Abstract
Recently it was shown that the design changes from the ABG-I to ABG-II hip stem resulted in a better, although not significant, proximal bone preservation. Our hypothesis was that by matching patients for preoperative bone quality, statistical power would increase and that the trend of better proximal bone preservation in ABG-II might become significant. Twenty-four ABG-II patients were compared to two different ABG-I groups: (1) 25 patients from our earlier prospective study and (2) a group of 24 patients selected to perfectly match the ABG-II group regarding gender, age and preoperative bone quality. Postoperative changes in periprosthetic bone mineral density (BMD) were quantified at 2 years postoperatively using DEXA scanning. Bone preservation (less BMD loss) was better for the ABG-II than the ABG-I (all two groups) in the proximal zones 1 and 7. In Gruen zone 7, a statistically significant difference was found for group B (p = 0.03). By matching patients for preoperative bone quality and gender, a statistical significant difference was found in proximal bone preservation in favour of ABG-II. In future comparative bone remodelling studies using DEXA, patients should be matched for preoperative bone quality and gender.
Résumé
Il a été démontré, récemment, que le changement de la pièce fémorale entre l’ABG-1 et l’ABG-2 entraînait une amélioration de la préservation du stock osseux mais ceci n’était pas significatif. En étudiant une série de patients, nous avons souhaité démontrer l’inverse. 24 patients avec une prothèse ABG-2 ont été comparés à différents groupes de patients avec une prothèse ABG-1: (1) 25 patients de notre étude prospective et (2) un groupe de 24 patients sélectionnés, avec une série parfaitement cohérente en termes de sexe, d’âge et de qualité osseuse préopératoire. Les modifications de la densité minérale osseuse (BMD) ont été mesurées à deux ans post opératoires en utilisant la technique DEXA. La préservation du capital osseux est meilleure dans l’ABG-2 que dans l’ABG-1 (y compris dans les deux groupes) au niveau de la partie proximale en zones 1 et 7. Dans la zone 7 de Gruen une différence significative a été trouvée pour le groupe B (p = 0.03). Cette étude montre qu’avec une bonne sélection de patients on peut mettre en évidence que la conservation osseuse est meilleure pour l’ABG-2. Dans une étude ultérieure, nous essayerons d’évaluer, selon la technique DEXA le capital osseux en sélectionnant des patients en préopératoire selon la qualité de l’os et le sexe.
Introduction
In 1989, the the Anatomic Benoist Girard (ABG-I) stem was developed, a cementless anatomical stem, made from Ti6Al4V (Young’s Modulus 110 GPa). Uniform and satisfactory radiological and clinical outcomes with a 10-year follow-up have been reported for the ABG-I stem by several authors [7, 10], but also proximal bone resorption due to stress shielding has been observed [7, 10, 14–17]. Stress shielding and bone resorption can be decisive for determining the long-term prognosis of a prosthesis [3, 5].
Therefore, the ABG-II femoral stem was developed and introduced in 1996 as a successor to the ABG-I. The ABG-II (Fig. 1) stem is made of a special, less stiff titanium alloy (TMZF, Young’s Modulus 85 GPa) and, for a given size, its length is shorter and the distal end is polished. Some extra HA coating is added proximally on the lateral shoulder [17]. The cervical-diaphyseal angle is reduced to 130° compared to a cervical diaphyseal angle of 135° of the ABG-I and the taper is standardised to V40.
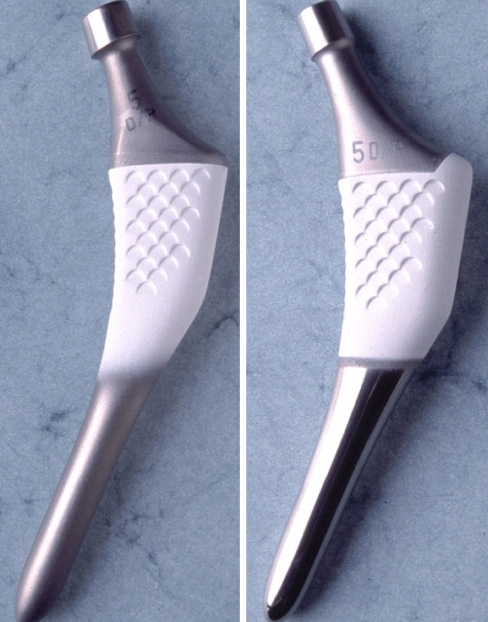
Fig. 1.
