Abstract
The mechanism for the short-term maintenance of information involves persistent neural activity during the retention interval, which forms a bridge between the cued memoranda and its later contingent response. Here, we used event-related functional magnetic resonance imaging to identify cortical areas with activity that persists throughout working memory delays with the goal of testing if such activity represents visuospatial attention or prospective saccade goals. We did so by comparing two spatial working memory tasks. During a memory-guided saccade (MGS) task, a location was maintained during a delay after which a saccade was generated to the remembered location. During a spatial item-recognition (SIR) task identical to MGS until after the delay, a button press indicated whether a newly cued location matched the remembered location. Activity in frontal and parietal areas persisted above baseline and was greater in the hemisphere contralateral to the cued visual field. However, delay-period activity did not differ between the tasks. Notably, in the putative frontal eye field (FEF), delay period activity did not differ despite that the precise metrics of the memory-guided saccade was known during the MGS delay and saccades were never made in SIR. Persistent FEF activity may therefore represent a prioritized attentional map of space, rather than the metrics for saccades.
Keywords: spatial, working memory, frontal eye field, saccade, attention
INTRODUCTION
Working memory allows animals to use information that is not currently available in the immediate environment. Without the ability to temporarily store information on-line, one’s behavior is stereotyped and largely depends upon learned stimulus-response associations. If, however, an internal representation of critical information can be created and maintained for a short period of time, flexible behavior emerges because one’s decisions can now utilize representations of temporally discontinuous events. Arguably, the most important scientific observation with regard to the neural mechanisms of working memory is that neuronal activity persists during the mnemonic delay period between a sensory cue (e.g., the position of a briefly flashed spot of light) and a later contingent motor response (e.g., a shift of gaze to the spot’s remembered location) (Funahashi et al., 1989; Fuster & Alexander, 1971; Gnadt & Andersen, 1988; Kubota & Niki, 1971). It is thought that this activity reflects some form of a memory. Yet, it remains unknown what information is carried by the persistent activity, whether it represents, for instance, a retrospective code of the past stimulus or it represents a prospective code for the memory-guided response.
The frontal and parietal cortices are most strongly linked to working memory processes. Damage to or inactivation of these areas can cause severe working memory impairments (Curtis & D'Esposito, 2004; Dias & Segraves, 1999; Funahashi et al., 1993; Li et al., 1999; Lynch, 1992; Muri et al., 1996; Ploner et al., 1999; Rivaud et al., 1994b; Wardak et al., 2002). Additionally, parts of the frontal and parietal cortices show evidence of persistent neural activity during the retention interval of spatial working memory tasks in both monkeys using electrophysiology (Bruce & Goldberg, 1985; Chafee & Goldman-Rakic, 1998; Funahashi et al., 1989; Sommer & Wurtz, 2001; Umeno & Goldberg, 2001) and humans using functional magnetic resonance imaging (Brown et al., 2004; Courtney et al., 1998; Curtis & D'Esposito, 2006; Curtis et al., 2004; Geier et al., 2007; Leung et al., 2002; Medendorp et al., 2006; Postle et al., 2000; Postle & Hamidi, in press; Rowe et al., 2000; Sala et al., 2003; Zarahn et al., 1999). These are critical pieces of evidence showing that parts of the frontal and parietal cortices are necessary for intact working memory and the mechanisms that underlie maintenance may be dependent upon persistent neural activity. Indeed, computational models of working memory hinge on the persistence of a signal throughout the retention interval until the memory-guided response can be generated (Machens et al., 2005; Wang, 2001).
Here, we used functional magnetic resonance imaging to identify the human cortical areas involved in maintenance and further tested hypotheses about what type of information is represented by persistent activity. With regard to our first goal – to identify areas whose activity persisted during memory delays – we used an event-related design that was optimized to evoke delay period activity that could be reliably measured. 1) We used memory delay periods that were variable in length and long in duration. The jittered delays helped statistically disambiguate blood oxygenation level dependent (BOLD) signals whose variance is best attributed to the delay period and not the bounding stimulus cue and motor response events. 2) It allowed us to unambiguously demonstrate that the activity persists until the response is made regardless of the delay length. 3) The variable delay helped keep subjects in an active state of maintenance since they could not predict when they were going to respond; with the use of fixed delay lengths, subjects learn that they do not need to remain in a state of readiness. Together, these features allow us to rigorously test for the presence of persistent delay period activity.
Our second goal was to test hypotheses about the nature of persistent activity, namely what type of information is carried by persistent activity. We asked if explicit foreknowledge about the metrics of the memory-guided response known throughout the retention interval influences persistent activity. We predicted that areas, like the human FEF, would show a greater magnitude of persistent activation when the precise metrics of the memory-guided saccade was known throughout the delay period. Our prediction was based on electrophysiological work in monkeys showing that presaccadic activity in FEF neurons is correlated with the metrics of saccades (Schall & Thompson, 1999). This hypothesis states that persistent activity is driven by neurons that code for the prospective saccade goal, a viable mechanism for performing memory-guided saccades.
