Abstract
Background
The N-Nitrosomethylurea breast cancer model induced in rats is used for the study of carcinogenesis in mammary cancer, prostate, pancreas, etc. This model is very similar to human neoplastic disease.
Methods
The present experimental study was designed to assess whether metoclopramide administration has any effect on development of MNU-induced tumours, and evaluate the treatment of goserelin acetate on PRL, TNF alpha and NO expression. NMU was administered to female Wistar rats on 2 occasions (5 mg/100 g body w/rat). PRL and TNF alpha were performed by immune-assay. Nitric Oxide by semi automated-assay and ploidy analyses by flow cytometry.
Results
The administration of metoclopramide made the induction time shorter and increased the incidence and average of tumours per rat. Tumours development was inhibited by a goserelin chronic administration. The ploidy of adenocarcinoma was polyploid-aneuploid type (average S = 60%). It was higher basal PRL plasma levels in rats with NMU induced tumours than in basal controls without tumour (p < 0.001). The goserelin "in bolus" administration showed maximal inhibition of plasma PRL at 90 min. Plasmatic TNF alpha expression was inhibited at 60 min and also remained inhibited in tissue homogenate post chronic treatment (P < 0.0125). Plasmatic NO expression is higher in rats with induced tumours than healthy controls (P < 0.001). In tissue homogenate NO values were inhibited at 90 min (P < 0.01), as well during chronically goserelin treatment (P < 0.005).
Conclusion
The increase of blood PRL levels in NMU-induced rats may be an indicator of a poor prognosis of mammary cancer evolution. The metoclopramide administration accelerates tumour growth. However goserelin administration achieves regression in tumour development associated to inhibition PRL, TNF alpha and NO expression.
Background
N-nitroso-N-methylurea (NMU) is an effective chemical carcinogen for the induction of mammary carcinoma in rats and a very good model for human mammary carcinomas [1-6]. Prolactin (PRL) is a mitogenic hormone similar to growth factors and rat mammary tumour express PRL messenger RNA and acts as a local growth factor that stimulates proliferation of mammary tumours [7]. The presence of specific receptors for PRL has been demonstrated and they belong to the cytokine family of receptors [8-10]. PRL may act as a positive growth factor in mammary tumour development and increase the proliferation of breast cancer cells in rats [7].
Metoclopramide (C14H22ClN3O2), their IUPAC name: 4-amino-5-chloro-N-(2-diethylamino)ethyl-2-methoxybenzamide is a potent dopamine receptor antagonist used for its antiemetic effect and by inhibiting the action of PRL inhibiting hormone and has sometimes been used to stimulate lactation. It thus may induce hyperprolactinemia in rats.
The agonistic analogue of gonadotrophin-releasing hormone (LH-RH) is known to suppress ovarian function and plasma prolactin levels and its antitumour activity has been evaluated extensively [11].
In chemical carcinogenesis, internal cytokines, such as interleukin (IL) 1α and tumour necrosis alpha (TNFα) contribute to tumour progression. However it has been suggested that TNF on tumour vasculature has a direct anti-tumour mechanism [12-14]. Indeed estradiol has inhibitory effects on IL 1α and TNFα mRNA and their proteins expression [15].
Inducible nitric oxide synthase (iNOS) and nitric oxide (NO) are implicated in tumour pathology and, it is well known that TNFα induces iNOS and NO release. While low concentrations of NO alter transcription factor and act as an antioxidant factor, high concentrations of NO can damage DNA and proteins. Moreover NO-induced alteration in cellular function can lead to cell death or stimulation of growth, and stimulate tumour growth by promoting angiogenic and invasive capacities [16-19].
The aim of the study was to assess the effect of metoclopramide on tumour development of NMU-induced tumours in Wistar rats, and at the same time to study acute and chronic administration of (LH-RH analogue) goserelin on PRL, TNFα and NO expression in this experimental model.
Methods
Animals
Wistar rats were used for all the experiments and were obtained at 45 days of age. Animals were housed 2/cage in a room illuminated for 12 h each day at a temperature of 25°C. The animals had free access to Purina laboratory chow and drinking water. All the experiments in this study were performed in compliance with the Royal Decree 223/1988 of March (BOE 8 18) and the Ministerial Order of 13 October 1989 (BOE 8) regarding protection of the experimental animals, as well as with the European Council Directive of 24 November 1986 (86/609/EEC).
Chemicals
Crystalline NMU (Sigma, No. N1517) was dissolved in 0.85% NaCl solution and acidified (acetic acid) at 5.0 pH. The concentration was then adjusted to 5 mg/100 g body wt/rat. Heparin-treated blood (0.2 ml) was obtained by cardiac puncture from rats lightly anesthetized with ether.
Tumour detection
Animals were palpated for detection of mammary tumours initialling after injection of NMU and the animals were examined by palpation for tumour masses thrice weekly post administration of carcinogen. Tumour sizes were measured with a calliper and their growth and histological parameters were tested.
Histology
The diagnoses of mammary gland cancer were made by histological examination of paraffin sections stained with haematoxylin/eosin in all tumours. Autopsy was performed when the neoplasms were large. A nodule of mammary cancer is designated an active tumour, the number of active tumours is expressed per rat with mammary cancer.
