Abstract
Juvenile Dermatomyositis (JDM) is the most common myopathy in children with characteristic skin rash and muscle weakness, in which longer duration of untreated disease was associated with less muscle weakness. The duration of untreated inflammation may alter the apoptotic pathways involved in skeletal muscle damage. Diagnostic muscle biopsies from 14 untreated patients were stained for apoptosis markers. TUNEL-positive nuclei and caspase 3 were detected within the laminin layer, indicating apoptosis of skeletal muscle nuclei. Untreated JDM disease duration greater than 2 months (“long”), was associated with higher Fas positive cell counts in the perivascular region compared with the “short” disease duration group, 2 months or less. Within the “long” duration group, higher Fas positive cell counts were positively associated with increased TUNEL-positive nuclei and caspase 3. We conclude that the duration of untreated disease (chronic inflammation) influences the mode of continuing cell damage and death in children with JDM.
Keywords: apoptosis, Juvenile Dermatomyositis (JDM), disease duration, skeletal muscle
Introduction
Juvenile dermatomyositis is the most common of the pediatric inflammatory myopathies, with an incidence in the U.S. of 3.1 children/million/year [1]. This systemic vasculitic disease is defined by a classic heliotrope rash (periorbital, malar and shawl areas, Gottron's papules), symmetrical proximal muscle weakness, elevated serum levels of muscle derived enzymes, and evidence of a myopathic process on electromyogram [2; 3]. The last criterion, known as the diagnostic “gold standard”, is the muscle biopsy in which perifasicular muscle fiber atrophy is associated with capillary occlusion and an inflammatory infiltrate composed primarily of mononuclear cells [4; 5; 6; 7]. Evidence of muscle fiber damage includes edema, centralization of nuclei, and localized atrophic cells. Histological evidence of muscle cell death is less common, even in children with florid weakness, which is attributed to the fact that the muscle cells are multinucleated and thus more resistant to lethal insult. The cause of muscle cell death was ascribed to the infiltrating monocytes and T cells [7].
The duration of untreated disease associated with the underlying chronic inflammation in patients with JDM impacts differently on the physical manifestations of the disease. Prolonged inflammation is associated with severe skin involvement, as evaluated by disease activity score (DAS) skin [8], as well as nailfold capillary end row loop drop out [9]. Conversely, the muscle symptoms, evaluated by DAS weakness, are inversely correlated with duration of untreated disease [10]. Disease chronicity is associated with the presence of the TNF-α 308 A allele and the related increased production of TNF-α by the peripheral blood mononuclear cells [11] and the muscle fibers themselves [12]. Laboratory indices of muscle inflammation are also impacted by the duration of untreated disease. Serum levels of muscle enzymes (aldolase, lactic dehydrogenase, creatine kinase, serum glutamic-oxaloacetic transaminase/aspartate aminotransferase) tend to be in the normal level with disease duration longer than 4.7 months, but the underlying mechanism for this observation is unknown [10]. Skeletal muscle fiber has been shown to upregulate Fas Ligand (FasL) and FLIP to counteract the apoptosis during inflammation [13]. Thus, longer disease duration may influence the type of apoptosis in JDM. Furthermore, when the impact of the duration of untreated disease on gene expression profiles were studied, children with JDM and a disease duration of greater than 2 months had significantly different gene expression profiles in untreated muscle biopsies from children with symptoms for 2 months or less, providing the rationale for using a 2 month cutoff to divide the patients into 2 groups [14].