To test that hypothesis we compared delay period activity during a memory-guided saccade (MGS) task, the field’s standard spatial working memory task, and during a spatial item recognition (SIR) task (Figure 1a). During the MGS task, a single cued location was maintained during a delay after which a saccade was generated to the cue’s remembered location. During the SIR task, which was identical to the MGS task until after the delay period, subjects never made an eye movement but instead made a button press that indicated whether a newly cued location exactly matched the remembered location. There were two key differences between the MGS and SIR tasks. First, subjects knew the precise metrics of the memory-guided response during the retention interval only in the MGS task; the button press response could not be predicted in the SIR task. This difference allowed us to test our prediction that cortical areas with saccade neurons (e.g., FEF) will show greater persistent activity during the delay period of the MGS compared to the SIR task. A second difference in the two tasks was that the motor effector used by the subjects to indicate their mnemonic response differed; saccades were used in the MGS task, while manual button presses were used in the SIR task. It was desirable to use a different response effector for a couple of reasons. Our main prediction, as described above, was that saccade neurons that code for the specific eye movement to the cue’s location exhibit persistent activity during delay periods. If we used another task in which saccades were the response effector, a population of saccade neurons may increase in activity in general anticipation of making a saccade even if the direction of the specific memory-guided saccade was not known. This would weaken our statistical power to detect differences. Since we intend to compare the two tasks, we ensured that they did not differ in difficulty. We equated the difficulty of the two tasks with the use of an adaptive psychophysical procedure that for each subject adjusted the spatial precision needed to make the match/non-match decision in the SIR task to be equal to the memory-guided saccade accuracy in the MGS task.
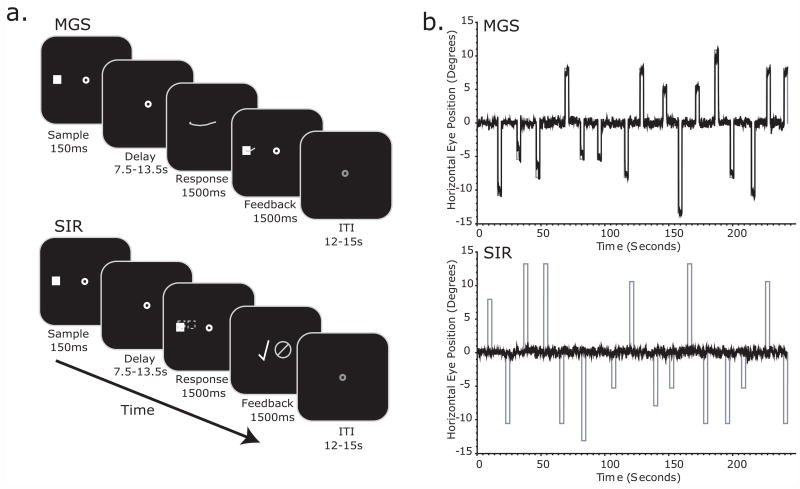
Figure 1.
a. Schematic of the memory-guided saccade (MGS) and spatial item-recognition (SIR) tasks. In both tasks, subjects maintained the position of a sample cue over a long and variable delay period. After the delay, subjects made a memory-guided saccade to the cued location in the MGS task. They decided whether a test cue matched the location of the sample cue (dotted box; was not visible to subject) in the SIR task. See methods for details. b. Horizontal eye position traces from single blocks of the MGS and SIR tasks in an example subject. (black line – eye position; grey line – target position).
MATERIALS AND METHODS
Subjects and behavioral procedures
Ten neurologically healthy individuals (7 male, 3 female; 8 right-handed, 1 left-handed, 1 ambidextrous; ages between 20 and 36) were recruited for participation and were paid for their time. All subjects gave written informed consent according to procedures approved by the human subjects Institutional Review Board at New York University.
