Abstract
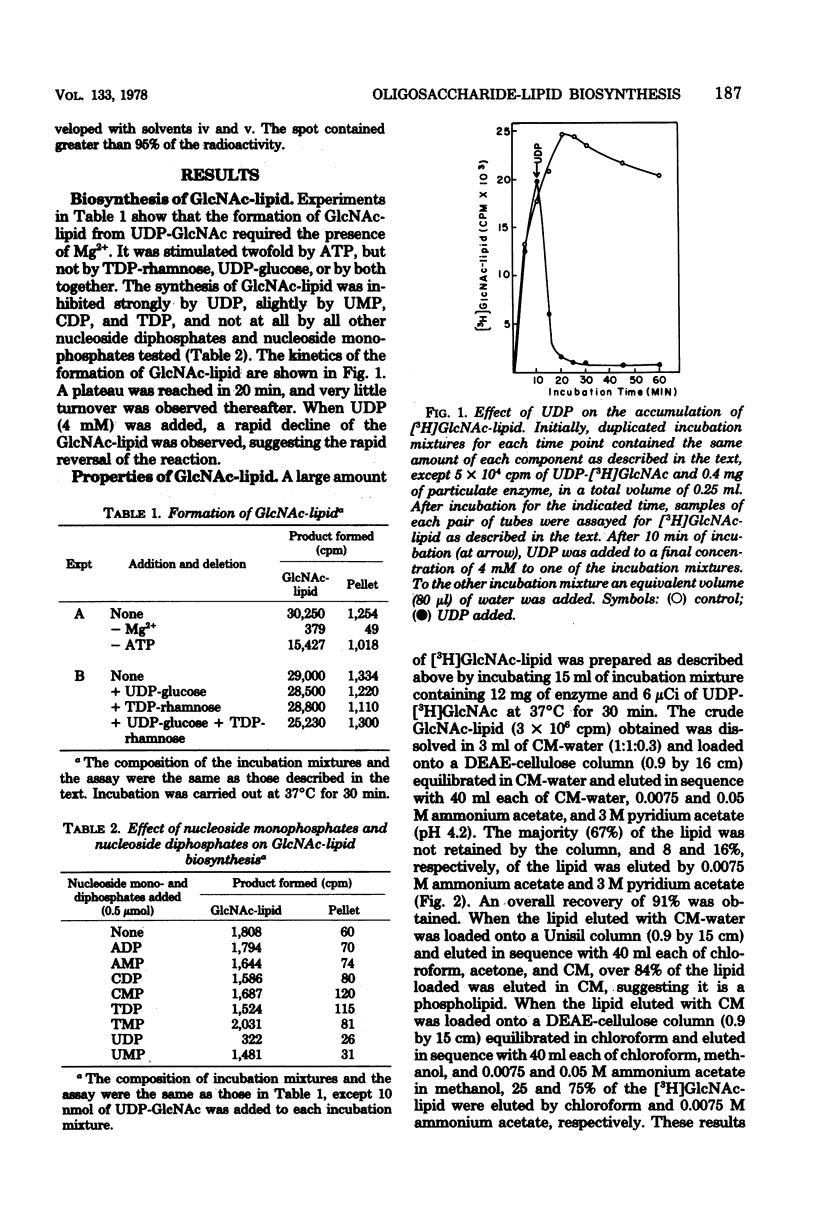
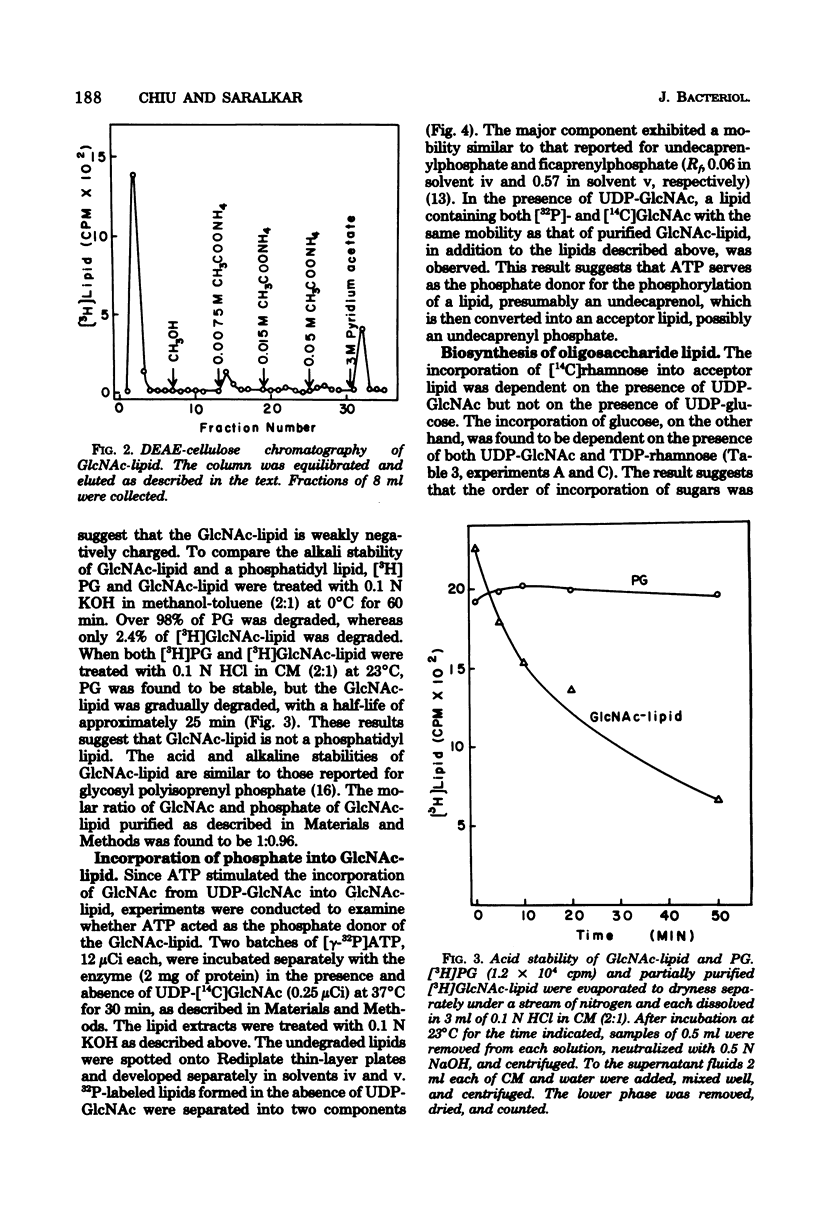
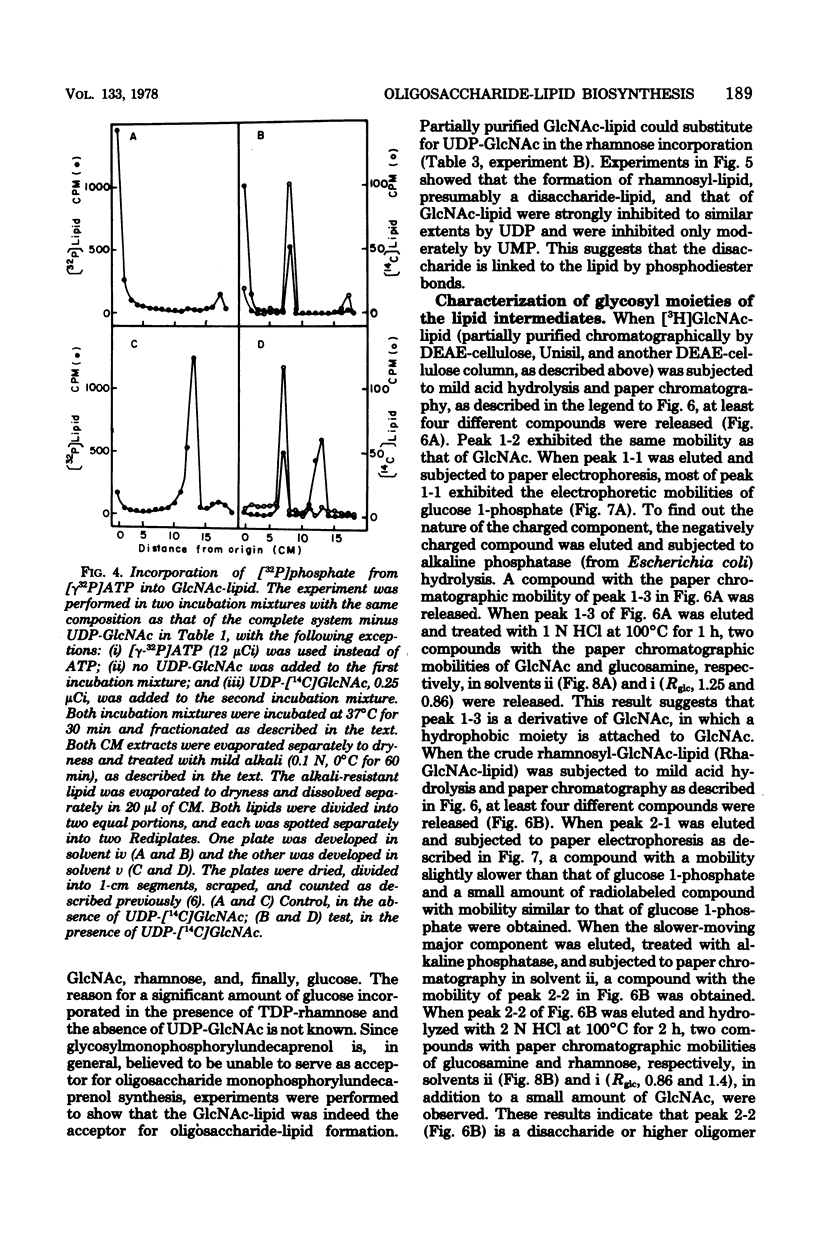
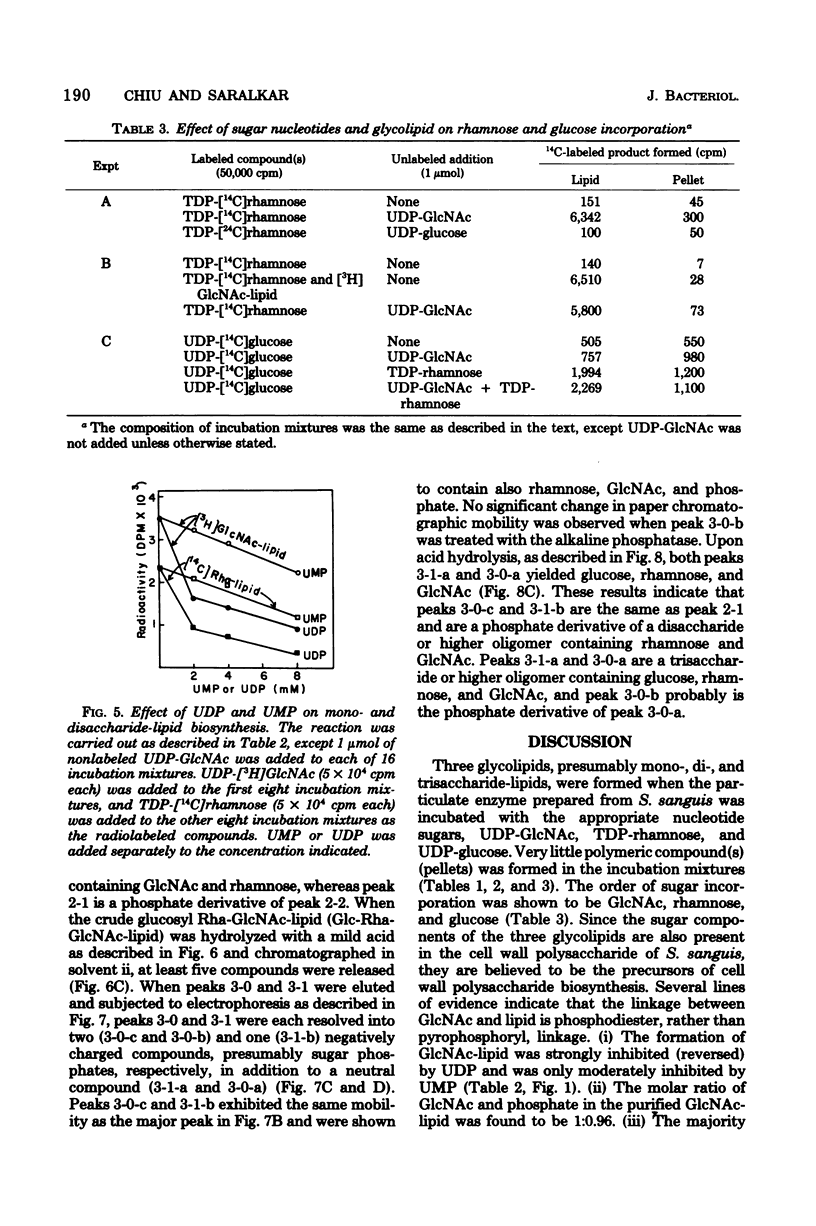
An oligosaccharide-lipid containing N-acetyl d-glucosamine (GlcNAc), l-rhamnose, and d-glucose was synthesized when the particulate enzyme from Streptococcus sanguis was incubated with UDP-GlcNAc, TDP-rhamnose, and UDP-glucose. The incorporation of d-glucose into the lipid was dependent on the preincorporation of l-rhamnose, which in turn was dependent on that of GlcNAc. This indicates that the order of sugar incorporation is GlcNAc, l-rhamnose, and d-glucose. The synthesis of GlcNAc-lipid was stimulated twofold by ATP and was inhibited strongly by UDP and slightly by UMP, CDP, and TDP, but not by all other nucleoside diphosphates and nucleoside monophosphates tested. A [γ-32P]ATP labeling experiment indicated that some acceptor lipid was present in nonphosphorylated form. The acid and alkaline stabilities of the GlcNAc-lipid were similar to those of glycosyl undecaprenylphosphate, and the thin-layer chromatographic mobility of the lipid was slightly faster than that of the mannosylphosphorylundecaprenol. The molar ratio of phosphate to GlcNAc in purified GlcNAc-lipid was found to be 0.96:1. These results suggested that the GlcNAc was attached to the lipid moiety, presumably undecaprenol, by phosphodiester bonds. The incorporation of l-rhamnose into the lipid was inhibited by UDP and UMP, respectively, in a manner similar to the incorporation of GlcNAc. This suggested that the oligosaccharide was also linked to the lipid moiety by phosphodiester bonds.