A clue to a mechanism of cellular injury came from a set of investigations in which we compared the profiles of genes expressed by muscle from children with untreated JDM with muscle from children with Duchene's Muscular dystrophy (DMD) [15]. We found that 43% of the upregulated genes specific to JDM (not shared with DMD), were those related to the immune response. In this group, the gene for TRAIL (Tumor necrosis factor Related Apoptosis Inducing Ligand) was up-regulated 3.9 fold, suggesting that this cell-death inducing pathway and apoptotic mechanism might play a role in JDM. TRAIL-mediated cell apoptosis proceeds by binding to two TRAIL receptors, DR4 and DR5, with subsequent activation of caspases via the receptor's intracellular death domains [16]. The characteristic changes of cell apoptosis are nuclear fragmentation, detected by TUNEL [17], and active forms of caspases [18]. Fas Ligand represents another extracellular cell death signal which mediates apoptosis through its receptor, Fas [19].
The purpose of the present study was to compare the distribution of apoptosis-inducing ligands and the extent of apoptosis in muscle biopsies between short disease duration of 2 months or less compared with long disease duration, greater than 2 months, in muscle biopsies from untreated children with JDM, using immunohistochemical and immunoflurescence techniques.
Materials and methods
Patient population
Fourteen children with newly diagnosed, untreated definite JDM (criteria of Bohan and Peter [2; 3]), were enrolled in the study after their families signed the IRB approved informed consent. The mean age of the children at time of muscle biopsy was 7.8 ± 0.7 years. The group consisted of 10 girls and 4 boys, with a female:male ratio of 2.5 (similar to the 2.3 ratio seen nationally [1]). The group was predominantly Caucasian (n=13), Hispanic (n=1) and the median disease duration from the first definite symptom of JDM (rash and/or proximal muscle weakness) to the date of muscle biopsy was 3.48 months. The group was equally divided into 7 children who were positive for the TNFα-308A and 7 children who had only G in the −308 position of the TNF allele. The mean disease activity score (DAS) was 6.4 ± 1.5 for skin (maximal possible score =9) and 6.8 ± 2.9 for muscle weakness (maximal possible score =11) and the total DAS was 13.1 +/− 0.87 (maximal possible score=20).
Genetic testing
The primers and probes were synthesized by Invitrogen. Peripheral blood mononuclear cells were isolated from anti-coagulated whole blood and stored at −80°C until DNA isolation was performed using a Puregene DNA isolation kit (Gentra Systems, Inc. Minneapolis, MN). The TNFα-308 polymorphism consists of a single base pair substitution of an A for the more common G. PCR was used to amplify a 107 bp fragment containing the region that incorporated a NcoI restriction site as previously described [20]. Digestion with NcoI confirmed genotype as GG, GA or AA.
Clinical definitions
At the time of the initial clinic visit, before the diagnostic biopsy date, the Disease Activity Score (DAS) was determined by one physician (LMP) on the untreated child. This score is a validated, simple, rapid and reliable clinical estimate of disease activity that rates the active involvement of both skin and muscle for a total score of 20 points [8]. Disease onset was defined as the time when the first definite symptom of JDM (rash or weakness) was recognized [21]. The duration of untreated disease was defined as the interval of time between the first symptom (disease onset) and the time when muscle biopsy was obtained prior to treatment.
Muscle Biopsy
Using MRI (T2 weighted image, fat suppression) to localize the maximal area of inflammation, a diagnostic open muscle biopsy was obtained in the operating room under general anesthesia. The muscle sample was immediately divided into two sections, one to be used for molecular studies and the other for immunohistochemistry. Serial transverse 8μm fresh frozen slides were prepared from the biopsy material. The areas of the muscle biopsy were categorized as perivascular, endomesial or perifascicular. Biopsies of normal muscle were obtained from two healthy children undergoing surgery, and who had given informed consent, as controls.