The experimental stimuli were controlled by E-Prime (Psychology Software Tools, Inc., PA) and projected (Eiki LC-XG100) into the bore of the scanner on a screen that was viewed by the subjects through an angled mirror. Participants were scanned while performing four runs of the memory-guided saccade (MGS) task and four runs of the spatial item recognition (SIR) task in the following order, MGS, MGS, SIR, SIR, MGS, SIR, MGS, SIR. A schematic of the experiments is illustrated in Figure 1a. In the MGS task, a trial began with a preparation cue (white dot, 1.5 s) at the center of the screen indicating that a new trial was beginning. Then, a sample cue (cyan square, 150 ms) was flashed between 5–15° left or right and 4–5° above or below the central fixation point. No cues were presented near the cardinal axes to prevent verbalization of the locations. Subjects were asked to remember the location of the sample for the duration of a long, variable, and unpredictable delay period (7.5, 9, 10.5, 12, or 13.5s). Subjects made a saccade to the remembered location and fixated that position; they did so when the fixation point disappeared. After 1.5 s, the sample cue was re-presented as feedback and subjects made corrections, if necessary, to the position of their fixation. A variable intertrial interval (ITI) (12–15 s) then followed to allow the hemodynamic response to return to baseline. In the SIR task, the stimuli, timing and instructions were the same as in the MGS, in fact, the experiments were identical except at the response and feedback periods. Note that no saccades were ever made during the SIR trials. After the delay, a test cue appeared at or near the previous location of the sample cue, and the subject indicated with a button press, made with their right index or ring finger, whether it matched the location of the sample cue. Feedback was then given, where correct was indicated by a green √ and incorrect was indicated by a red Ø. We also included partial catch trials, where unpredictably after the sample cue appeared, the trial was aborted 1.5 s into the delay and went to the ITI. The partial catch trials were included to help in deconvolution and allowed us to examine the effects of encoding the sample cue’s location without maintenance or responding. Within each block there were 18 trials, 2 of which were catch trials. Overall, there were 64 MGS, 64 SIR, and 16 catch trials. Importantly, since we wanted to compare MGS and SIR task evoked neural activity, we tried to equate task difficulty across the two tasks for each subject. Since we could not control parameters that might affect MGS performance, we modified the difficulty of the SIR task using on-line estimates of MGS accuracy. We visually approximated the accuracy of the subject’s memory-guided saccades with the use of a grid with 1-deg cells overlaid of the video display of eye position. Then, we used that estimate from the end of each MGS run to adjust the difficulty of the next SIR run with the use of a staircase procedure that determined how close the test cue was to the sample cue on non-match trials. By placing the non-matching test cue closer, we made the decision more difficult.
Oculomotor procedures
Eye position was monitored in the scanner at 60 Hz with an infrared videographic camera equipped with a telephoto lens (ASL 504LRO; Applied Sciences Laboratories, Bedford, MA; custom modified with a Sony HAD CCD) that focused on the right eye viewed from the flat surface mirror mounted inside the RF coil. Nine-point calibrations were performed at the beginning of the session and between runs when necessary. Eye-movement data were transformed to degrees of visual angle, calibrated using a third-order polynomial algorithm that fit eye positions to known spatial positions, and scored offline with in-house software (GRAPES; See Figure 1b). Trials in which subjects did not comply with task instructions (e.g., looking at the sample cue before the delay, anticipatory saccades, breaking fixation in the SIR task, excessive blinks) were modeled to remove their variance, but were excluded from further analysis. All 10 subjects completed the 8 blocks of the experiment (4 MGS blocks and 4 SIR blocks). However, due to technical difficulty while recording, 1 subject did not have oculomotor data for 2 blocks (1 MGS, 1 SIR) and another subject for 4 blocks (4 SIR). These subjects' eye movements were reviewed for task compliance by carefully watching the offline videotape of the session. A total of 147 trials (11.05%) from all subjects were discarded from analyses because of non-compliance. In the MGS task, the difference between the position of fixation after the memory-guided saccade and the fixation to acquire the feedback cue was used as an index of memory accuracy and was stored in terms of degrees of visual angle (Figure 3a). Most often subjects made a single MGS, but in cases where they changed fixation before the feedback was presented, we used the last fixation as their response.
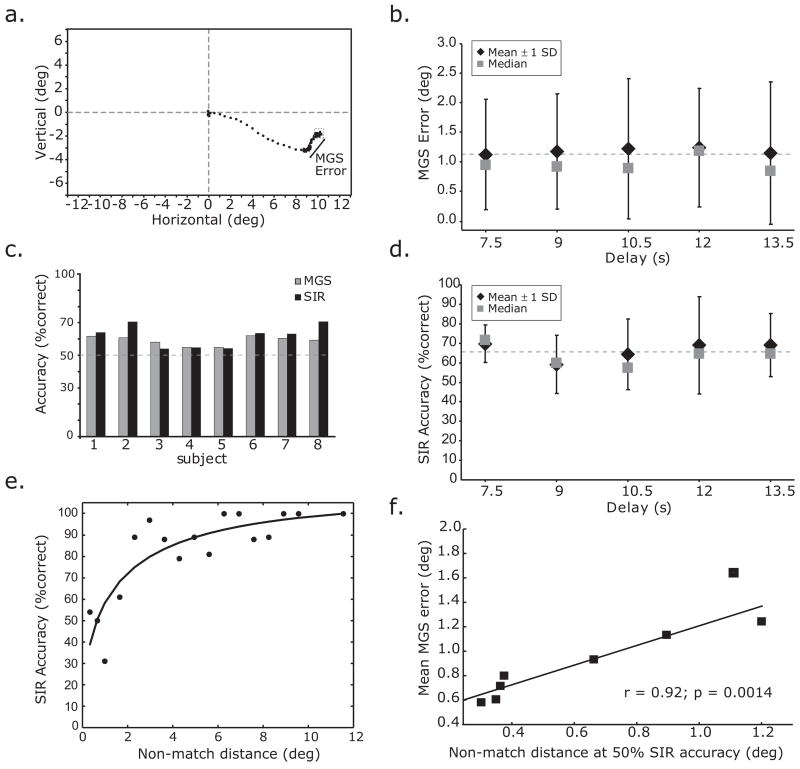
Figure 3.
