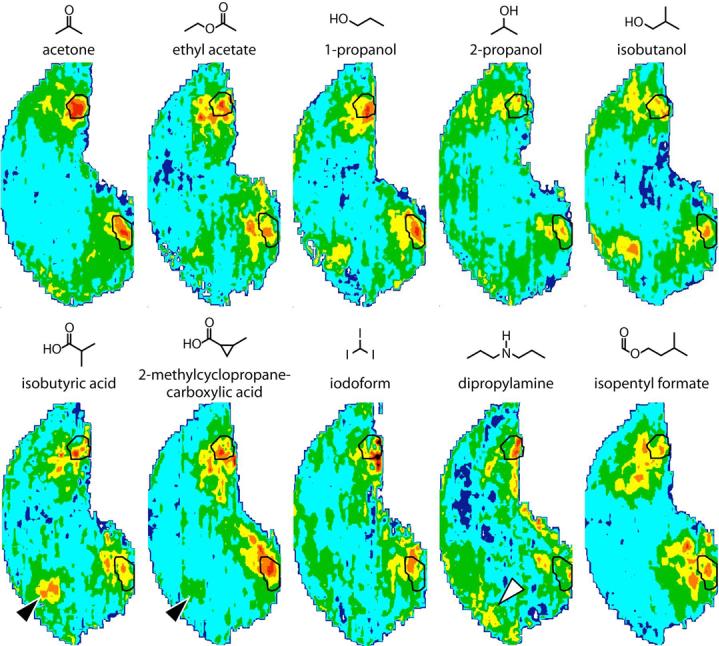
Anatomically standardized contour charts illustrating the average distribution of 2DG uptake across the entire glomerular layer in rats exposed to other odorants that stimulate the posterior glomeruli. Orientation and color scale are the same as in Figure 1. The outlined areas represent the boundaries of the modules that were analyzed in Figure 5. Black arrowheads show the location of a module that responds to most carboxylic acids, but that is not activated by the alicyclic odorant, 2-methylcyclopropanecarboxylic acid. The open arrowhead shows a very dorsal region that shows some evidence of being activated by the secondary amine, dipropylamine.
Three of these odorants (2- methylcyclopropanecarboxylic acid, iodoform, and dipropylamine) have unique structural elements that distinguish them from other odorants we have investigated. The alicyclic odorant 2-methylcyclopropanecarboxylic acid, while possessing five carbons total, has the hydrocarbon hydrogen content of a four-carbon aliphatic acid due to the additional carbon-carbon bond involved in the ring. It therefore is more soluble in water than five-carbon aliphatic acids, and its stimulation of posterior glomeruli was very remarkable (Fig. 7). 2-Methylcyclopropanecarboxylic acid did not cause very much activation of the anterior modules that very typically are stimulated by carboxylic acids, an example of which can be seen in the same figure for isobutyric acid (Fig. 7, black arrowheads). It seems possible that the cyclopropyl ring hindered access of receptors to the acid group, similar to what we proposed for a triple bond at a similar location in 2-octynoic acid and methyl 2-octynoate (Johnson et al., manuscript submitted).
Iodoform is the only halogen-substituted odorant we have studied. Although it contains only one carbon atom, it was one of the least water-soluble molecules that effectively stimulated very posterior glomeruli, and it is not very volatile. Iodoform reliably stimulated posterior glomeruli in all of the rats that were exposed, although there was a tendency toward a more ventral location for these responses compared to other small molecules (Fig. 7).
Dipropylamine, one of only a few amines that we have studied as odorants, weakly stimulated very posterior glomeruli, especially in the lateral aspect of the bulb (Fig. 7). In addition to these posterior glomeruli, there was some evidence for stimulation of very dorsal glomeruli (Fig. 7, white arrowhead), which would be in agreement with results of optical imaging studies (Takahashi et al., 2004b).