Immunohistochemistry
In brief, frozen sections of muscle biopsies were fixed in acetone for 10 min at −20°C. After blocking with 5% BSA for 1 h at room temperature, the sections were incubated with primary antibodies (1:50−1:200 dilution in blocking buffer) for 1 h at room temperature or overnight at 4°C. After washing (PBS), the sections were incubated with biotinylated secondary antibody (Jackson ImmunoResearch Laboratory, West Grove, PA) at room temperature for 30 min. Avidin-biotin complex and diaminobenzidine (DAB) reagent kits (Vector Laboratories, Burlingame, CA) were used according to the manufacturer's protocol to detect the secondary antibody. Counterstaining with hematoxylin was performed for 30 seconds. The following primary antibodies were used, active caspase 3 (BD Biosciences Inc., San Diego, CA), caspase 9, cytochrome c, DR4, DR5, FAS, TRAIL (D-3) (Santa Cruz Biotechnology Inc., Santa Cruz, CA), laminin B2 chain (Chemicon, Temecula, CA). TUNEL staining was performed according to the instruction from manufacturer Chemicon (DAB) and Roche Diagnostic (Rhodamine). Images of stained muscle sections were acquired using Openlab computer software 4.04 (Improvision Inc., Lexington, MA) and a Leica DMR-HC microscope (Leica Microsystems GmbH, Wetzlar, Germany) coupled to a Photometric Cool Snap charge-coupled device camera and then edited using Adobe Photoshop CS2 software. Positive cells or nuclei were counted under a light microscope in three different regions: perivascular, perifascicular, endomysial at 40X magnification and the counts were repeated at three random sites within each sample. The average count within each region was used for statistical analysis.
Immunofluorescence staining
Frozen sections were fixed as described above and stained with TUNEL (Rhodamine-label, Roche Inc.) followed by laminin B2 chain (Chemicon), caspase 3 (BD Biosciences) staining. The primary antibodies were diluted 1:10 to 1:50 and the flurochrome-conjugated secondary antibodies (Jackson ImmunoResearch Laboratory) were diluted 1:100 to 1:250. At the end, the tissue section was counterstained with DAPI. Images were taken with Zeiss 510 META confocal laser scanning microscope (Carl Zeiss, Jena, Germany).
Data analysis
Pearson correlations between duration of untreated disease and DAS, between death receptors (DR4, DR5 and Fas) and apoptosis endpoint markers (caspase 3 and TUNEL), were tested. Patients were divided into two groups, long duration (>2 mo) and short duration (≤2 mo), based on their durations of untreated disease. Previous studies showed that gene expression profiles were distinct between short and long duration groups using same criteria [14]. The difference of distribution of each antigen between two duration groups or between two TNFα-308A groups (AA+AG vs. GG) was examined using General Linear Models with SPSS statistical software (v11.0, Chicago, IL). P value less than 0.05 was considered as statistically significant.
Results
The association of increased disease duration with less muscle symptoms
In this group of children with JDM, the length of time between the first symptom and the first clinic visit (duration of untreated disease) was inversely correlated with DAS muscle (Figure 1).
Figure 1.
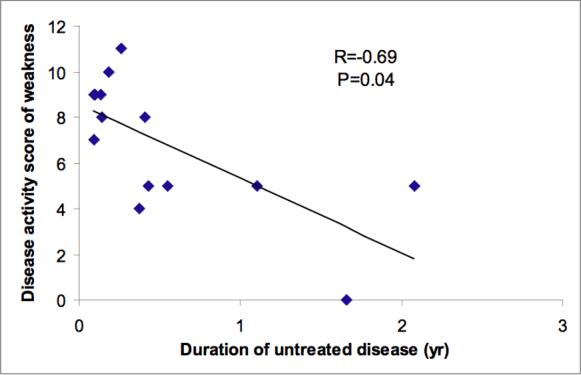
Fourteen JDM patients were evaluated for the disease activity score (DAS) of muscle weakness at the time of muscle biopsies. The duration of untreated disease is inversely associated with DAS weakness (p<0.05).