Behavioral data. a. Eye trace of a memory guided saccade (MGS) to a cued location in the lower right visual field. The difference between the fixation following the MGS and the corrective saccade after feedback was given was used to calculate the error in degrees of visual angle. b, d. Performance on the MGS and SIR tasks as a function of the delay period interval. Notice that the delay length does not affect performance. c. Performance was statistically the same in each subject for MGS and SIR tasks. e. Psychometric function for performance on the SIR task as function of the distance of the non-match test cue from the sample cue location. Each dot represents the average SIR accuracy when the sample cue location and the test cue did not match by the various distances. Notice that performance was successfully modified by this manipulation. f. Scatter plot illustrating that the separation distance between the sample cue and the non-match test cue that resulted in 50% SIR accuracy predicts the subject’s average MGS error.
Neuroimaging procedures
We used functional magnetic resonance imaging at 3 T (Allegra; Siemens, Erlangen, Germany) to measure blood-oxygen level-dependent (BOLD) changes in cortical activity. During each fMRI scan, a time series of volumes was acquired using a T2*-sensitive echo planar imaging pulse sequence (repetition time, 1500 ms; echo time, 30 ms; flip angle 75°; 24 slices; 3 mm3 isotropic voxels; in-plane field of view of 192 mm2). Images were acquired using custom radio frequency coils (NM-011 transmit head-coil and NMSC-021 four-channel phased array receive coil; NOVA Medical, Wakefield, MA) placed over lateral frontal and parietal cortices. High-resolution (1 mm3 isotropic voxels) MP-RAGE three-dimensional T1-weighted scans were acquired for anatomical registration, segmentation, and display. To minimize head motion, subjects were stabilized with foam padding around the head.
fMRI data preprocessing and surface based statistical analysis
Post hoc image registration was used to correct for residual head motion [MCFLIRT (motion correction using FMRIB's Linear Image Registration Tool)]. Additional preprocessing of the fMRI data were as follows. We bandpass filtered the time series of each voxel (0.01 to 0.33 Hz) to compensate for the slow drift typical in fMRI measurements (Biswal & Hyde, 1997; Zarahn et al., 1997), divided the time series of each voxel by its mean intensity to convert to percent signal modulation and compensate for the decrease in mean image intensity with distance from the receive coil, and spatially smoothed the data to arrive at smoothness of 6mm at FWHM.
We modeled each within-trial event (i.e., sample cue, delay, and response) for MGS and SIR trials separately. The encoding of the sample cue and the generation motor response were short transient events and were thus modeled with an impulse time-locked to the event convolved with a canonical hemodynamic response function (HRF) (Polonsky et al., 2000). The memory delay spanned 7.5 – 13.5 seconds and was modeled very well by the linear combination of a zero-order polynomial (i.e., boxcar) and a first-order polynomial (i.e., linear ramp). Both delay regressors spanned the delay period and were time-shifted by 4000 ms to account for the hemodynamic lag. The parameter estimates from the first-order polynomial was used to estimate delay period activity at the group level because at the individual subject level it predicted significant delay period activity, confirmed by plotting the time series of the voxels identified by this parameter. Each of the independent variable regressors were entered into a modified general linear model (GLM) (Worsley & Friston, 1995) for statistical analysis using VoxBo (http://www.voxbo.org).
For each subject, we used Caret (http://brainmap.wustl.edu/caret) for anatomical segmentation, gray-white matter surface generation, flattening, and multi-fiducial deformation mapping to the PALS atlas (Van Essen, 2005). Registering subjects in a surface space using precise anatomical landmark constraints (e.g., central sulcus, sylvian and calcarine fissures, etc.) results in greater spatial precision of the alignment compared to standard volumetric normalization methods (Van Essen, 2005). Further, statistical maps for contrasts of interest were created using the beta-weights estimated from each subject’s GLM. These parameter maps were then deformed into the same atlas space, and t-statistics were computed for each contrast across subjects in spherical atlas space. We used a nonparametric statistical approach based on permutation tests to help address the problem of multiple statistical comparisons (Holmes, 1996; Nichols, 2002), which are even more problematic when one performs statistical analyses on surfaces. First, we constructed a permuted distribution of clusters of neighboring surface nodes with t-values > 3.0. We chose a primary t-statistic cutoff of 3.0 because it is strict enough that intense focal clusters of activity would pass but not so strict that diffuse large clusters of activity are lost. In the case of a one-sample comparison, where measured values are compared to the test value of 0, the signs of the beta values for each node were randomly permuted for each subject’s surface, prior to computing the statistic. One thousand iterations, N, of this procedure were performed to compute a permutation distribution for each statistical test performed. Then, we ranked the resulting suprathreshold clusters by their area. Finally, corrected p-values at α = 0.05 for each suprathreshold cluster were obtained by comparing their area to the area of the top 5% of the clusters in the permuted distribution, where the critical suprathreshold cluster size, C, at a t-score threshold of t > 3.0 was C = Nα+1. The permutation tests controlled for Type I error by allowing us to formally compute the probability that an activation of a given magnitude could cluster together by chance.