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bracha R., Glaser L. An intermediate in telchoic acid biosynthesis. Biochem Biophys Res Commun. 1976 Oct 4;72(3):1091–1098. doi: 10.1016/s0006-291x(76)80244-6. [DOI] [PubMed] [Google Scholar]
- Brooks D., Baddiley J. A lipid intermediate in the synthesis of a poly-(N-acetylglucosamine 1-phosphate) from the wall of Staphylococcus lactis N.C.T.C. 2102. Biochem J. 1969 Nov;115(2):307–314. doi: 10.1042/bj1150307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks D., Baddiley J. The mechanism of biosynthesis and direction of chain extension of a poly-(N-acetylglucosamine 1-phosphate) from the walls of Staphylococcus lactis N.C.T.C. 2102. Biochem J. 1969 Jul;113(4):635–642. doi: 10.1042/bj1130635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu T. H., Emdur L. I., Platt D. Lipoteichoic acids from Streptococcus sanguis. J Bacteriol. 1974 May;118(2):471–479. doi: 10.1128/jb.118.2.471-479.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M., Panos C. Cell wall polysaccharide biosynthesis by membrane fragments from Streptococcus pyogenes and stabilized L-form. J Bacteriol. 1971 May;106(2):347–355. doi: 10.1128/jb.106.2.347-355.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emdur L. I., Chiu T. H. Turnover of phosphatidylglycerol in Streptococcus sanguis. Biochem Biophys Res Commun. 1974 Aug 5;59(3):1137–1144. doi: 10.1016/s0006-291x(74)80097-5. [DOI] [PubMed] [Google Scholar]