Stryker ABG-I (left) in comparison to the Stryker ABG-II (right) implant made of a low modulus titanium alloy and having a shorter small-diameter polished stem, increased proximal HA coating and neck lateralization (130° vs. 135°)
In a prospective randomised trial, it was shown that the design changes from the ABG-I to ABG-II stem result in a trend towards better proximal bone preservation [18]. However, differences in bone mineral density failed to become statistically significant. Recently it was shown that preoperative bone quality is a major factor influencing bone loss around a newly inserted femoral stem [12]. Thus, it was postulated that different preoperative bone quality between both groups may have reduced statistical power of this original study and that matching patient groups for preoperative bone quality would increase statistical power and lead to significant differences between the implants.
Materials and Methods
Hip arthroplasty patients (n = 24) having received the Stryker ABG-II total hip were compared to two different patient groups having received the ABG-I total hip system:
Twenty-five patients from a previously published prospectively randomised comparison [18]
A group of 24 patients selected from the previously published randomised comparison [18] and from another study [12] to perfectly match the ABG-II group for gender, age and preoperative bone quality.
Preoperative bone quality was measured using preoperative dual-energy X-ray absorptiometry (DEXA) scans and defined as either normal, osteopenic or osteoporotic bone using the WHO classification [1].
The ABG-II group consisted of 11 males and 13 females with a mean weight of 83.0 kg and mean age of 60.0 years. The mean Merle d’Aubigne (MdA) hip score of the ABG-II group was 10.0 preoperatively. Index diagnosis for the ABG-II group was osteoarthritis in 20 patients, post-traumatic arthritis in 2 patients and rheumatoid arthritis in 2 patients. Three patients were classified as having normal bone (3/24 = 13%), 16 patients had osteopenic bone (16/24 = 67%) and 5 patients had osteoporotic bone (5/24 = 21%; Table 1).
Table 1.
Patient demographics
| ABG-II | Group A | Group B | |
|---|---|---|---|
| Male/female | 11/13 | 8/17 | 11/13 |
| Mean weight | 83 | 77.5 | 75 |
| Mean age | 60 | 61.7 | 60 |
| Preop MdA | 10 | 10.5 | 10 |
| Osteoarthritis | 20 | 22 | 21 |
| Post-traumatic arthritis | 2 | ||
| Avascular necrosis | 1 | 1 | |
| Rheumatoid arthritis | 2 | 1 | 2 |
| Morbus Paget | 1 | ||
| Normal bone | 3 | 3 | 3 |
| Osteopenic | 16 | 15 | 16 |
| osteoporosis | 5 | 7 | 5 |
Group A consisted of 17 males and 8 females with a mean weight of 77.5 kg and mean age of 61.7 years. The mean Merle d’Aubigne (MdA) hip score of the ABG-I group was 10.5 preoperatively. Index diagnosis for group A was osteoarthtitis in 22 patients, avascular head necrosis in one patient, rheumatoid arthriits in one patient and Paget’s disease in one patient. Three patients were classified as having normal bone (3/25 = 12%), 15 patients had osteopenic bone (15/25 = 60%) and 7 patients had osteoporotic bone (7/25 = 28%).