Preparation of tumour fraction and blood samples
The tumour samples were prepared in the following manner: after sacrifice of the animals by decapitation the tumours were removed immediately. All further steps in the preparation were performed at 0–4°C. After being washed in ice-cold PBS buffer (pH 7.4) the tumours were homogenized in this buffer (10 ml/5 g of wet tumour). The homogenate was isolated by centrifugation for 30 min at 9000 × g and the supernatant were stored in aliquots at -80°C until used in each of the corresponding assays.
Measurement of prolactin, TNF α and NO concentration in plasma and mammary tumour homogenate
Tumour homogenate and plasma were analyzed for prolactin concentration using a commercially available ELISA kit (PRL, kit ELISA, Amersham Pharmacia Biotech, Barcelona, Spain). The basal level of rat plasma was in accord to that indicated and obtained using standard rat plasma provided by the ELISA kit and indicating that rat stress was avoided, as confirmed by the variability of the plasma blood levels in controls.
For TNF α concentration was using a commercially available kit: (Factor-Test™, mTNF-α ELISA Test Kit, Genzyme, Spain).
An assay for Nitric Oxide concentration was performed according to Navarro-Gonzáles et al [20].
The blood was centrifuged at 1500 g for 15 min; plasma was harvested and stored at -80°C until the hormonal concentration and biomarkers concentration were measured.
Total protein concentration was determined by the Lowry assay (Bio-Rad, Madrid, Spain) for tumour homogenates. In homogenate TNF α, NO concentrations were expressed relative to total protein.
Histological analysis was performed on primary NMU tumours on haematoxylin-eosin stained sections. Flow cytometry (FACScan, Becton Dickinson) was performed of rat NMU induced tumours. Flow cytometry was performed on nuclei prepared from 40 μm thick sections from formalin fixed paraffin tissue by the modified technique of Hedley et al. and McLemore et al [21,22].
Experimental Design
At 55 days of age, the female rats were divided into the following groups:
1- Healthy Controls (n = 10) without NMU-induction.
2- NMU controls (n = 20) received weekly an injection of NMU [a set of 2 injections during 2 weeks NMU (5 mg/100 g weight/rat)] and was observed after carcinogen administration for tumour development.
3- NMU plus metoclopramide (M) (n = 20) in drinking water (0.125 mg/L), "at libitum", was observed weekly for tumour development. The elimination half-life for (M) is 5–6 hours.
4- NMU plus goserelin (n = 40) and the metoclopramide administration was suspended in this group when tumour was detected and they were subdivided into 2 groups for (a) acute and (b)chronic study, respectively:
a) Received goserelin administration "in bolus" (0.25 mg/ml/rat, i.v.) and killed 2 h after injection.
b) Received goserelin (subcutaneous/daily) during 60 days (0.25 mg/ml/day).
Statistical Analysis
Differences in tumour incidence were demonstrated using x2 analysis and Yates correction. Inter-group comparisons were performed by Student t test. Values of p < 0.05 were considered to be significantly different.
Results
Post administration of NMU in control group, mammary cancer was detected in 9 of 20 (45%) rats on days 90–120. Metoclopramide treatment had effect on NMU-induced tumours, where the incidence was 85%. Latency to the appearance of first palpable tumour tended to be shorter in all rats that received metoclopramide. Indeed the average of tumours was higher than in controls, Table 1.
Table 1.
Metoclopramide (M) administration on NMU tumours.
| Wistar rats | Induction time (days) | Incidence (%) | Average of tumours |
| Control NMU | 90 – 120 | 45% | 0.5 t/rat |
| NMU + M | 55 | 85% | 1.4 t/rat |
NMU = N-nitroso-N-methylurea. M = metoclopramide. t = tumour. The tumour induction time, tumour incidence and average of tumours were increased in rats with metoclopramide administration.
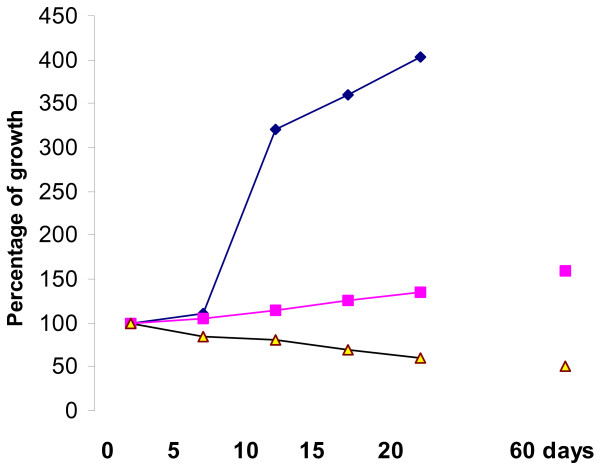
In NMU-induced tumours plus (M) the size of tumours observed an increase in growth at 400% on 20th day, compared with the administered goserelin, where the tumours stop in their development or diminish in size (last one observed at 60th day, Fig 1.
Figure 1.