Localization of apoptosis markers in JDM muscle
TRAIL was detected primarily at the perivascular region and expressed along the cell surfaces of mononuclear cells (Figure 2). Receptors of TRAIL including DR4, DR5, as well as Fas (receptor of Fas ligand) were not only expressed in the perivascular region but also the perifasicular and endomysial regions, especially along the edges of skeletal muscle fibers (Figure 2). Although perforin, cytochrome c, caspase 9 and caspase 3 were detected in all three regions, the perivascular region had the highest expression of these antigens. These markers were also present in vasculatures. Conversely, the endomysial region contained the lowest expression of these antigens (Figure 2 & 3). TUNEL staining revealed dead nuclei in all three regions examined, which was also more common in the perivascular region (Figure 3). In muscles from healthy children, only the death receptors, DR4, DR5 and Fas were detected (Figure 2 & 3).
Figure 2.

Frozen sections of muscle biopsies from JDM patients and healthy children were stained with antibodies against TNF-related apoptosis-inducing ligands (TRAIL), receptors (DR4, DR5 and Fas), perforin. Arrows indicate positive stains. Perforin was present in vasculature.
Figure 3.

Frozen sections of muscle biopsies from JDM patients and healthy children were stained with TUNEL or antibodies against apoptosis-related proteins (cytochrome c, caspase 9, caspase 3). Arrows indicate positive stains. TUNEL, caspase 9, caspase 3 were present in vasculature.
Costaining studies
Using a fluorochrome-labeld TUNEL kit, we confirmed the results of the histochemical staining. Laminin staining revealed the border of individual muscle fibers and allowed us to distinguish the nuclei of the infiltrating immune cells from the myonuclear cells. TUNEL-positive nuclei were present at both sub-lamina and extra-lamina. Some TUNEL-positive nuclei at sub-lamina were also caspase 3 positive as shown in Figure 4.
Figure 4.

Frozen sections of muscle biopsies were co-stained with TUNEL (red), antibodies against laminin (violet), caspase 3 (green) and counterstained with DAPI (blue). TUNEL co-localized with DAPI indicating nuclear death. Caspase 3 and TUNEL double positive cells were present within the laminin layer of the skeletal muscle fibers. Arrows indicate an example of an apoptotic myonucleus.
The impact of the duration of untreated disease on the distribution of the apoptotic markers
There was no difference in detection of TRAIL, DR4, DR5, perforin, cytochrome c, caspase 3, or caspase 9 in any of the three regions between the short and long disease duration groups. Longer disease duration (>2months) was associated with higher Fas positive cell counts in the perivascular region (p=0.02). Conversely, counts of TUNEL positive nuclei in the endomysial region were lower in patients with longer disease duration (p=0.03) (Figure 5). When the correlation analysis between death receptors (DR4, DR5 and Fas) and apoptosis/necrosis endpoint markers (caspase 3 and TUNEL) in the same region was performed, significant associations were observed between DR4 and caspase 3 in the perivascular region irrespective of disease chronicity (R=0.63, P=0.02). There was also an association between Fas in the perivascular region and TUNEL in the endomysial region (R=0.74, p=0.02), and between Fas and caspase 3 in the perivascular region from the longer duration group (R=0.65, p=0.05). There was more Fas expression in the perivascular region than either DR4 or DR5 (p<0.05). TNF-α 308A allele was not associated with localization of any of the apoptosis markers in any region.
Figure 5.
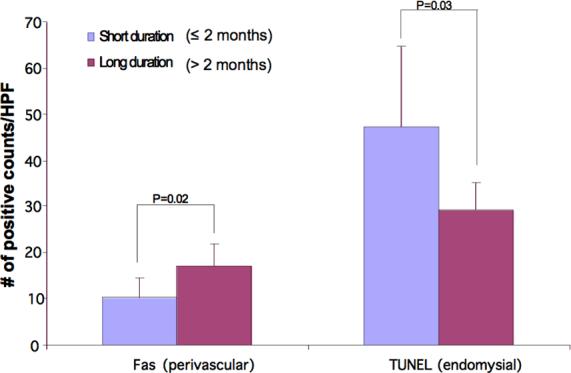
The counts of positively stained antigens tested per region (perivascular, perfasicular, endomysial) were compared between long (>2 months) and short (≤ 2 months) disease duration groups. A short duration of untreated disease is associated with lower Fas expression at perivascular region and higher TUNEL counts at endomysial region (p< 0.05).