The matched group B consisted of 11 males and 13 females with a mean weight of 75.0 kg and mean age of 60.0 years. The mean Merle d’Aubigne (MdA) hip score of the ABG-I group was 10.0 preoperatively. Index diagnosis for group B was osteoarthritis in 21 patients, avascular necrosis in one patient and rheumatoid arthritis in 2 patients. Three patients were classified as having normal bone (3/24 = 13%), 16 patients had osteopenic bone (16/24 = 67%) and 5 patients had osteoporotic bone (5/24 = 21%).
There were no significant differences between all three groups comparing age, weight and preoperative MdA score. The ABG-II group and group B were also not different regarding gender ratio and preoperative bone quality. The surgical approach was straight lateral and the surgical technique (no reaming) was the same for all series. Bone defects which needed allografting were not encountered in this series.
BMD measurements
Bone mineral density (BMD) measurements were performed using a QDR-2000plus bone densitometer (Hologic Inc, Bedford, Mass., USA). Preoperative scans were acquired of the posteroanterior (PA; L1–L4) and lateral (L2–L4) lumbar spine, the contralateral hip (femoral neck, trochanter, intertrochanteric, total hip and Ward’s triangle sites) and the non-dominant distal forearm (ultra-distal, mid-, one-third and total radius sites). Based on these measurements, patients were categorised for bone quality using the T-score and the WHO classification (normal, osteopenic or osteoporotic bone).
The patient’s leg was positioned in a foam bag to control rotation [11]. The scans were analysed using the manufacturer’s metal exclusion software with a template to automatically create seven Gruen zones [6] which were manually adjusted to the anatomy of each individual. BMD was measured laterally (Gruen zones 1, 2 and 3) and medially (Gruen zones 5, 6 and 7) around the stem of each prosthesis and 1 cm distally to the tip of the stem (Gruen zone 4). The manufacturer’s scan comparison software was used to transfer the Gruen zones regions of interest (ROI) onto the follow-up scans of each individual with care taken in patient positioning and scan analysis to ensure that the area measured coincided as closely as possible with the baseline postoperative scan. The coefficient of variation (CV) for BMD measurements using this technique has previously been established at 2.4% overall with a range of 1.4–4.1%, depending on the Gruen zone assessed [11].
Postoperative DEXA scans were performed to measure BMD in periprosthetic bone at 10 days (treated as baseline) and at 2 years to compare the results of the bone remodelling process. Results were expressed as the percentage change from baseline and the data examined for the differences in periprosthetic bone loss between between the ABG-I and ABG-II stems for each of the different Gruen zones (unpaired, double-sided Student’s t test). Clinical Merle d’Aubigne (MdA) hip scores were evaluated at the same moment of BMD measurements and the stem position was defined on postoperative X-rays (varus >2°, neutral or valgus >2°).
Results
The mean Merle d’Aubigne (MdA) hip score of group A was 10.5 preoperatively and increased to 17.0 2 years postoperatively. The mean Merle d’Aubigne (MdA) hip score of group B and the ABG-II group was 10 preoperatively and increased to 17.0 2 years postoperatively.
The mean stem size used for group A was 3.6 (range 2–5), and group B 3.4 (range 2–5). The mean stem size used for the ABG-II group was 3.3 (range 1–5), (p > 0.05, not significant), implying that the fit/fill configuration was of the similar for all three groups.
Stem alignment of both implants also was not significantly different in the three groups with varus positions (>2°) recorded in 45.8% for the ABG-II stems. In group A, varus position was recorded in 48% of the stems and in group B 37.5% (p > 0.05; Table 2).
Table 2.
Postoperative MdA values, stem size and stem alignment
| ABG-II | Group A | Group B | |
|---|---|---|---|
| Preop MdA | 10 | 10.5 | 10 |
| Postop MdA | 17 | 17 | 17 |
| Mean stem size | 3.3 | 3.6 | 3.4 |
| Stem alignment varus % | 45.8 | 48 | 37.5 |
Bone preservation (less BMD loss) was better for the ABG-II than the ABG-I (both groups) in the proximal zones 1 and 7 but also in the mid-stem zones 2 and 6 (Table 3). In Gruen zone 6, the ABG-II even recorded an increase in BMD. In zone 7, BMD loss at 2 years for the ABG-II was only −4.1% but between −11.9 (group A) and −14.5% (group B) for the ABG-I. In Gruen zone 1, BMD loss was also less for the ABG-II (−7.9%) than the ABG-I (−9.3% to −11.3%), depending on the group. In the distal zones 3–5, the BMD loss was slightly less for the ABG-I than the ABG-II (except Gruen zone 4 for group B).