DISCUSSION
Absence of combinatorial, modular coding of multiple functional group-related features
Earlier experiments mapping odorant-evoked 2-DG uptake or optical signals in the olfactory bulb suggested that individual odorant functional groups might be represented in particular glomerular clusters, modules, or domains in the olfactory bulb (Leon and Johnson, 2003; Mori et al., 2006). These experiments showed that many aliphatic odorants sharing the same functional group activated overlapping glomerular regions independently of their hydrocarbon structure and that changing the functional group resulted in the activation of different glomerular regions (Johnson et al., 1998, 1999, 2002, 2004, 2005a; Johnson and Leon, 2000a,b; Uchida et al., 2000; Takahashi et al., 2004a,b). Changes in hydrocarbon structure in odorants with the same functional group were associated with changes in the activity of glomerular clusters in other parts of the bulb (Johnson et al., 1999, 2004; Johnson and Leon, 2005a). This type of observation led to a simple notion of feature coding in the system wherein glomerular modules were viewed as responding to particular chemical substructures of the odorant molecule (Leon and Johnson, 2003).
More recent work with a larger number of combinations of hydrocarbon structures and functional groups has indicated that this simple notion does not generalize to all combinations. Instead, odorant functional groups and hydrocarbon structures in many cases appear to interact to produce new features that are recognized by new sets of receptors and glomeruli that are not predictable from the responses to the individual elements (Johnson et al., 2005b). In other cases, structural elements such as unsaturated bonds (Johnson et al., 2007) and hydrocarbon branching (Johnson et al., 2005b) appear to disrupt the recognition of functional group-related features. The position of a functional group within an aliphatic hydrocarbon structure also can influence which glomerular modules are activated (Johnson et al., 2005a).
The present work shows that, like the interactions between functional groups and certain hydrocarbon structures, combinations of different oxygenic functional groups also produce responses that are not predictable from the responses to corresponding odorants possessing only single oxygenic functional groups. The presence of a second oxygenic functional group both prevented responses seen for odorants with single functional groups and induced novel activity in regions not stimulated by the odorants with only one oxygenic functional group. All combinations of oxygenic groups that we studied produced activity in the same general posterior area of the bulb, suggesting that the combination itself might be considered a feature, possibly related to the overall water solubility of the odorant. The activation of posterior glomeruli by odorants with two oxygenic functional groups is consistent both with early 2-DG mapping studies that used ethyl acetoacetate as an odorant (Jourdan et al., 1980; Astic and Cattarelli, 1982) and with morphometric studies of the sparing of bulbar anatomy after rearing rats in the presence of only ethyl acetoacetate as an odorant (Royet et al., 1989a,b).
Activation of the posterior bulb by water-soluble odorants
We have shown that there is a significant correlation between odorant water solubility and the activation of glomeruli in the posterior portions of the olfactory bulb, halfway between the dorsal and ventral extremes, and that the odorants that are most soluble in water are the best stimuli for these glomeruli. Other molecular properties related to water solubility also were correlated with 2-DG uptake in posterior regions. Water-soluble odorants stimulating caudal glomeruli included aliphatic odorants with two oxygenic functional groups, small aliphatic odorants with only one oxygenic functional group, and heterocyclic odorants with low hydrocarbon content.
More polar and water-soluble odorants tend to absorb more strongly into olfactory mucosa (Mozell and Jagodowicz, 1973; Hornung and Mozell, 1977; Hornung et al., 1987; Kurtz et al., 2004), so that they have been predicted to interact more with odorant receptors in the anterior part of the central channel of the epithelium, where inspired air first passes (Kimbell et al., 1997; Schoenfeld and Cleland, 2006; Zhao et al., 2006). Indeed, the water-soluble odorants ethyl acetoacetate, 1-propanol, and propyl acetate preferentially activated sensory neurons located in the anterior and dorsal parts of both the nasal septum and the nasal turbinates in experiments where voltage-sensitive dye responses were imaged in ex vivo preparations during artificially simulated nasal air flow (Youngentob et al., 1995, 2003; Kent et al., 1996). The central channel of the epithelium, however, projects exclusively to the dorsal aspect of the olfactory bulb and not to the posterior, midlateral and midmedial regions that were activated by such odorants in the present study (Mori et al., 1985; Schwob and Gottlieb, 1986; Tsuboi et al., 2006). It therefore seems most likely that the in vivo activation of these posterior bulbar regions by highly water-soluble odorants in our studies involves activation of sensory neurons located more ventrally and peripherally in the epithelium. Soluble odorants can be dispersed more thoroughly throughout the nose when they are inspired at a higher flow rate (Scott, 2006), raising the possibility that vigorous sniffing of these odorants by the awake rats in our study might contribute to the relatively ventral activation.