Region-of-Interest (ROI) time series procedures
We used ROI-based analyses of the time courses of BOLD signal change. First, on each subject’s high resolution anatomical scans, we traced around grey matter of several a priori ROIs motivated by past studies of spatial working memory (Brown et al., 2004; Connolly et al., 2002; Curtis & D'Esposito, 2006; Curtis et al., 2004; Medendorp et al., 2005, 2006; Schluppeck et al., 2006) and preliminary inspection of single subject activations, including the superior precentral sulcus (sPCS), inferior precentral sulcus (iPCS), posterior portion of inferior frontal sulcus (pIFS), intraparietal sulcus (IPS), and transverse parietal sulcus (tPS). The sPCS was defined as the dorsal segment of the precentral sulcus at the junction of the superior frontal sulcus. sPCS were further divided into dorsolateral (dl) sPCS and dorsomedial (dm) sPCS segments depending on whether it was lateral or medial to the junction. iPCS was ventral segment of precentral sulcus extended to the junction of the inferior frontal sulcus, and pIFS was defined as the portion of inferior frontal sulcus just anterior to that junction. IPS was defined as the sulcus that divides the superior and inferior parietal lobules. The tPS was defined as the descending sulcus on the medial wall of the parietal lobe posterior to the cingulate and anterior to the posterior occipital sulcus. Example ROIs are illustrated in Figure 2 projected on a subject’s inflated surface. Next, within each ROI, we selected the 20 voxels (540 mm3) with the strongest main effect of the linear combination of all the task covariates. These voxels showed some consistent deviation from baseline during the task without being biased by any task component. Using a combined structural-functional criteria to select voxels for study is similar to the way electrophysiologists first identify neurons that respond to the task and then subject those neurons to further study.
Figure 2.
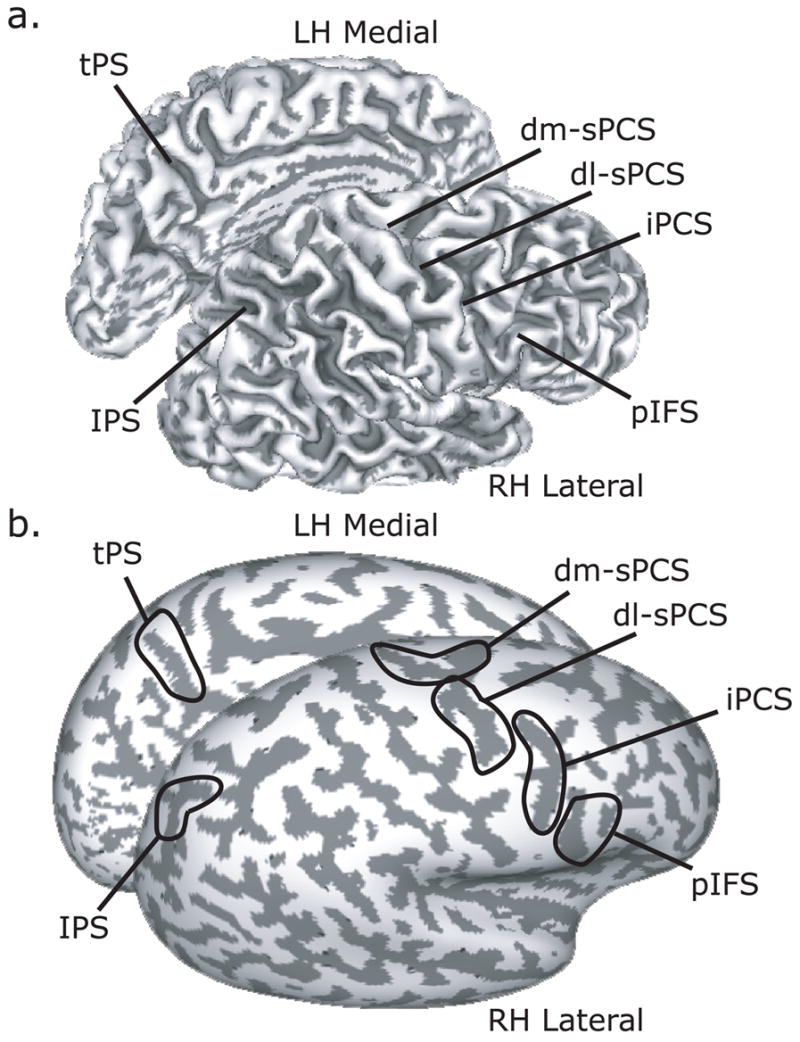
Key regions of interest (ROI) are denoted on the grey-white matter boundary of the right lateral and left medial hemisphere folded (a) and inflated (b). Dark grey overlay indicates sulci, while light grey indicates gyri. Abbreviations: dm-sPCS = dorsal medial superior precentral sulcus; dl-sPCS = dorsal lateral superior precentral sulcus; iPCS = inferior precentral sulcus; pIFS = posterior inferior frontal sulcus; IPS = intraparietal sulcus; tPS = transverse parietal sulcus.