Table 3.
Relative BMD loss from baseline 2-years postop (mean ± SEM) per ABG-I/II group
| Relative BMD loss 2 years postop (%) | ||||||
|---|---|---|---|---|---|---|
| ABG-I | ABG-I | ABG-II | ||||
| Gruen zone | Unmatched [19] group A | Matched group B | Reference | |||
| Mean | SEM | Mean | SEM | Mean | SEM | |
| R1 | −9.3 | 2.6 | −11.3 | 3.2 | −7.9 | 2.5 |
| R2 | −4.1 | 2.6 | −7.8 | 3.3 | −3.5 | 2.0 |
| R3 | −2.9 | 1.7 | −4.0 | 2.0 | −5.9 | 2.5 |
| R4 | −1.5 | 1.3 | −3.8 | 1.6 | −2.8 | 1.2 |
| R5 | −1.7 | 2.4 | −3.6 | 1.9 | −4.5 | 1.5 |
| R6 | −1.4 | 2.2 | −2.6 | 2.2 | +2.8a | 2.8 |
| R7 | −11.9 | 3.3 | −14.5 | 3.0 | −4.1 | 3.7 |
aIncreased BMD
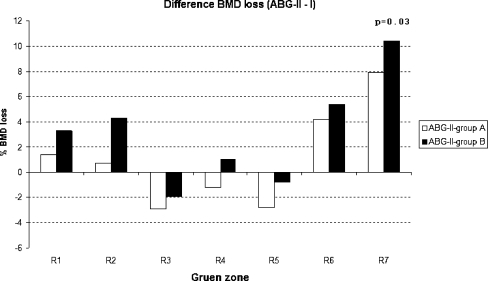
All these distinct differences between both ABG stems became more pronounced when the ABG-II was compared to the matched group (group B) than the unmatched group (group A; Table 4). In Gruen zone 7 the positive difference in BMD preservation increased from +7.9% (group A; unmatched ABG-I) to 10.4% (group B; matched ABG-I). These difference became also statistically significant at p = 0.03. The same was observed in Gruen zone 1, where the difference (ABG-II minus ABG-I) increased from +1.4 to 3.3% (group B, Fig. 2). The p-value went down but the comparison remained statistically non-significant. The same trend of increasing BDM advantage for the ABG-II combined with lower p-values applied to mid-stem Gruen zones 2 and 6 (Table 4).
Table 4.
Difference in BMD loss between ABG-II and ABG-I groups
| Difference BMD loss (ABG-II minus I; %) | ||||
|---|---|---|---|---|
| Gruenzone | Unmatched (AGB-I) group A | Matched group B | ||
| II-I | p | II-I | p | |
| R1 | +1.4 | 0.70 | +3.3 | 0.42 |
| R2 | +0.7 | 0.84 | +4.3 | 0.28 |
| R3 | −2.9 | 0.34 | −1.9 | 0.56 |
| R4 | −1.2 | 0.49 | +1.0 | 0.62 |
| R5 | −2.8 | 0.33 | −0.8 | 0.72 |
| R6 | +4.2 | 0.24 | +5.4 | 0.14 |
| R7 | +7.9 | 0.12 | +10.4a | 0.03 |
ap < 0.05
Fig. 2.