Many of the odorants activating posterior glomeruli were small molecules that had high vapor pressures, leading to an overall correlation of posterior uptake with vapor pressure as well as with water solubility (Figure 7). High vapor pressure could contribute to a greater tendency of the odorants to remain in the air phase and thereby to travel more peripherally and ventrally in the nose despite their water solubility, which might further explain the more ventral bulbar representation of these odorants (Schoenfeld and Knott, 2004; Schoenfeld and Cleland, 2005). However, the odorants possessing two oxygenic functional groups were actually far less volatile than their single-oxygen counterparts, so that volatility cannot explain the more ventral activation by these odorants.
The central channel, or zone 1, of the epithelium contains a population of sensory neurons expressing the so-called phylogenetic class I, or fish-like, odorant receptors, and these sensory neurons have been shown to project to the dorsal aspect of the bulb (Tsuboi et al., 2006). Although rodent class I receptors have been described as being relevant to the detection of “water-soluble” odorants (Zhang and Firestein, 2002), this notion is based primarily on the fact that class I receptors in fish and amphibians detect non-volatile odorants such as amino acids, which are not only soluble in water but also of great importance in aquatic, as opposed to terrestrial, environments (Mezler et al., 2001). Particular rodent class I receptors respond to aliphatic acids, aldehydes, and alcohols (Malnic et al., 1999; Saito et al., 2004), which have much less solubility in water than the odorants stimulating caudal glomeruli in the present study. Indeed, mapping studies show that aliphatic acids, aldehydes, and alcohols typically evoke 2DG uptake in modules located in anterior portions of the dorsal bulbar aspect where class I receptors are known to project (Johnson et al., 1999, 2002, 2004, 2005a,b; Johnson and Leon, 2000a,b; Uchida et al., 2000; Takahashi et al., 2004). Further bulbar mapping studies have shown dorsal activation by aromatic odorants with oxygenic functional groups and by high concentrations of ketones (Johnson and Leon, 2000a; Uchida et al., 2000; Johnson et al., 2002, 2004, 2005a,b; Takahashi et al., 2004a; Farahbod et al., 2006), suggesting that these odorants might be good candidates for ligands of mammalian class I odorant receptors.
The promise of molecular property-based data mining of odorant-evoked activity patterns
The present work represents the completion of a series of studies we have performed to assess the effect of systematic changes in odorant chemistry on the spatial representations of odorants in the glomerular layer of the rat olfactory bulb (Johnson et al., 1998, 1999, 2002, 2004, 2005a,b, 2006; 2007; Johnson and Leon, 2000a,b; Linster et al., 2001; Farahbod et al., 2006; Ho et al., 2006a,b). We have mapped 2DG uptake using a consistent method that results in anatomically standardized data matrices that can be compared statistically across the multiple studies. In addition to the systematic studies, we also have been mapping responses to a wide variety of different pure odorant molecules differing greatly in chemical structure and perceived odor. As a result of this work, we now have constructed a large database of activity patterns that can be used to formulate or test hypotheses regarding the relationships between odorant chemistry and anatomy (http://leonserver.bio.uci.edu). In the present study, we were able to draw upon this entire database to discover a correlation between the activation of posterior glomeruli and properties related to water solubility. In future work, we hope to extend this type of data mining to other bulbar areas and molecular properties, as well to make the underlying data matrices publicly available so that other researchers can use this resource to help answer their own questions about odorant chemistry coding and its relation to perception.