Discussion
Skeletal muscle weakness is often associated with florid inflammation in children with JDM. Muscle injury is accompanied by infiltration of macrophages and T lymphocytes [7]. Our previous work showed that the gene expression profile of TRAIL, an apoptosis-inducing ligand expressed on the surface of activate T lymphocytes and natural killer cells, was upregulated in children with JDM compared to children with Duchene disease [15]. The results from the present study confirm the expression of TRAIL as well as its receptors, DR4 and DR5, in the muscle biopsies from JDM patients. Our results suggested that DR4 was more involved than DR5 in apoptosis signaling for there was a positive correlation between DR4 and caspase 3 in the perivascular region. TRAIL has been shown to induce apoptosis of vascular smooth muscle cells from the atherosclerotic plague while anti-TRAIL and anti-DR5 antibodies blocked TRAIL-mediated apoptosis [22]. In contrast, osteoprotegerin (OPG), another receptor for TRAIL, inhibits TRAIL-induced apoptosis, due to the absence of a death domain [23]. In related studies, we found that sera from untreated patients with JDM contained a significantly lower level of OPG and patients with longer disease duration had an even lower serum OPG level [24], which may be associated with reduced inhibition of TRAIL-mediated apoptosis. However, the role of OPG in JDM requires further investigation.
Another pathway by which apoptosis is induced utilizes the Fas/Fas ligand system. This system is thought to be important in regulating the immune system as mutations of Fas and FasL in humans cause autoimmune lymphoproliferative syndrome (ALPS) [25; 26]. Fas receptor was previously identified both on muscle fibers and on the infiltrating mononuclear cells from patients with either polymyositis or dermatomyositis [27], and FasL was localized to muscle fibers and mononuclear cells in adults with polymyositis [13]. In our study, Fas was expressed on mononuclear cells as well as muscle fibers in the diagnostic muscle biopsy from JDM patients, which is similar to the previous observation. More cells were Fas-positive than DR4+ or DR5+ only in the long-duration group. In addition, Fas expression was correlated with caspase 3 in the perivascular region and TUNEL in the endomysial region. These results support the conclusion that Fas played, if not more, at least as significant role as DR4 in apoptosis if the inflammation of JDM was untreated for more than 2 months.
Cell apoptosis in response to TRAIL or FasL may or may not involve mitochondria, depending on the cell type involved. Activation of caspase 8 is sufficient to induce apoptosis through activation of caspase 3 in some cell types, whereas an amplification of the extrinsic pathway through release of cytochrome c from mitochondria is necessary to commit the cells to apoptosis in others. In the latter pathway, caspase 9 activates caspase 3 to induce apoptosis [28]. In our study, there was expression of caspase 3 along with TUNEL observed on staining the JDM muscle biopsies, documenting the process of apoptosis. The detection of caspase 9 and cytochrome c released from mitochondria suggested that damage to mitochondria also contributes to the apoptosis.
The apoptosis of skeletal muscle is poorly understood because muscle fibers are multinucleated cells. Most reports did not find apoptotic nuclei of muscle fibers in patients with myositis using the TUNEL method [29; 30; 31]. However, Sugiura and collegues [27] identified apoptotic nuclei of muscle fibers using TUNEL and CD45 double staining in the muscle from patients with polymyositis and dermatomyositis. We confirmed the apoptosis of nuclei of muscle fibers using TUNEL, caspase 3 and laminin B2 co-staining and found the nuclei that were double positive for TUNEL and caspase 3 resided within laminin layer in the diagnostic muscle biopsies from the children. A similar approach has been used to study the myonuclear apoptosis in rabbits [32] and rats [33]. The duration of untreated disease is inversely correlated with clinical symptoms (DAS), which is in agreement with our previous observation [10]. This may be due to the fact that children who were weaker sought medical treatments earlier. This argument was supported by our finding that the counts of TUNEL positive nuclei in the endomysial region were lower in patients with longer disease duration >2 months.