Difference (ABG-II minus ABG-I) BMD loss for all seven Gruen zones
The previously reported influence of preoperative bone quality on bone remodelling [18] which led to matching our patients for normal, osteopenic and osteoporotic bone was also confirmed in our study (Table 5). Patients with normal bone had less bone loss than osteopenic and osteoporotic patients, especially in the critical proximal zone (Zone1: −5.4% vs. −10.5 and −9.5%, Zone7: −6.5% vs. −9.4 and −10.7%, p > 0.05).
Table 5.
Difference in bone loss per bone quality category
| Bone loss per bone quality category | ||||||
|---|---|---|---|---|---|---|
| Gruen zone | Normal | Osteopenia | Osteoporosis | |||
| Mean | SEM | Mean | SEM | Mean | SEM | |
| R1 | −5.4 | 4.7 | −10.5 | 2.5 | −9.5 | 4.9 |
| R2 | −4.5 | 3.4 | −3.3 | 2.1 | −13.9 | 5.9 |
| R3 | −4.3 | 1.1 | −2.2 | 1.6 | −6.9 | 3.4 |
| R4 | −1.4 | 1.0 | −1.8 | 0.9 | −8.9 | 3.3 |
| R5 | −3.3 | 1.3 | −2.3 | 1.2 | −9.9 | 3.6 |
| R6 | −0.8 | 1.5 | 1.2 | 1.9 | −2.8 | 6.5 |
| R7 | −6.5 | 4.9 | −9.4 | 3.1 | −10.7 | 5.8 |
Discussion
Uncemented, HA-coated anatomic stems like the ABG have proven to be reliable implants and several studies with a long-term follow-up revealed excellent and consistent results for the ABG-I hip prosthesis [7, 10]. Stress-shielding induced bone resorption in the proximal Gruen zones, and distal cortical thickening leading to pedestal formation is not uncommon in hip arthroplasty in general [8, 21] and has also been described for the ABG-I hip prosthesis by several authors [7, 10, 14–17]. These studies of bone density changes in the ABG-I series were based on serial X-ray interpretations which are not as accurate in describing BMD changes as DEXA studies. Nevertheless, recently it was shown that even with conventional radiography, statistically significant differences in bone remodelling quality could be detected between two implant variants (Zweymuller stem; cementless, straight tapered femoral stem) following a small design change of an original stem design [22]. Using DEXA, even smaller changes in bone mineral density (BMD) can be accurately detected near the prosthesis [20] so that a bone remodelling comparison between two generations of the ABG seemed feasible.
Confirming this assumption, a first investigation comparing the ABG-I with the ABG-II in a prospectively randomised study showed different BMD developments over a 2-year period with an advantage for the ABG-II especially in the proximal Gruen zones [18]. However, the strong influence of preoperative bone quality on bone remodelling [12] was not known at the initiation of the study so that the two patient groups differed significantly with regards to the preoperative T-scores and thus confounded the study.
In this retrospective study, it was shown that by matching the patients for preoperative bone quality, statistical power could be improved and a statistically significant difference could be validated for zone 7 in favour of the ABG-II. At the same time, the advantage of the ABG-II over the ABG-I with regards to better bone preservation increased also in Gruen zones 1, 2 and 6 while the p-values for the comparison decreased. Thus the statistical power of comparing DEXA values could be increased by matching the patients for preoperative bone quality.
The effect of matching the patients for preoperative bone quality can also be confounded by the patients’ gender. The ABG-II group and group B were also matched for gender, as it has been suggested that postmenopausal women lose more periprosthetic bone than men of the same age [20]. Patients with a low preoperative BMD risk more bone loss near the prosthesis and women seem to be at an ever higher risk. It has been shown that bisphosphonate drugs can reduce or even avoid periprosthetic bone loss following THA [2], which suggests that especially female arthroplasty patients with a low preoperative T-score may benefit from a bisphosphonate therapy.
Several factors might be responsible for the significantly better proximal bone preservation in favour of ABG-II. The increased elasticity of the ABG-II stem (TMZF vs. Ti6Al4V) enhances the implant-to-bone load transfer and induces a more physiological stress pattern in the periprosthetic bone which may lead to reduced bone atrophy due to stress shielding [3].