Supplementary Material
ACKNOWLEDGMENTS
We thank Zhe Xu, Paige Pancoast, Jennifer Kwok, Joan Ong, and Sepideh Saber for technical assistance and Dr. Cynthia Woo for a critical reading of the manuscript.
Supported by United States Public Health Service Grant DC03545
Footnotes
Associate editor: Thomas E. Finger
LITERATURE CITED
- Araneda RC, Kini AD, Firestein S. The molecular receptive range of an odorant receptor. Nat Neurosci. 2000;3:1248–1255. doi: 10.1038/81774. [DOI] [PubMed] [Google Scholar]
- Astic L, Cattarelli M. Metabolic mapping of functional activity in the rat olfactory system after a bilateral transection of the lateral olfactory tract. Brain Res. 1982;245:17–25. doi: 10.1016/0006-8993(82)90335-3. [DOI] [PubMed] [Google Scholar]
- Astic L, Saucier D. Anatomical mapping of the neuroepithelial projection to the olfactory bulb in the rat. Brain Res Bull. 1986;16:445–454. doi: 10.1016/0361-9230(86)90172-3. [DOI] [PubMed] [Google Scholar]
- Buck L, Axel R. A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Clancy AN, Schoenfeld TA, Forbes WB, Macrides F. The spatial organization of the peripheral olfactory system of the hamster. Part II: Receptor surfaces and odorant passageways within the nasal cavity. Brain Res Bull. 1994;34:211–241. doi: 10.1016/0361-9230(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Farahbod H, Johnson BA, Minami SS, Leon M. Chemotopic representations of aromatic odorants in the rat olfactory bulb. J Comp Neurol. 2006;497:350–366. doi: 10.1002/cne.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SL, Johnson BA, Leon M. Long hydrocarbon chains serve as unique molecular features recognized by ventral glomeruli of the rat olfactory bulb. J Comp Neurol. 2006a doi: 10.1002/cne.20973. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SL, Johnson BA, Chen AL, Leon M. Differential responses to branched and unsaturated aliphatic hydrocarbons in the rat olfactory system. J Comp Neurol. 2006b doi: 10.1002/cne.21139. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung DE, Mozell MM. Factors influencing the differential sorption of odorant molecules across the olfactory mucosa. J Gen Physiol. 1977;69:343–361. doi: 10.1085/jgp.69.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung DE, Youngentob SL, Mozell MM. Olfactory mucosa/air partitioning of odorants. Brain Res. 1987;413:147–154. doi: 10.1016/0006-8993(87)90163-6. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Leon M. Modular representations of odorants in the glomerular layer of the rat olfactory bulb and the effects of stimulus concentration. J Comp Neurol. 2000a;422:496–509. doi: 10.1002/1096-9861(20000710)422:4<496::aid-cne2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Leon M. Odorant molecular length: one aspect of the olfactory code. J Comp Neurol. 2000b;426:330–338. doi: 10.1002/1096-9861(20001016)426:2<330::aid-cne12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Woo CC, Leon M. Spatial coding of odorant features in the glomerular layer of the rat olfactory bulb. J Comp Neurol. 1998;393:457–471. doi: 10.1002/(sici)1096-9861(19980420)393:4<457::aid-cne5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Woo CC, Hingco EE, Pham KL, Leon M. Multidimensional chemotopic responses to n-aliphatic acid odorants in the rat olfactory bulb. J Comp Neurol. 1999;409:529–548. [PubMed] [Google Scholar]