- Emdur L. I., Saralkar C., McHugh J. G., Chiu T. H. Glycerolphosphate-containing cell wall polysaccharides from Streptococcus sanguis. J Bacteriol. 1974 Nov;120(2):724–732. doi: 10.1128/jb.120.2.724-732.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emdur L., Chiu T. The role of phosphatidylglycerol in the in vitro biosynthesis of teichoic acid and lipoteichoic acid. FEBS Lett. 1975 Jul 15;55(1):216–219. doi: 10.1016/0014-5793(75)80995-1. [DOI] [PubMed] [Google Scholar]
- GLASER L., KORNFELD S. The enzymatic synthesis of thymidine-linked sugars. II. Thymidine diphosphate L-rhamnose. J Biol Chem. 1961 Jun;236:1795–1799. [PubMed] [Google Scholar]
- Hancock I. C., Baddiley J. Biosynthesis of the wall teichoic acid in Bacillus licheniformis. Biochem J. 1972 Mar;127(1):27–37. doi: 10.1042/bj1270027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann H., Manniello J. M., Barkulis S. S. Structure of streptococcal cell walls. V. Phosphate esters in the walls of group A Streptococcus pyogenes. Biochem Biophys Res Commun. 1967 Feb 21;26(4):486–491. doi: 10.1016/0006-291x(67)90574-8. [DOI] [PubMed] [Google Scholar]
- Lahav M., Chiu T. H., Lennarz W. J. Studies on the biosynthesis of mannan in Micrococcus lysodeikticus. II. The enzymatic synthesis of mannosyl-l-phosphoryl-undecaprenol. J Biol Chem. 1969 Nov 10;244(21):5890–5898. [PubMed] [Google Scholar]
- Lambert P. A., Hancock I. C., Baddiley J. Occurrence and function of membrane teichoic acids. Biochim Biophys Acta. 1977 May 31;472(1):1–12. doi: 10.1016/0304-4157(77)90012-0. [DOI] [PubMed] [Google Scholar]