HA-coatings have been shown to reduce the bone loss in general [13], because such a coating increases the speed, strength, and amount of bony ingrowth, which may lead to better biological fixation proximally and a better sealing of the implant against wear particles increasing long-time survival [4, 9, 13]. The ABG-II has some extra HA coating most proximal on the lateral implant shoulder and this may have maintained higher proximal BMD as found in this study. While the distal 2/3 of the ABG-I stem has a grit-blasted surface texture, the ABG-II stem is distally polished, shorter and thinner to counteract distal bone ongrowth which can reduce or prevent distal off-loading and with it, cortical thickening and pedestal forming as frequently observed for the ABG-I. The slightly lower distal BMD loss measured for the ABG-I may thus not be a sign of superior bone preservation of the old stem design, but an indication that distal bone ongrowth, which expresses itself in locally higher BMD values, does happen less with the ABG-II as intended by the design change. The design changes from ABG-I to ABG-II have lead to the desired clinical effects but, based on this study, it cannot be identified which of the individual factors was most influential.
It was shown that implant design can improve periprosthetic bone preservation at 2 years postoperatively from when according to literature [23] BMD changes less and becomes steady. In the long term, the influence of implant design on periprosthetic bone remodelling will be less as other factors such as wear-particle-induced osteolytic effects may dominate the bone remodelling process and eventually initiate failure. However, periprosthetic bone density which is well preserved by implant design features during the initial postoperative period may reduce or delay late osteolysis by sealing the bone-implant interface against wear-particle ingress. In addition, increased proximal bone preservation in the early postoperative phase may also provide long-term benefits by reducing the periprosthetic fracture rate as indicated by a slightly elevated periprosthetic fracture rate reported for the less proximal bone preserving ABG-I [19].
In conclusion, in future studies using DEXA scanning to compare the effects of different implant designs or treatments on periprosthetic bone remodelling, patients should be matched for preoperative bone quality and gender to limit the number of patients while maintaining maximum statistical power.
References
- 1.WHO (1994) Study group, Geneva
- 2.Bhandari M, Bajammal S, Guyatt GH, Griffith L, Busse JW, Schunemann H, Einhorn TA (2005) Effect of bisphosphonates on periprosthetic bone mineral density after total joint arthroplasty: a meta-analysis. J Bone Jt Surg, Am Vol 87:293–301 [DOI] [PubMed]
- 3.Bobyn JD, Mortimer ES, Glassman AH, Engh CA, Miller JE, Brooks CE (1992) Producing and avoiding stress shielding: laboratory and clinical observations of noncemented total hip arthroplasty. Clin Orthop 274:79–96 [PubMed]
- 4.Coathup MJ, Blackburn J, Goodship AE, Cunningham JL, Smith T, Blunn GW (2005) Role of hydroxyapatite coating in resisting wear particle migration and osteolysis around acetabular components. Biomaterials 26:4161–9 [DOI] [PubMed]
- 5.Engh CA, McGovern TF, Bobyn JD, Harris WH (1992) A quantitative evaluation of periprosthetic bone-remodeling after cementless total hip arthroplasty. J Bone Jt Surg, Am Vol 74:1009–20 [PubMed]
- 6.Gruen TA, McNeice GM, Amstutz HC (1979) “Modes of failure” of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res:17–27 [PubMed]