We plotted the time series of BOLD responses, averaged across voxels within an ROI and averaged across subjects from analogous ROIs, time-locked to the presentation of the sample cue. The average signal was baselined against the average response of the last two TRs before the trial began. Since the delays varied in length, contributions to the average plot only included data from TRs up to the end of the delay so as not to contaminate the estimation with activity evoked by the motor response after the delay. The error bands were computed by taking the average of each individual’s standard error, which appropriately estimates the mean of the within-subject variance.
In order to quantitatively evaluate the time course data, we created separate cue and maintenance indices for each ROI. Activity related to encoding the sample cue’s location was defined as the average of the time points in the epoch between 1.5 and 6 seconds following the presentation of the sample cue. Activity related to maintenance was defined as the average of the time points in the delay period, specifically, between 7.5 seconds following the sample cue until the end of the delay period, which was variable. Since we found very similar patterns of activation in the left and right hemispheres for our ROIs, we combined data from left and right homologous ROIs. This procedure doubled the number of observations and increased our power to test for effects of laterality of responses with regard to the location of the sample cue in the visual field. Contralateral activation was defined as activation in the left ROIs when the sample cue fell in the right visual field, plus activation in the right ROIs when the cue fell in the left visual field. Ipsilateral activation was defined as activation in the left ROIs to left visual field cues, plus activation in the right ROI to right visual field cues. The cue and maintenance indices were plotted against each other with contralateral values on the y-axis and ipsilateral values on the x-axis and fitted with a linear function. Further, we calculated a laterality index for each subject as the contrast ratio between contralateral and ipsilateral activity [(contra−ipsi)/(contra+ipsi)]. To exclude the possibility that the index could reflect a difference of deactivations, on the rare occasion that both contra and ipsi activations were negative, we set the index to zero.
RESULTS
Behavioral data analyses
The accuracy of the memory-guided saccade, in degrees of visual angle, was used as the metric of performance in the MGS task (Figure 3a). On the MGS task, memory-guided saccades tended to land 1.2±0.3 degrees away from the sample cue, and almost always slightly short or “hypometric” of the cued location. On the SIR task, subjects correctly made the match/non-match decision 62.6±8.0 percent of the time. We tested three important assumptions with these behavioral data. 1) Performance did not worsen as the memory delay lengthens. Figure 3b and 3d show that performance did not worsen within the range of delays that we used, 7.5s – 13.5s. This justifies collapsing the different delays in our analyses of the functional neuroimaging data. 2) Performance on the MGS and SIR were equated for difficulty. In order to test this assumption, we needed to transform the MGS error, a continuous measure of error in degrees of visual angle, to a binary variable (i.e., correct/error) so that we could compare performance on MGS with SIR. To do so, for each subject we classified memory-guided saccade errors that were smaller than or equal to their mean as correct and memory-guided saccade errors that were larger as errors. Figure 3c plots the results of transforming the MGS errors into percent correct so a comparison could be made with SIR percent correct. As can be seen, no subject showed a significant difference between MGS and SIR performance (Fisher’s exact test, all p’s>0.05). Therefore, our ability to rigorously compare the neuroimaging activations during MGS and SIR was strengthened. We used a staircase that changed the difficulty of the SIR task by changing the distance between the non-matching test cue and the sample cue. Figure 3e plots the psychometric function that illustrates the relationship between SIR performance and the distance that the non-match test cue was from the sample cue. As expected, performance improved as the distance increased. It was this manipulation that allowed us to match the MGS and SIR performance on a subject-by-subject basis. 3) Performance on the MGS and SIR task taps a common cognitive resource. Testing this assumption requires that we relate a subject’s ability on the MGS task to their ability on the SIR task. We correlated each subject’s mean MGS error (in degrees of visual angle) with the separation distance between the sample cue and the non-match test cue that resulted in 50% accuracy on the SIR task. We derived the latter parameter from each subject’s psychometric functions, where we fit Weibull functions to each subject’s SIR accuracy as a function of the separation distance of the sample and non-match test cue (see Figure 3e). Subjects’ mean MGS error correlated with the distance between the sample cue and the target cue that resulted in 50% performance on the SIR task (Figure 3f). Therefore, subjects who had very accurate memory-guided saccades on the MGS task were able to make finer spatial discriminations between the sample and test locations on the SIR task. Interestingly, this indicates that the MGS and SIR task tap a common cognitive resource, or perceptual ability, that may depend on a similar neural mechanism.
Surface-based statistical tests
Group statistical maps of activity specific to the encoding and maintenance of the sample cue’s location are shown in Figure 4a and 4b. Encoding the sample cue’s location resulted in a pattern of activity in the frontal and parietal cortex that was indistinguishable between the MGS and SIR task. Multiple foci of robust activation can be seen along the dorsal precentral sulcus and along the intraparietal sulcus and supramarginal gyrus. Other significant activations can be seen on the medial wall in the transverse parietal sulcus and superior frontal gyrus. No significant differences were found when we contrasted MGS and SIR at the sample cue epoch, even when the threshold was lowered to an uncorrected value (p<0.05).