- Johnson BA, Ho SL, Xu Z, Yihan JS, Yip S, Hingco EE, Leon M. Functional mapping of the rat olfactory bulb using diverse odorants reveals modular responses to functional groups and hydrocarbon structural features. J Comp Neurol. 2002;449:180–194. doi: 10.1002/cne.10284. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Farahbod H, Xu Z, Saber S, Leon M. Local and global chemotopic organization: general features of the glomerular representations of aliphatic odorants differing in carbon number. J Comp Neurol. 2004;480:234–249. doi: 10.1002/cne.20335. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Farahbod H, Saber S, Leon M. Effects of functional group position on spatial representations of aliphatic odorants in the rat olfactory bulb. J Comp Neurol. 2005a;483:192–204. doi: 10.1002/cne.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Farahbod H, Leon M. Interactions between odorant functional group and hydrocarbon structure influence activity in glomerular response modules in the rat olfactory bulb. J Comp Neurol. 2005b;483:205–216. doi: 10.1002/cne.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Xu Z, Pancoast P, Kwok J, Ong J, Leon M. Differential specificity in the glomerular response profiles of alicyclic, bicyclic, and heterocyclic odorants. J Comp Neurol. 2006;499:1–16. doi: 10.1002/cne.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Ong J, Lee K, Ho SL, Arguello S, Leon M. Effects of double and triple bonds on the spatial representations of odorants in the rat olfactory bulb. J Comp Neurol. 2007;500:720–733. doi: 10.1002/cne.21198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan F, Duveau A, Astic L, Holley A. Spatial distribution of [14C]2-deoxyglucose uptake in the olfactory bulbs of rats stimulated with two different odours. Brain Res. 1980;188:139–154. doi: 10.1016/0006-8993(80)90563-6. [DOI] [PubMed] [Google Scholar]
- Kent PF, Youngentob SL, Sheehe PR. Odorant-specific spatial patterns in mucosal activity predict perceptual differences among odorants. J Neurophysiol. 1995;74:1777–1781. doi: 10.1152/jn.1995.74.4.1777. [DOI] [PubMed] [Google Scholar]
- Kent PF, Mozell MM, Murphy SJ, Hornung DE. The interaction of imposed and inherent olfactory mucosal activity patterns and their composite representation in a mammalian species using voltage-sensitive dyes. J Neurosci. 1996;16:345–353. doi: 10.1523/JNEUROSCI.16-01-00345.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbell JS, Godo MN, Gross EA, Joyner DR, Richardson RB, Morgan KT. Computer simulation of inspiratory airflow in all regions of the F344 rat nasal passages. Toxicol Appl Pharmacol. 1997;145:388–398. doi: 10.1006/taap.1997.8206. [DOI] [PubMed] [Google Scholar]
- Kurtz DB, Zhao K, Hornung DE, Scherer P. Experimental and numerical determination of odorant solubility in nasal and olfactory mucosa. Chem Senses. 2004;29:763–773. doi: 10.1093/chemse/bjh079. [DOI] [PubMed] [Google Scholar]
- Leon M, Johnson BA. Olfactory coding in the mammalian olfactory bulb. Brain Res Rev. 2003;42:23–32. doi: 10.1016/s0165-0173(03)00142-5. [DOI] [PubMed] [Google Scholar]
- Levai O, Breer H, Strotmann J. Subzonal organization of olfactory sensory neurons projecting to distinct glomeruli within the mouse olfactory bulb. J Comp Neurol. 2003;458:209–220. doi: 10.1002/cne.10559. [DOI] [PubMed] [Google Scholar]
- Linster C, Johnson BA, Morse A, Yue E, Xu Z, Hingco EE, Choi Y, Choi M, Messiha A, Leon M. Perceptual correlates of neural representations evoked by odorant enantiomers. J Neurosci. 2001;21:9837–9843. doi: 10.1523/JNEUROSCI.21-24-09837.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- Mezler M, Fleischer J, Breer H. Characteristic features and ligand specificity of the two olfactory receptor classes from Xenopus laevis. J Exp Biol. 2001;204:2987–2997. doi: 10.1242/jeb.204.17.2987. [DOI] [PubMed] [Google Scholar]