Tumours evolution post-NMU administration (0–60 days) post-first measurement (= 100% as initial size)-: -■- NMU control rats without treatment -◆- NMU tumours treated with metoclopramide. -▲- NMU tumours treated with goserelin.
The histology revealed that all the tumours were adenocarcinoma.
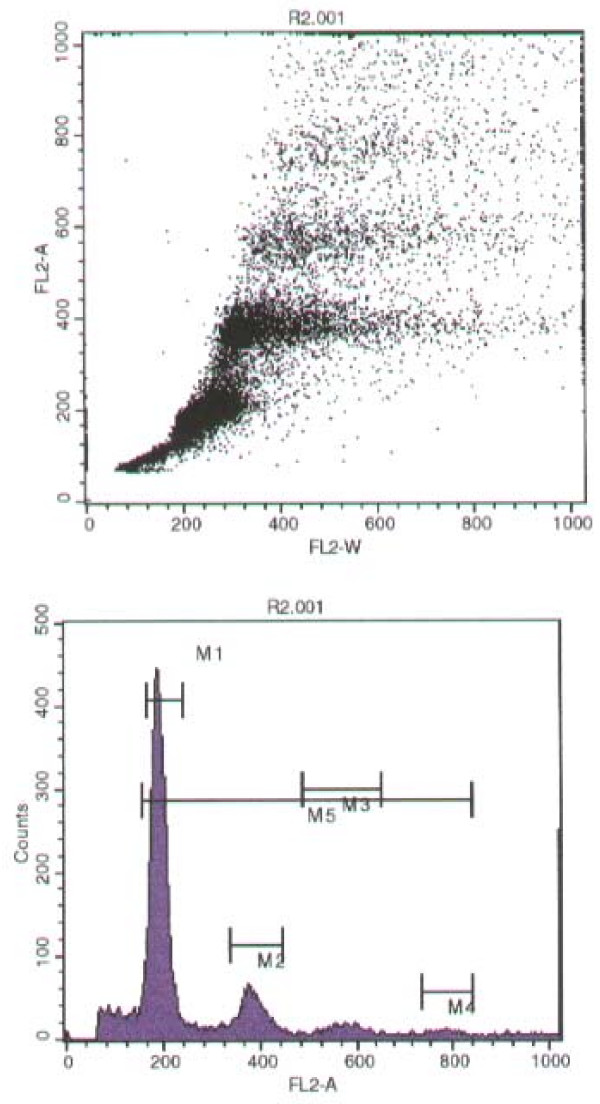
Analyses by flow cytometry of nuclear DNA patterns revealed 100% polyploidy in NMU induced tumours. According to this study all tumour samples were polyploidy-aneuploid and none of them was diploid type, percentage of S = 60%, Mitosis (ratio) = 1.99. DNA Index = 1.50 – 2.00, and with a CV (G0/G1) = 9%. Fig 2.
Figure 2.
Flow cytometry analyses of NMU induced mammary tumours. The dot plot images (top) and histogram (bottom) of the NMU-induced adenocarcinoma sample. They observed polyploidy distribution in all samples.
PRL measurements
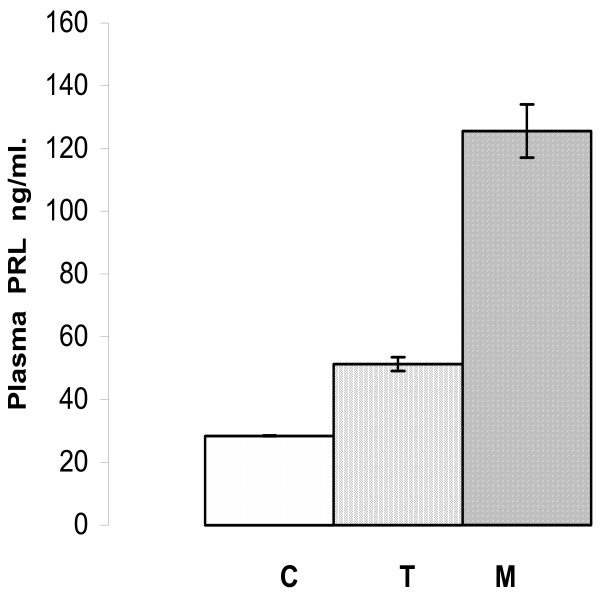
In plasma the mean value of PRL levels was lower in healthy controls [C] (28.5 ± 0.1 ng/ml, n = 20) than in rats with NMU tumours [T] (51.3 ± 2.2 ng/ml, n = 20), P < 0.01. During metoclopramide (M) administration the PRL basal mean levels were 125.6 ± 8.5 ng/ml, n = 20 in healthy controls, Fig 3.
Figure 3.
Immunoreactive plasma PRL means levels. In healthy controls (C) without NMU-induced tumours were lower than rats with NMU-induced tumours (T), and lower than with metoclopramide treatment (M). (Mean ± SEM).
The PRL plasma mean levels of NMU tumour rats [T], were higher than in tumour homogenates [TH] (corresponding to the same group of rats and extraction time) 5.8 ± 1.2 ng/ml (n = 20); these results were statistically significant (P < 0.0005).