We found that Fas positive cell counts in the perivascular region, where most infiltrating lymphocytes were located, was associated with longer disease duration. This suggests that if left untreated, JDM patients may suffer from more muscle damage regardless of the extent of the disease status at the onset. Indeed, apoptosis at both perivascular and endomysial regions indicated by caspase 3 and TUNEL respectively were positively associated with increased Fas positive cell counts. The presence of caspase 3 and TUNEL in vasculature suggested the involvement of endothelial or smooth muscle cell death.
We conclude that both TRAIL and FasL are involved in apoptosis observed in muscle biopsies of children with JDM. These data provide the evidence that the disease duration is an important factor in evaluating untreated children with JDM, and is associated with the increased apoptotic damage to their skeletal muscle if left untreated.
Acknowledgement
The authors thank Dr. Maxine Kuroda for assisting statistical analysis, Annette L. Urganus for technical assistance.
Supported by: R01 AR48289 (NIAMS) and CureJM (to LMP)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
presented partially at the American College of Rheumatology Annual Conference 2006.
References
- 1.Mendez EP, Lipton R, Ramsey-Goldman R, Roettcher P, Bowyer S, Dyer A, Pachman LM. US incidence of juvenile dermatomyositis, 1995−1998: results from the National Institute of Arthritis and Musculoskeletal and Skin Diseases Registry. Arthritis Rheum. 2003;49:300–5. doi: 10.1002/art.11122. [DOI] [PubMed] [Google Scholar]
- 2.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344–7. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 3.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403–7. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 4.Engel AG, Arahata K. Mononuclear cells in myopathies: quantitation of functionally distinct subsets, recognition of antigen-specific cell-mediated cytotoxicity in some diseases, and implications for the pathogenesis of the different inflammatory myopathies. Hum Pathol. 1986;17:704–21. doi: 10.1016/s0046-8177(86)80180-0. [DOI] [PubMed] [Google Scholar]
- 5.Hohlfeld R, Goebels N, Engel AG. Cellular mechanisms in inflammatory myopathies. Baillieres Clin Neurol. 1993;2:617–35. [PubMed] [Google Scholar]
- 6.Mizuno K, Yachie A, Nagaoki S, Wada H, Okada K, Kawachi M, Toma T, Konno A, Ohta K, Kasahara Y, Koizumi S. Oligoclonal expansion of circulating and tissue-infiltrating CD8+ T cells with killer/effector phenotypes in juvenile dermatomyositis syndrome. Clin Exp Immunol. 2004;137:187–94. doi: 10.1111/j.1365-2249.2004.02500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDouall RM, Dunn MJ, Dubowitz V. Nature of the mononuclear infiltrate and the mechanism of muscle damage in juvenile dermatomyositis and Duchenne muscular dystrophy. J Neurol Sci. 1990;99:199–217. doi: 10.1016/0022-510x(90)90156-h. [DOI] [PubMed] [Google Scholar]
- 8.Bode RK, Klein-Gitelman MS, Miller ML, Lechman TS, Pachman LM. Disease activity score for children with juvenile dermatomyositis: reliability and validity evidence. Arthritis Rheum. 2003;49:7–15. doi: 10.1002/art.10924. [DOI] [PubMed] [Google Scholar]
- 9.Smith RL, Sundberg J, Shamiyah E, Dyer A, Pachman LM. Skin involvement in juvenile dermatomyositis is associated with loss of end row nailfold capillary loops. J Rheumatol. 2004;31:1644–9. [PubMed] [Google Scholar]