- Miyamichi K, Serizawa S, Kimura HM, Sakano H. Continuous and overlapping expression domains of odorant receptor genes in the olfactory epithelium determine the dorsal/ventral positioning of glomeruli in the olfactory bulb. J Neurosci. 2005;25:3586–3592. doi: 10.1523/JNEUROSCI.0324-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Mori K, Fujita SC, Imamura K, Obata K. Immunohistochemical study of subclasses of olfactory nerve fibers and their projections to the olfactory bulb in the rabbit. J Comp Neurol. 1985;242:214–229. doi: 10.1002/cne.902420205. [DOI] [PubMed] [Google Scholar]
- Mori K, Takahashi YK, Igarashi KM, Yamaguchi M. Maps of odorant molecular features in the mammalian olfactory bulb. Physiol Rev. 2006;86:409–433. doi: 10.1152/physrev.00021.2005. [DOI] [PubMed] [Google Scholar]
- Mozell MM, Jagodowicz M. Chromatographic separation of odorants by the nose: retention times measured across in vivo olfactory mucosa. Science. 1973;181:1247–1249. doi: 10.1126/science.181.4106.1247. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB. A zonal organization of odorant receptor gene expression in the olfactory epithelium. Cell. 1993;73:597–609. doi: 10.1016/0092-8674(93)90145-g. [DOI] [PubMed] [Google Scholar]
- Royet JP, Sicard G, Souchier C, Jourdan F. Specificity of spatial patterns of glomerular activation in the mouse olfactory bulb: Computer-assisted image analysis of 2-deoxyglucose autoradiograms. Brain Res. 1987;417:1–11. doi: 10.1016/0006-8993(87)90173-9. [DOI] [PubMed] [Google Scholar]
- Royet JP, Jourdan F, Ploye H. Morphometric modifications associated with early sensory experience in the rat olfactory bulb: I. Volumetric study of the bulbar layers. J Comp Neurol. 1989a;289:586–593. doi: 10.1002/cne.902890405. [DOI] [PubMed] [Google Scholar]
- Royet JP, Jourdan F, Ploye H, Souchier C. Morphometric modifications associated with early sensory experience in the rat olfactory bulb: I. Stereological study of the population of olfactory glomeruli. J Comp Neurol. 1989b;289:594–609. doi: 10.1002/cne.902890406. [DOI] [PubMed] [Google Scholar]
- Saito H, Kubota M, Roberts RW, Chi Q, Matsunami H. RTP family members induce functional expression of mammalian odorant receptors. Cell. 2004;119:679–91. doi: 10.1016/j.cell.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Sato T, Hirono J, Tonoike M, Takebayashi M. Tuning specificities to aliphatic odorants in mouse olfactory receptor neurons and their local distribution. J Neurophysiol. 1994;72:2980–2989. doi: 10.1152/jn.1994.72.6.2980. [DOI] [PubMed] [Google Scholar]
- Saucier D, Astic L. Analysis of the topographical organization of olfactory epithelium projections in the rat. Brain Res Bull. 1986;16:455–462. doi: 10.1016/0361-9230(86)90173-5. [DOI] [PubMed] [Google Scholar]
- Schaefer ML, Finger TE, Restrepo D. Variability of position of the P2 glomerulus within a map of the mouse olfactory bulb. J Comp Neurol. 2001;436:351–362. [PubMed] [Google Scholar]
- Schoenfeld TA, Cleland TA. The anatomical logic of smell. Trends Neurosci. 2005;28:620–627. doi: 10.1016/j.tins.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TA, Cleland TA. Anatomical contributions to odorant sampling and representation in rodents: zoning in on sniffing behavior. Chem Senses. 2006;31:131–144. doi: 10.1093/chemse/bjj015. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TA, Knott TK. NADPH diaphorase activity in olfactory receptor neurons and their axons conforms to a rhinotopically-distinct dorsal zone of the hamster nasal cavity and main olfactory bulb. J Chem Neuroanat. 2002;24:269–285. doi: 10.1016/s0891-0618(02)00070-4. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TA, Knott TK. Evidence for the disproportionate mapping of the olfactory airspace onto the main olfactory bulb of the hamster. J Comp Neurol. 