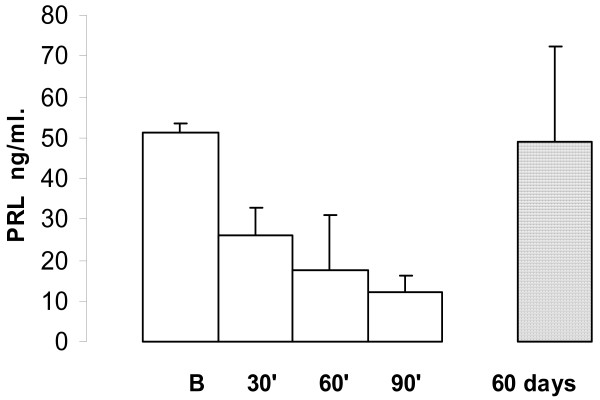
The PRL plasma levels decreased in NMU rats when they were treated with goserelin. During acute administration the time course was, basal: 51.3 ± 2.2 ng/ml (n = 20); 30 min 26.2 ± 6.5 ng/ml (n = 20), P < 0.001; 60 min 17.5 ± 13.5 ng/ml (n = 20), P < 0.0125; and at 90 min: 12.2 ± 4.2 ng/ml (n = 20), P < 0.0005.
During chronic administration of goserelin the mean PRL levels were similar to those in healthy control rats: 49.0 ± 23.4 ng/ml (n = 20) P = 0.40, Fig 4.
Figure 4.
The plasma PRL time course during goserelin administration. In the NMU induced mammary tumours the values observed maximal inhibition at 90 minutes (P < 0.005). PRL value in chronically treated rats with goserelin was similar to basal (B) value; each point represents the mean ± SEM.
In tumour homogenate [TH] there was no statistically significant variations of PRL mean levels during acute administration of goserelin neither post chronically treated: Basal 5.8 ± 1.2 ng/ml (n = 20); 30 min 6.9 ± 0.1 ng/ml (n = 20), P = 0.20; 60 min 5.6 ± 0.3 ng/ml (n = 20); at 90 min 6.8 ± 0.2 ng/ml (n = 20), P = 0.35. At 60 days treatment the mean value was 5.9 ± 0.1 ng/ml (P = 0.35).
TNF alpha measurements
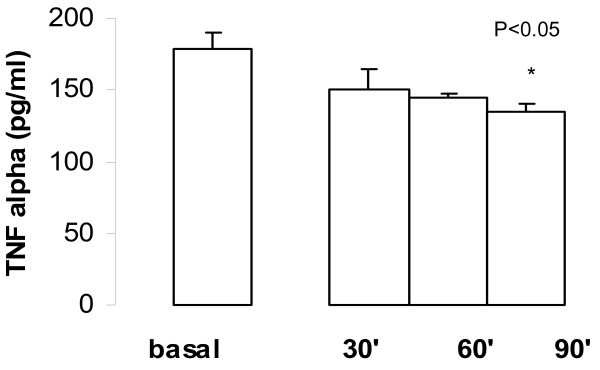
TNFα expression during time course of acute administration of goserelin observed inhibition in plasma. The mean values were: Basal 178.7 ± 11.6 pg/ml (n = 20); at 30 min 150.0 ± 15.0 pg/ml (n = 20), P = 0.10; at 60 min 145.2 ± 2.5 pg/ml (n = 20), P < 0.05; and at 90 min. 135.0 ± 5.0 (n = 20), P < 0.05; Fig 5.
Figure 5.
Time course of TNF α expression in plasma during acute administration of goserelin. The values observed maximal inhibition at 90 min from basal values; each point represents the mean ± SEM.
The inhibition of TNF α expression was observed during post-chronic treatment with goserelin: 132.1 ± 52.2 pg/ml, n = 20 (P < 0.0125).
In tumour homogenates mean basal values of TNFα were: 255.0 ± 30.0 pg/mg of protein (n = 20), at 30 min 210.0 ± 12.5 pg/mg of protein (n = 18), P = 0.20; at 60 min 200.0 ± 15.0 pg/mg of protein (n = 18), P < 0.05; and at 90 min 210.0 ± 15.0 pg/mg of protein (n = 18), P = 0.10.
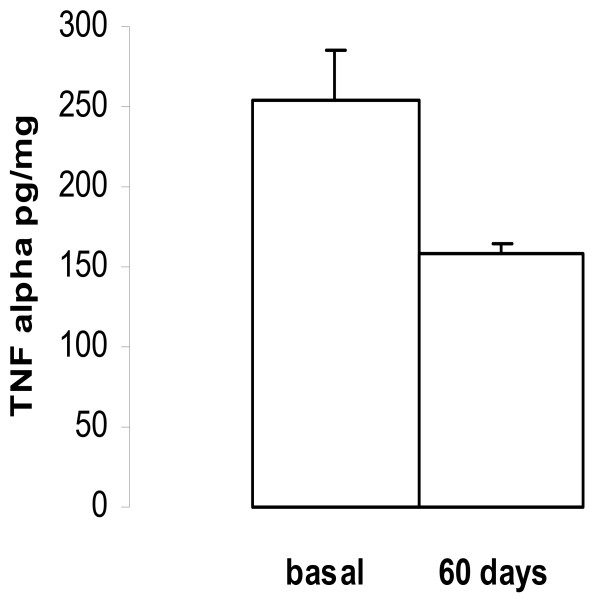
At 60 days of goserelin treatment, the TNFα expression observed a statistically significant inhibition when compared with the basal values, 255.0 ± 30.5 vs. 159.0 ± 5.2 pg/mg of protein (n = 18), P < 0.0125, Fig 6.