- 10.Pachman LM, Abbott K, Sinacore JM, Amoruso L, Dyer A, Lipton R, Ilowite N, Hom C, Cawkwell G, White A, Rivas-Chacon R, Kimura Y, Ray L, Ramsey-Goldman R. Duration of illness is an important variable for untreated children with juvenile dermatomyositis. J Pediatr. 2006;148:247–53. doi: 10.1016/j.jpeds.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 11.Pachman LM, Liotta-Davis MR, Hong DK, Kinsella TR, Mendez EP, Kinder JM, Chen EH. TNFalpha-308A allele in juvenile dermatomyositis: association with increased production of tumor necrosis factor alpha, disease duration, and pathologic calcifications. Arthritis Rheum. 2000;43:2368–77. doi: 10.1002/1529-0131(200010)43:10<2368::AID-ANR26>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 12.Fedczyna TO, Lutz J, Pachman LM. Expression of TNFalpha by muscle fibers in biopsies from children with untreated juvenile dermatomyositis: association with the TNFalpha-308A allele. Clin Immunol. 2001;100:236–9. doi: 10.1006/clim.2001.5063. [DOI] [PubMed] [Google Scholar]
- 13.Nagaraju K, Casciola-Rosen L, Rosen A, Thompson C, Loeffler L, Parker T, Danning C, Rochon PJ, Gillespie J, Plotz P. The inhibition of apoptosis in myositis and in normal muscle cells. J Immunol. 2000;164:5459–65. doi: 10.4049/jimmunol.164.10.5459. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y-W, Abbott K, Pachman LM. Juvenile Dermatomyositis (JDM): Impact of duration of untreated disease and pathological calcification (pCa) on gene expression profiles. Clin Invest Med. 2004;27:#W51.139. [Google Scholar]
- 15.Tezak Z, Hoffman EP, Lutz JL, Fedczyna TO, Stephan D, Bremer EG, Krasnoselska-Riz I, Kumar A, Pachman LM. Gene expression profiling in DQA1*0501+ children with untreated dermatomyositis: a novel model of pathogenesis. J Immunol. 2002;168:4154–63. doi: 10.4049/jimmunol.168.8.4154. [DOI] [PubMed] [Google Scholar]
- 16.Mariani SM, Matiba B, Armandola EA, Krammer PH. Interleukin 1 beta-converting enzyme related proteases/caspases are involved in TRAIL-induced apoptosis of myeloma and leukemia cells. J Cell Biol. 1997;137:221–9. doi: 10.1083/jcb.137.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srinivasan A, Roth KA, Sayers RO, Shindler KS, Wong AM, Fritz LC, Tomaselli KJ. In situ immunodetection of activated caspase-3 in apoptotic neurons in the developing nervous system. Cell Death Differ. 1998;5:1004–16. doi: 10.1038/sj.cdd.4400449. [DOI] [PubMed] [Google Scholar]
- 19.Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol. 1999;11:255–60. doi: 10.1016/s0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- 20.Bouma G, Oudkerk Pool M, Scharenberg JG, Kolkman JJ, von Blomberg BM, Scheper RJ, Meuwissen SG, Pena AS. Differences in the intrinsic capacity of peripheral blood mononuclear cells to produce tumor necrosis factor alpha and beta in patients with inflammatory bowel disease and healthy controls. Scand J Gastroenterol. 1995;30:1095–100. doi: 10.3109/00365529509101613. [DOI] [PubMed] [Google Scholar]
- 21.Pachman LM, Lipton R, Ramsey-Goldman R, Shamiyeh E, Abbott K, Mendez EP, Dyer A, Curdy DM, Vogler L, Reed A, Cawkwell G, Zemel L, Sandborg C, Rivas-Chacon R, Hom C, Ilowite N, Gedalia A, Gitlin J, Borzy M. History of infection before the onset of juvenile dermatomyositis: results from the National Institute of Arthritis and Musculoskeletal and Skin Diseases Research Registry. Arthritis Rheum. 2005;53:166–72. doi: 10.1002/art.21068. [DOI] [PubMed] [Google Scholar]