- 7.Herrera A, Canales V, Anderson J, Garcia-Araujo C, Murcia-Mazon A, Tonino AJ (2004) Seven to 10 years followup of an anatomic hip prosthesis: an international study. Clin Orthop Relat Res:129–37 [DOI] [PubMed]
- 8.Mulliken BD, Bourne RB, Rorabeck CH, Nayak N (1996) A tapered titanium femoral stem inserted without cement in a total hip arthroplasty: radiographic evaluation and stability. J Bone Jt Surg, Am Vol 78:1214–25 [DOI] [PubMed]
- 9.Munting E (1996) The contributions and limitations of hydroxyapatite coatings to implant fixation: a histomorphometric study of load bearing implants in dogs. Int Orthop 20:1–6 [DOI] [PubMed]
- 10.Oosterbos CJ, Rahmy AI, Tonino AJ, Witpeerd W (2004) High survival rate of hydroxyapatite-coated hip prostheses: 100 consecutive hips followed for 10 years. Acta Orthop Scand 75:127–33 [DOI] [PubMed]
- 11.Rahmy A, Tonino AJ, Tan W, ter Riet G (2000) Precision of dual energy X-ray absorptiometry in determining periprosthetic bone mineral density of the hydroxyapatite coated hip prosthesis. Hip Int 10:83–90
- 12.Rahmy AI, Gosens T, Blake GM, Tonino A, Fogelman I (2004) Periprosthetic bone remodelling of two types of uncemented femoral implant with proximal hydroxyapatite coating: a 3-year follow-up study addressing the influence of prosthesis design and preoperative bone density on periprosthetic bone loss. Osteoporos Int 15:281–9 [DOI] [PubMed]
- 13.Rosenthall L, Bobyn JD, Tanzer M (1999) Bone densitometry: influence of prosthetic design and hydroxyapatite coating on regional adaptive bone remodelling. Int Orthop 23:325–9 [DOI] [PMC free article] [PubMed]
- 14.Rossi P, Sibelli P, Fumero S, Crua E (1995) Short-term results of hydroxyapatite-coated primary total hip arthroplasty. Clin Orthop Relat Res:98–102 [PubMed]
- 15.Theis JC, Beadel G (2003) Changes in proximal femoral bone mineral density around a hydroxyapatite-coated hip joint arthroplasty. J Orthop Surg (Hong Kong) 11:48–52 [DOI] [PubMed]
- 16.Tonino AJ, Rahmy AI (2000) The hydroxyapatite-ABG hip system: 5- to 7-year results from an international multicentre study: The International ABG Study Group. J Arthroplast 15:274–82 [DOI] [PubMed]
- 17.Tonino AJ, Therin M, Doyle C (1999) Hydroxyapatite-coated femoral stems. Histology and histomorphometry around five components retrieved at post mortem. J Bone Jt Surg, Br Vol 81:148–54 [DOI] [PubMed]
- 18.van der Wal BC, Rahmy AIA, Grimm B, Blake GM, Heyligers IC, Tonino AJ (2006) The influence of implant design on periprosthetic bone remodelling of two types of uncemented HA-coated hip stems: a two-year follow-up study using DEXA. Hip Int 16:8–17 [PubMed]
- 19.van der Wal BC, Vischjager M, Grimm B, Heyligers IC, Tonino AJ (2005) Periprosthetic fractures around cementless hydroxyapatite-coated femoral stems. Int Orthop 29:235–40 [DOI] [PMC free article] [PubMed]
- 20.Venesmaa PK, Kroger HP, Jurvelin JS, Miettinen HJ, Suomalainen OT, Alhava EM (2003) Periprosthetic bone loss after cemented total hip arthroplasty: a prospective 5-year dual energy radiographic absorptiometry study of 15 patients. Acta Orthop Scand 74:31–6 [DOI] [PubMed]
- 21.Wan Z, Dorr LD (1996) Natural history of femoral focal osteolysis with proximal ingrowth smooth stem implant. J Arthroplast 11:718–25 [DOI] [PubMed]
- 22.Wick M, Lester DK (2004) Radiological changes in second- and third-generation Zweymuller stems. J Bone Jt Surg, Br Vol 86:1108–14 [DOI] [PubMed]
- 23.Wixson RL, Stulberg SD, Van Flandern GJ, Puri L (1997) Maintenance of proximal bone mass with an uncemented femoral stem analysis with dual-energy x-ray absorptiometry. J Arthroplast 12:365–72 [DOI] [PubMed]