Figure 6.
Effect of goserelin on TNF α concentration in tumour homogenate. At 60 days of LH-RH treatment, the mean value of TNF α was lower than basal value (P < 0.0125) (mean ± SEM).
Nitric Oxide measurement
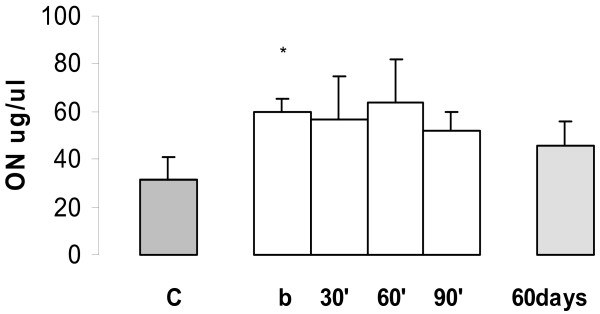
In female Wistar rats without NMU induced tumour, the basal mean NO concentration in plasma was 31.5 ± 9.5 μg/μl, n = 20. The mean NO concentrations were higher in plasma of female Wistar rats with NMU induced tumours than basal healthy controls (59.6 ± 5.5 μg/μl n = 20, P < 0.001). In plasma inhibition of NO expression during time course of acute administration of goserelin has been observed but these differences were not statistically significant. Basal mean values were: 59.6 ± 5.5 μg/μl (n = 20), 30 min 56.7 ± 17.8 μg/μl (n = 20), P = 0.10; at 60 min 64.0 ± 17.9 μg/μl (n = 20) P = 0.15 and at 90 min 52.2 ± 7.5 μg/μl (n = 20) P = 0.25. Lack of inhibition of NO expression by goserelin was observed during chronic treatment 46.0 ± 19.9 μg/μl, n = 20 (P = 0.25) when compared with NMU induced basal values, Fig 7.
Figure 7.
Expression of NO in plasma in the NMU-induced mammary tumours. Healthy control without tumours (C) versus basal (b) NMU induced rats *P < 0.001 (Values expressed as mean ± SEM). During the time course for NO expression acute and chronic administration of goserelin, between them the differences were not statistically significant.
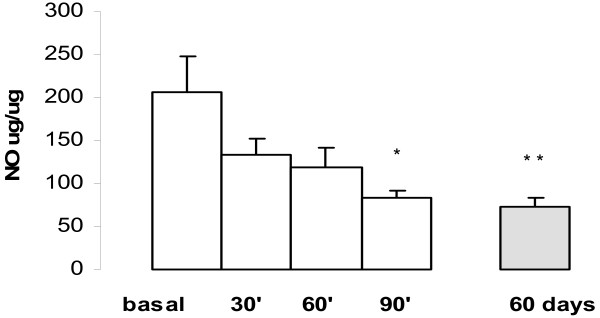
The NO concentration was inhibited by goserelin acute treatment in tumour homogenate. The mean basal concentration was: 205.9 ± 42.3 μg/μg of protein (n = 20), at 30 min 134.0 ± 17.9 μg/μg of protein (n = 20) P = 0.05, at 60 min: 118.0 ± 22.9 μg/μg of protein (n = 20), P < 0.05, and at 90 min. 82.3 ± 9.9 μg/μg of protein (n = 20), P < 0.01.
In chronically administered goserelin (at 60 day) the NO values lowered to 72.4 ± 9.9 μg/μg of protein and when compared with basal NO values statistically significant differences was observed between them (P < 0.005), Fig 8.
Figure 8.
Concentration of NO in NMU-induced tumour homogenate during administration of goserelin (time course). The maximal inhibition of NO was at 90 min (*P < 0.01) and remained at 60 days (** P < 0.005), each point represents the mean ± SEM.
Discussion
The MNU breast cancer model induced in rats is used for the study of carcinogenesis in mammary cancer, prostate, pancreas, etc. This model is very similar to human neoplastic disease [23]. The developmental stage of mammary gland at the time of exposure of the gland to NMU has an important effect of the gland to carcinogenesis [1].
A great variability of various mouse and rat strains for sensitivity on mammary tumours with chemical initiating agents was studied and considered that their results support the need to use more then one rat strain for the initiation of mammary carcinogenesis, due to "very low"-sensitivity for the NMU tumour development in Wistar-Han rats [24]. Nevertheless, in Wistar rats the incidence of tumours per rat and percentage of rats with NMU tumours was lower than observed in Sprague-Dawley rats as observed in a previous study. In our experiments we administered intra-peritoneal carcinogen at twice and in both studies the rats received metoclopramide daily, as inductor of PRL release and, as has been demonstrated PRL acts as positive growth factor in mammary tumour development suggesting that the hormonal mileu around the time of carcinogen exposure affects not only the incidence and phenotype of mammary transformants but also the molecular events associated with mammary carcinogenesis [3,7,25]. Experimental studies proposed that PRL could act as a local growth factor and may stimulate the proliferation of mammary NMU-induced tumours. [7,10,26].