- 22.Sato K, Niessner A, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM. TRAIL-expressing T cells induce apoptosis of vascular smooth muscle cells in the atherosclerotic plaque. J Exp Med. 2006;203:239–50. doi: 10.1084/jem.20051062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emery JG, McDonnell P, Burke MB, Deen KC, Lyn S, Silverman C, Dul E, Appelbaum ER, Eichman C, DiPrinzio R, Dodds RA, James IE, Rosenberg M, Lee JC, Young PR. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem. 1998;273:14363–7. doi: 10.1074/jbc.273.23.14363. [DOI] [PubMed] [Google Scholar]
- 24.Rouster-Stevens KA, Langman CB, Price HE, Seshadri R, Shore RM, Abbott K, Pachman LM. RANKL:osteoprotegerin ratio and bone mineral density in children with untreated juvenile dermatomyositis. Arthritis Rheum. 2007;56:977–83. doi: 10.1002/art.22433. [DOI] [PubMed] [Google Scholar]
- 25.Jackson CE, Puck JM. Autoimmune lymphoproliferative syndrome, a disorder of apoptosis. Curr Opin Pediatr. 1999;11:521–7. doi: 10.1097/00008480-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Jackson CE, Fischer RE, Hsu AP, Anderson SM, Choi Y, Wang J, Dale JK, Fleisher TA, Middelton LA, Sneller MC, Lenardo MJ, Straus SE, Puck JM. Autoimmune lymphoproliferative syndrome with defective Fas: genotype influences penetrance. Am J Hum Genet. 1999;64:1002–14. doi: 10.1086/302333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugiura T, Murakawa Y, Nagai A, Kondo M, Kobayashi S. Fas and Fas ligand interaction induces apoptosis in inflammatory myopathies: CD4+ T cells cause muscle cell injury directly in polymyositis. Arthritis Rheum. 1999;42:291–8. doi: 10.1002/1529-0131(199902)42:2<291::AID-ANR11>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 28.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–89. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 29.Schneider C, Gold R, Dalakas MC, Schmied M, Lassmann H, Toyka KV, Hartung HP. MHC class I-mediated cytotoxicity does not induce apoptosis in muscle fibers nor in inflammatory T cells: studies in patients with polymyositis, dermatomyositis, and inclusion body myositis. J Neuropathol Exp Neurol. 1996;55:1205–9. doi: 10.1097/00005072-199612000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Olive M, Martinez-Matos JA, Montero J, Ferrer I. Apoptosis is not the mechanism of cell death of muscle fibers in human muscular dystrophies and inflammatory myopathies. Muscle Nerve. 1997;20:1328–30. doi: 10.1002/(sici)1097-4598(199710)20:10<1328::aid-mus20>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 31.Inukai A, Kobayashi Y, Ito K, Doyu M, Takano A, Honda H, Sobue G. Expression of Fas antigen is not associated with apoptosis in human myopathies. Muscle Nerve. 1997;20:702–9. doi: 10.1002/(sici)1097-4598(199706)20:6<702::aid-mus7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 32.McLoon LK, Rowe J, Wirtschafter J, McCormick KM. Continuous myofiber remodeling in uninjured extraocular myofibers: myonuclear turnover and evidence for apoptosis. Muscle Nerve. 2004;29:707–15. doi: 10.1002/mus.20012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dupont-Versteegden EE, Strotman BA, Gurley CM, Gaddy D, Knox M, Fluckey JD, Peterson CA. Nuclear translocation of EndoG at the initiation of disuse muscle atrophy and apoptosis is specific to myonuclei. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1730–40. doi: 10.1152/ajpregu.00176.2006. [DOI] [PubMed] [Google Scholar]