- Lennarz W. J., Scher M. G. Metabolism and function of polyisoprenol sugar intermediates in membrane-associated reactions. Biochim Biophys Acta. 1972 Aug 4;265(3):417–441. doi: 10.1016/0304-4157(72)90015-9. [DOI] [PubMed] [Google Scholar]
- Mescher M. F., Hansen U., Strominger J. L. Formation of lipid-linked sugar compounds in Halobacterium salinarium. Presumed intermediates in glycoprotein synthesis. J Biol Chem. 1976 Dec 10;251(23):7289–7294. [PubMed] [Google Scholar]
- Munoz E., Ghuysen J. M., Heymann H. Cell walls of Streptococcus pyogenes, type 14. C polysaccharide-peptidoglycan and G polysaccharide-peptidoglycan complexes. Biochemistry. 1967 Dec;6(12):3659–3670. doi: 10.1021/bi00864a007. [DOI] [PubMed] [Google Scholar]
- Osborn M. J. Structure and biosynthesis of the bacterial cell wall. Annu Rev Biochem. 1969;38:501–538. doi: 10.1146/annurev.bi.38.070169.002441. [DOI] [PubMed] [Google Scholar]
- Owen P., Salton M. R. Isolation and characterization of a mannan from mesosomal membrane vesicles of Micrococcus lysodeikticus. Biochim Biophys Acta. 1975 Oct 6;406(2):214–234. doi: 10.1016/0005-2736(75)90006-1. [DOI] [PubMed] [Google Scholar]
- PAZUR J. H., ANDERSON J. S. ENZYMIC TRANSFER OF RHAMNOSYL UNITS FROM THYMIDINE DIPHOSPHATE RHAMNOSE TO BACTERIAL CELL-WALL FRAGMENTS. Biochim Biophys Acta. 1963 Sep 10;74:788–790. doi: 10.1016/0006-3002(63)91435-5. [DOI] [PubMed] [Google Scholar]