Our study demonstrated that metoclopramide (as dopaminergic antagonist) has a very high capacity to induce hyperprolactinemia in rat, indeed the tumour growth accelerated during the observation period and this effect could be influenced or stimulated by PRL release. These results are in agreement with different previous experimental and clinical studies on PRL-induced proliferation. [9,27-33]. Thus sulpiride a selective dopamine D2 antagonist has been documented in a clinical study the administration of LH-RH inhibits sulpiride-induced hyperprolactinemia [34].
The histological study of our tumours revealed adenocarcinoma type, similar to observed in human breast cancer. Flow cytometric S-phase fraction contributes to diagnosis of tumours. At present to our knowledge, only flow cytometric analyses were performed in NMU-induced solid tumours in rat prostate [35]. Monakov et al [36] observed chromosomal damages and polyploidy and hiperpolyploid in somatic cells after the NMU administration and considered that they can serve as a prognostic factor of carcinogenesis. Our study is the first communication about the flow cytometry analysis of the NMU-induced tumours in female rats and it showed polyploidy (aneuploid) in all the tumour samples. These results contributed to discriminate from possible benign-induced tumours since benign tumours would be diploid type.
Initially an acute administration of LH-RH agonist induces a release of LH and FSH. However, continuous and chronic administration produces an inhibition of the hypophyseal-gonadal-axis though the process of "down regulation" of pituitary receptors for LH-RH. Furthermore the expression on LH-RH receptor gene was established in tumours [37]. In our study the tumour growth was inhibited by chronically administration of goserelin and an important tumour regression was observed at 60 days of treatment. During chronic administration of goserelin the mean PRL levels were similar to that in healthy control rats, in this way previous metoclopramide-induced hyperprolactinemia and by tumour presence, PRL expression was inhibited. During our study we observed an increase of plasmatic TNF α in rats with NMU-induced and estradiol dependent tumours when compared to controls without NMU-induced tumours. Moreover, it is well known that a variety of chemical carcinogens can adversely affect the immune system and influence tumour evolution and exert toxic effect on lymphoid organs, thus possibly affecting the production of cytokines at the initiation of carcinogenesis. This increment was inhibited by LH-RH analogues treatment and according to Tagami et al. may act by inhibition of estrogens production, because estrogens are also responsible of COX-2 induction, the same way as c-fos, IL-1 and TNF alpha [38].
It is well known that NO is a free radical that mediates a variety of cell functions. The plasmatic NO expression of our study showed a statistically significant increase of NO concentration in rats with NMU-induced tumours when compared to controls, which was observed in basal conditions. These results suggest that NO can stimulate tumour growth. Additionally, TNFα induces iNOS and NO production and could mediate tumour promoter-induced transformation.
In this study we have examined PRL, TNF alpha and NO levels in plasma and in tumour homogenates during goserelin administration and, similar behaviour was observed. PRL levels in homogenate presented no statistical significant differences. It could be due to the initial low values in homogenate or because the sensitivity of the assay was insufficient.
It is well known that NO levels in cancer cells contribute to tumour angiogenesis and during acute administration of goserelin it did not completely inhibit this increase. At present, the practical importance of these values or relevance with respect to predicting the disease course is not clear.
This study demonstrates firstly the role "in vivo" of metoclopramide on tumour growth and secondly for goserelin in NMU-induced mammary tumours in which inhibitory effects were observed on growth as well as with significant tumour regression, besides it also exerted inhibitory effects on plasmatic and tissular PRL, TNFα and ON expression. In summary, these findings could reveal a possible new inhibitory pathway in the tumour development regulation by LH-RH analogues.
Authors' contributions
IV designed and carried out the experiments and wrote the manuscript and REdeS participated in study analysis and drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
We Thank Dr. MT Sotelo for her contribution in the histopathological study of tumours and we are grateful for the collaboration on this project to M. Grau, Veterinary of Centro de Investigación of Hospital Universitario 12 de Octubre. We thank Astra-Zéneca, Manchester, UK, that kindly provided the pure LH-RH (goserelin acetate, ICI 118630).
Contributor Information
Irene Vegh, Email: i_vegh@hotmail.com.
Rafael Enríquez de Salamanca, Email: enriquez@h12o.es.