2004;476:186–201. doi: 10.1002/cne.20218. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TA, Clancy AN, Forbes WB, Macrides F. The spatial organization of the peripheral olfactory system of the hamster. I. Receptor neuron projections to the main olfactory bulb. Brain Res Bull. 1994;34:183–210. doi: 10.1016/0361-9230(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Gottlieb DI. The primary olfactory projection has two chemically distinct zones. J Neurosci. 1986;6:3393–3404. doi: 10.1523/JNEUROSCI.06-11-03393.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JW. Sniffing and spatiotemporal coding in olfaction. Chem Senses. 2006;31:119–130. doi: 10.1093/chemse/bjj013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JW, Brierley T, Schmidt FH. Chemical determinants of the rat electroolfactogram. J Neurosci. 2000;20:4721–4731. doi: 10.1523/JNEUROSCI.20-12-04721.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard G, Holley A. Receptor cell responses to odorants: Similarities and differences among odorants. Brain Res. 1984;292:283–296. doi: 10.1016/0006-8993(84)90764-9. [DOI] [PubMed] [Google Scholar]
- Stewart WB, Kauer JS, Shepherd GM. Functional organization of rat olfactory bulb analysed by the 2-deoxyglucose method. J Comp Neurol. 1979;185:715–734. doi: 10.1002/cne.901850407. [DOI] [PubMed] [Google Scholar]
- Strotmann J, Conzelmann S, Beck A, Feinstein P, Breer H, Mombaerts P. Local permutations in the glomerular array of the mouse olfactory bulb. J Neurosci. 2000;20:6927–6938. doi: 10.1523/JNEUROSCI.20-18-06927.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi YK, Kurosaki M, Hirono S, Mori K. Topographical representation of odorant molecular features in the rat olfactory bulb. J Neurophysiol. 2004a;92:2413–2427. doi: 10.1152/jn.00236.2004. [DOI] [PubMed] [Google Scholar]
- Takahashi YK, Nagayama S, Mori K. Detection and masking of spoiled food smells by odor maps in the olfactory bulb. J Neurosci. 2004b;24:8690–8694. doi: 10.1523/JNEUROSCI.2510-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi A, Miyazaki T, Imai T, Sakano H. Olfactory sensory neurons expressing class I odorant receptors converge their axons on an antero-dorsal domain of the olfactory bulb in the mouse. Eur J Neurosci. 2006;23:1436–1444. doi: 10.1111/j.1460-9568.2006.04675.x. [DOI] [PubMed] [Google Scholar]
- Uchida N, Takahashi YK, Tanifuji M, Mori K. Odor maps in the mammalian olfactory bulb: domain organization and odorant structural features. Nat Neurosci. 2000;3:1035–1043. doi: 10.1038/79857. [DOI] [PubMed] [Google Scholar]
- Vassar R, Ngai J, Axel R. Spatial segregation of odorant receptor expression in the mammalian olfactory epithelium. Cell. 1993;74:309–318. doi: 10.1016/0092-8674(93)90422-m. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Kent PF, Sheehe PR, Schwob JE, Tzoumaka E. Mucosal inherent activity patterns in the rat: Evidence from voltage-sensitive dyes. J Neurophysiol. 1995;73:387–398. doi: 10.1152/jn.1995.73.1.387. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Kent PF, Margolis FL. OMP gene deletion results in an alteration in odorant-induced mucosal activity patterns. J Neurophysiol. 2003;90:3864–3873. doi: 10.1152/jn.00806.2002. [DOI] [PubMed] [Google Scholar]
- Zhang X, Firestein S. The olfactory receptor gene superfamily of the mouse. Nat Neurosci. 2002;5:124–133. doi: 10.1038/nn800. [DOI] [PubMed] [Google Scholar]
- Zhao K, Dalton P, Yang GC, Scherer PW. Numerical modeling of turbulent and laminar airflow and odorant transport during sniffing in the human and rat nose. Chem Senses. 2006;31:107–118. doi: 10.1093/chemse/bjj008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.