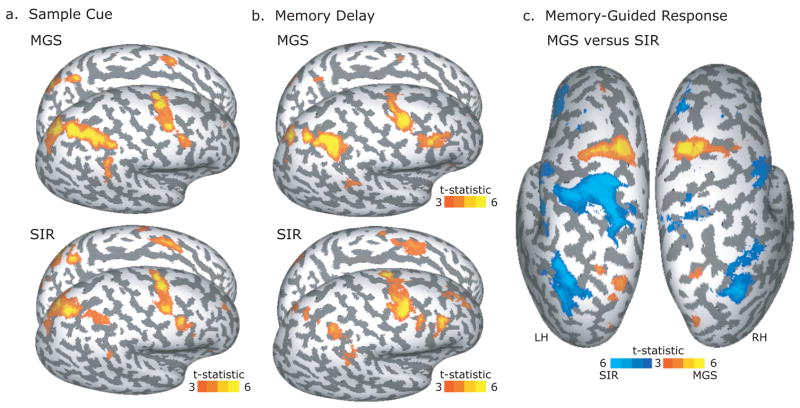
Figure 4.
Sample cue, memory delay and memory-guided response related activity. a. Encoding the position of the sample cue evoked a similar pattern of BOLD activity during the MGS and SIR tasks. b. Similarly, maintaining the sample cue’s position evoked a remarkable similar pattern of BOLD activity during the MGS and SIR tasks. After the delay period, memory for the sample cue’s location was tested by having subjects make an eye movement in the MGS task or a manual button response with the right hand in the SIR task. Panel c. shows the direct MGS vs SIR contrast at the response epoch rendered on the left and right dorsal cortical hemispheres.
Compared to the cue epoch, a similar pattern of frontal and parietal cortical activation was continued into and throughout the memory delay (Figure 4b). We see multiple foci of activation in the dorsal precentral sulcus and the intraparietal sulcus, as well as the supramarginal gyrus. Additionally, the posterior portion of the inferior frontal sulcus becomes significantly active during the delay period. Although there appears to be more activity during the MGS compared to SIR memory delay in the posterior parietal cortex, no significant differences were found in the direct contrast in any brain areas, even when the threshold was lowered. When we combined the data from the MGS and SIR task, we saw an additional focus of activity in the right middle frontal gyrus on the gyral surface above the intermediate frontal sulcus in the right hemisphere only (Supplementary Figure S1).
Large BOLD activations were evoked time-locked to the generation of the memory-guided responses in each of the tasks (Supplementary Figure S2). A direct comparison between MGS and SIR at the response epoch yielded several significant differences shown in Figure 4c. Compared to the response in the SIR task, memory-guided saccades in the MGS task evoked significantly greater activity in the dorsal precentral sulcus and dorsal transverse parietal sulcus bilaterally. Compared to the response in the MGS task, making the match/nonmatch decision and corresponding button press with the index and middle fingers on the right hand in the SIR task evoked significantly greater activity in the contralateral (left) central sulcus and postcentral gyrus. This contrast also revealed greater activity in the ventral central sulci bilaterally and the lateral transverse occipital sulci bilaterally (Figure 4c). Too many factors differ between the tasks at the response epoch to make any strong interpretations of the results, but at the very least the contrast indicates that we have enough statistical power to measure differences between the two tasks, in this case the type of memory-guided movement. Clearly, we can see that generating a memory-guided saccade evokes more activity in the dorsal precentral sulcus and making a button press generates more activity in the contralateral motor cortex.