References
- Gullino PM, Pettigrew HM, Grantham FH. N-nitrosomethylurea as mammary gland carcinogen in rats. J Natl Cancer Inst. 1975;54:401–414. [PubMed] [Google Scholar]
- Russo J, Gusterson BA, Rogers AE, Russo IH, Wellings SR, van Zwieten MJ. Comparative study of human and rat mammary tumorigenesis. Lab Invest. 1990;62:244–278. [PubMed] [Google Scholar]
- Martin G, Davio C, Rivera E, Melito G, Cricco G, Andrade N, Caro D, Bergoc R. Hormone dependence of mammary tumors induced in rats by intraperitoneal NMU injection. Cancer Invest. 1997;15:8–17. doi: 10.3109/07357909709018912. [DOI] [PubMed] [Google Scholar]
- Curtis JR. The role of 7, 12 DMBA and three hormones in 7, 12 DMBA – induced rat mammary cancer: three hypotheses. Med Hypotheses. 1982;9:489–507. doi: 10.1016/0306-9877(82)90018-4. [DOI] [PubMed] [Google Scholar]
- Russo IH, Russo J. Mammary gland neoplasia in long term rodent studies. Environ Health Perspet. 1996;104:938–967. doi: 10.2307/3433143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi S, Guzman RC, Yang J. Hormones and mammary carcinogenesis in mice, rats and humans: A unifying hypothesis. Proc Natl Acad Sci USA. 1995;92:3650–3657. doi: 10.1073/pnas.92.9.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mershon J, Sall W, Mitchner N, Ben-Jonathan N. Prolactin is a local growth factor in rat mammary tumors. Endocrinology. 1995;136:3619–3623. doi: 10.1210/en.136.8.3619. [DOI] [PubMed] [Google Scholar]
- Kelly PA, Dijane J, Postel-Vinay MC, Edery M. The prolactin growth hormone receptor family. Endocr Rev. 1991;12:235–251. doi: 10.1210/edrv-12-3-235. [DOI] [PubMed] [Google Scholar]
- Schroeder MD, Symowicz J, Schuler LA. Prolactin modulates cell cycle regulators in mammary tumor epithelial cells. Mol Endocrinol. 2002;16:45–57. doi: 10.1210/me.16.1.45. [DOI] [PubMed] [Google Scholar]
- Vonderhaar BR. Prolactin involvement in breast cancer. Endocrine-Related Cancer. 1999;6:389–404. doi: 10.1677/erc.0.0060389. [DOI] [PubMed] [Google Scholar]
- Schally AV, Comaru-Schally AM, Nagy A, Kovacs M, Szepeshazi K, Plonowski A, Varga JL, Halmos G. Hypothalamic hormones and cancer. Front Neuroendocrinol. 2001;22:248–291. doi: 10.1006/frne.2001.0217. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Niitsu Y, Neda H, Sone H, Yamauchi N, Umetsu T, Urushizaki I. Antitumor effect of tumor necrosis factor against various primarily cultured human cancer cells. Jpn J Cancer Res. 1985;76:1115–1119. [PubMed] [Google Scholar]
- Watanabe N, Niitsu Y, Umeno H, Kuriyama H, Neda H, Yamauchi N, Maeda M, Urushizaki I. Toxic effect of tumor necrosis factor on tumor vasculature in mice. Cancer Res. 1988;48:2179–2183. [PubMed] [Google Scholar]
- Weeler DL, Ness KJ, Oberley TD, Verma AK. Protein kinase C epsilon is linked to 12-O-tetradecanoylphorbol-13-acetate-induced tumor necrosis factor-alpha ectodomain shedding and the development of metastatic squamous cell carcinoma in protein kinase C epsilon transgenic mice. Cancer Res. 2003;63:6547–6555. [PubMed] [Google Scholar]
- Srivastava S, Weitzmann MN, Cenci S, Ross FP, Adler S, Pacifici R. Estrogen decreases TNF gene expression by blocking JNK activity and the resulting production of c-Jun and JunD. J Clin Invest. 1999;104:503–513. doi: 10.1172/JCI7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisavljevic Z. Nitric oxide suppression triggers apoptosis through the FKHRL1 (FOXO 3A)/ROK kinase pathway in human breast carcinoma cells. Cancer. 2003;97:1358–1363. doi: 10.1002/cncr.10081. [DOI] [PubMed] [Google Scholar]
- Dhar A, Brindley JM, Stark C, Citro ML, Keefer LK, Colburn NH. Nitric Oxide does not mediate but inhibits transformation and tumor phenotype. Mol Cancer Ther. 2003;2:1285–1293. [PubMed] [Google Scholar]
- Wink DA, Miranda KM, Espey MG, Pluta RM, Hewett SJ, Colton C, Vitek M, Feelisch M, Grisham MB. Mechanism of the oxidant effects of nitric oxide. Antioxid Redox Signal. 2001;3:203–213. doi: 10.1089/152308601300185179. [DOI] [PubMed] [Google Scholar]
- Lala PK, Chakraborty C. Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol. 2001;2:149–156. doi: 10.1016/S1470-2045(00)00256-4. [DOI] [PubMed] [Google Scholar]
- Navarro Gonzálvez JA, García Benayas C, Arenas J. Semiautomated measurement of nitrate in biological fluids. Clin Chem. 1998;44:679–681. [PubMed] [Google Scholar]