- Pless D. D., Schmit A. S., Lennarz W. J. The characterization of mannan of Micrococcus lysodeikticus as an acidic lipopolysaccharide. J Biol Chem. 1975 Feb 25;250(4):1319–1327. [PubMed] [Google Scholar]
- Powell D. A., Duckworth M., Baddiley J. A membrane-associated lipomannan in micrococci. Biochem J. 1975 Nov;151(2):387–397. doi: 10.1042/bj1510387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch V. M., Jr, Panos C. Synthesis of "group polysaccharide" by membranes from Streptococcus pyogenes and its stabilized L-form. J Bacteriol. 1977 Mar;129(3):1407–1414. doi: 10.1128/jb.129.3.1407-1414.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher M., Lennarz W. J., Sweeley C. C. The biosynthesis of mannosyl-1-phosphoryl-polyisoprenol in Micrococcus lysodeikticus and its role in mannan synthesis. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1313–1320. doi: 10.1073/pnas.59.4.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waechter C. J., Lennarz W. J. The role of polyprenol-linked sugars in glycoprotein synthesis. Annu Rev Biochem. 1976;45:95–112. doi: 10.1146/annurev.bi.45.070176.000523. [DOI] [PubMed] [Google Scholar]
- Wyke A. W., Ward J. B. Biosynthesis of wall polymers in Bacillus subtilis. J Bacteriol. 1977 Jun;130(3):1055–1063. doi: 10.1128/jb.130.3.1055-1063.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZELEZNICK L. D., BOLTRALIK J. J., BARKULIS S. S., SMITH C., HEYMANN H. Biosynthesis of streptococcal cell walls: A rhamnose polysaccharide. Science. 1963 Apr 26;140(3565):400–401. doi: 10.1126/science.140.3565.400. [DOI] [PubMed] [Google Scholar]