- Hedley DW, Friedlander ML, Taylor IW, Rugg CA, Musgrove EA. Method for analysis of cellular DNA content of paraffin-embedded pathological material being flow cytometry. J Histochem Cytochem. 1983;31:1333–1335. doi: 10.1177/31.11.6619538. [DOI] [PubMed] [Google Scholar]
- McLemore DD, el Naggar A, Stephens LC, Jardine JH. Modified methodology to improve flow cytometric DNA histograms from paraffin-embedded material. Stain Technol. 1990;65:279–291. doi: 10.3109/10520299009105619. [DOI] [PubMed] [Google Scholar]
- Chan MM, Lu X, Merchant FM, Iglehart JD, Miron P. Gene expression profiling of NMU-induced rat mammary tumors: cross species comparison with human breast cancer. Carcinogenesis. 2005;26:1343–1353. doi: 10.1093/carcin/bgi100. [DOI] [PubMed] [Google Scholar]
- Ahlers I, Solar P, Buresova A, Ahlersova E. Very low sensitivity of Wistar:Han female rats to chemocarcinogen in mammary carcinogenesis induction. Neoplasma. 1998;45:373–376. [PubMed] [Google Scholar]
- Guzman RC, Osborn RC, Swanson SM, Sakthivel R, Hwang SI, Miyamoto S, Nandi S. Incidence of c-Ki-ras activation in N-methyl-N-nitrosourea-induced mammary carcinomas in pituitary-isografted mice. Cancer Res. 1992;52:5732–5737. [PubMed] [Google Scholar]
- Manni A, Raineri J, Arafah BM, Finegan HM, Pearson OH. Role of estrogen and prolactin in the growth and receptor levels of N-nitroso-methylurea – induced rat mammary tumours. Cancer Res. 1982;42:3492–3495. [PubMed] [Google Scholar]
- Welsch CW, Nagasawa H. Prolactin and murine mammary tumorigenesis: a review. Cancer Res. 1977;37:951–963. [PubMed] [Google Scholar]
- Shaw-Bruha CM, Pirrucello SJ, Shull JD. Expression of the prolactin gene in normal and neoplastic human breast tissues and human mammary cell lines: promoter usage and alternative mRNA splicing. Breast Cancer Res Treat. 1997;44:243–253. doi: 10.1023/A:1005879103367. [DOI] [PubMed] [Google Scholar]
- Rycyzyn MA, Clevenger CV. The intranuclear prolactin/Cyclophilin B complex as a transcriptional inducer. Proc Natl Acad Sci USA. 2002;99:6790–6795. doi: 10.1073/pnas.092160699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalldecker GB, Pigni J, Fuentes AM, Vegh I. Prolactin response to dopamine and valproate administration in breast cancer patients. Biomed Pharmacother. 1989;43:301–306. doi: 10.1016/0753-3322(89)90012-7. [DOI] [PubMed] [Google Scholar]
- Clevenger CV, Furth PA, Hankinson SE, Schuler LA. The role of prolactin in mammary carcinoma. Endocr Rev. 2003;24:1–27. doi: 10.1210/er.2001-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutzman JH, Rugowski DE, Schroeder MD, Watters JJ, Schuler LA. Multiple kinase cascades mediate prolactin signals to activating protein-1 in breast cancer cells. Mol Endocrinol. 2004;18:3064–3075. doi: 10.1210/me.2004-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glikman P, Vegh I, Pollina MA, Mosto AH, Levy CM. Ornithine decarboxylase activity, prolactin blood levels, and estradiol and progesterone receptors in human breast cancer. Cancer. 1987;60:2237–2243. doi: 10.1002/1097-0142(19871101)60:9<2237::AID-CNCR2820600923>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Rubio MA, Torres-Aleman I, Calle JR, Cabranes JA, Schally AV, Charro AL. D-Trp6-luteinizing hormone-realising hormone inhibits Sulpiride-induced hyperprolactinemia in normal men. J Clin Endocr Metab. 1987;65:368–369. doi: 10.1210/jcem-65-2-368. [DOI] [PubMed] [Google Scholar]
- Tomita K, Hosaka Y, Aso Y, Akasa H, Shirai T. Histological and flow cytometric analyses of carcinogenesis of rat prostate cancer. Nippon Hinyokika Gakkai Zasshi. 1990;81:1396–1403. doi: 10.5980/jpnjurol1989.81.1396. [DOI] [PubMed] [Google Scholar]
- Monakhov AS, Iakovleva TK, Anisimov VN. [Chromosomal damage in peripheral blood lymphocytes of rats in nitrosomethylurea-induced carcinogenesis] Eksp Onkol. 1990;12:40–43. [Article in Russian] [PubMed] [Google Scholar]
- Schally AV, Halmos G, Rekasi Z, Arencibia JM. The actions of LH-RH agonists, antagonists and cytotoxic analogs on the LH-RH receptors on the pituitary and tumors. In: Devroey P, editor. Infertility and Reproductive Medicine Clinics of North America. Philadelphia: Saunders; 2001. pp. 17–44. [Google Scholar]
- Tagami K, Niwa K, Lian Z, Gao J, Mori H, Tamaya T. Preventive effect of Juren-taiho-to on endometrial carcinogenesis in mice is based on Shimotsu-to constituent. Bio Pharm Bull. 2004;27:156–161. doi: 10.1248/bpb.27.156. [DOI] [PubMed] [Google Scholar]