Region of interest time series analyses
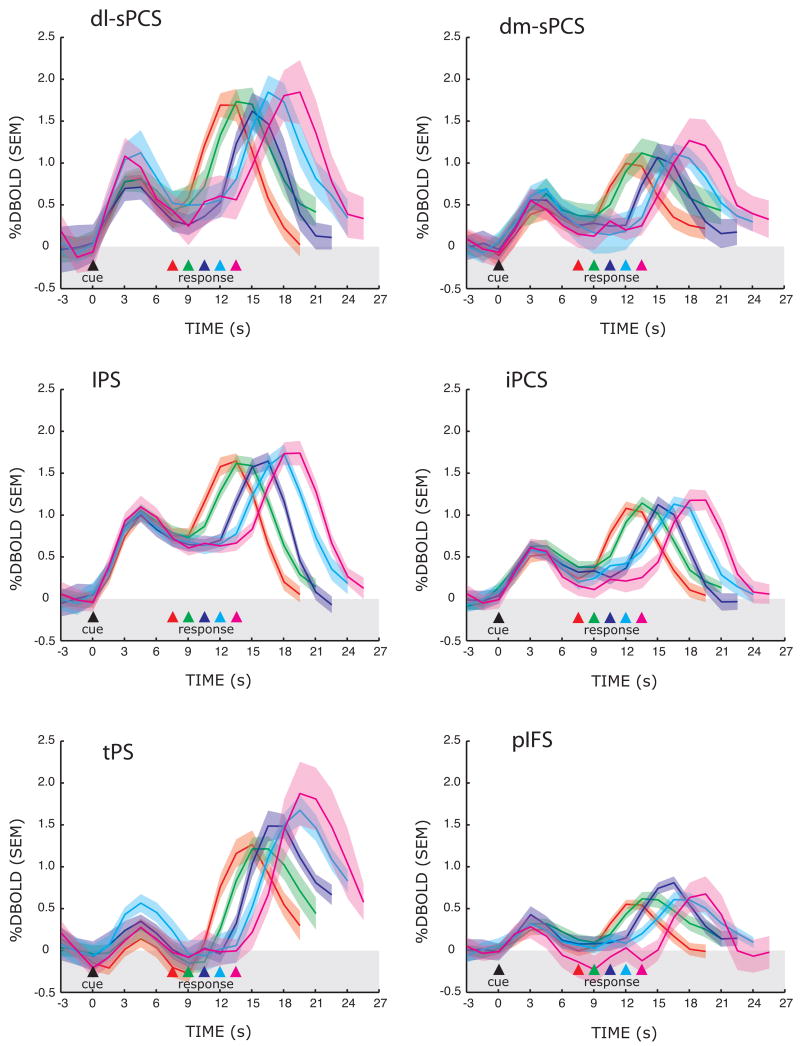
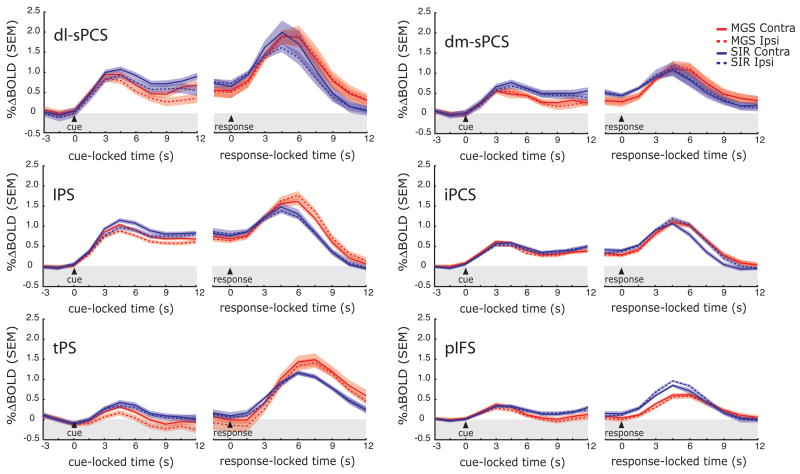
Next, we plotted the time series of BOLD responses in the various ROIs (see Figure 4) to test several hypotheses. First, a region that plays a critical role in working memory maintenance should show activity above baseline throughout the entire memory delay irrespective of the length of the delay. For each ROI, we plotted the BOLD time course data for each of the five different delay lengths of the MGS task, time-locked to the presentation of the sample cue (Figure 5). Initially, a transient response can be seen time-locked to the presentation of the sample cue. Then, one can clearly see that several ROIs show persistent activity above baseline that spans the entire delay, even when the subjects had maintained the cue’s position for nearly 14 seconds. We found strong persistent delay period activity in the dorsolateral segment of the superior precentral sulcus (dl-sPCS) and in the intraparietal sulcus (IPS) and modest delay period activity in the dorsomedial segment of the superior precentral sulcus (dm-sPCS) and the inferior precentral sulcus (iPCS). Other regions, like the transverse parietal sulcus (tPS) and posterior inferior frontal sulcus (pIFS) did not sustain throughout the delay period. Identical results were found during the SIR task (Supplementary Figure S3). Finally, one can see another later transient response time-locked to the response that is staggered in the order of the delay length. Notice that in the precentral suclus ROIs, the late phase of the delay period activity begins to increase or ramp up well before the end of the memory delay.
Figure 5.
Trial-averaged BOLD time courses aligned on the presentation of the sample cue during the MGS task. Only data from the hemisphere contralateral to the visual field within which the sample cue was presented are included. Separate lines represent the different delay lengths, where the time of the memory-guided saccade is indicated by the colored triangle. The peaks of the responses are staggered according to the memory delay length. Importantly, several regions of interest show evidence of sustained activity regardless of the length of the delay, even at very long delays. See Figure 2 for a list of the abbreviated regions.
Second, we tested the hypothesis that the time course of activation, especially during the memory delay differs between the MGS and SIR tasks. We collapsed the time course data across the different delay length by time-locking the data to the sample cue and averaging trials up to the end of the delay. We also plotted the data time-locked to the response cue. Remarkably, the BOLD time courses looked very similar during the MGS and SIR tasks. Following the initial response time-locked to the sample cue, we observed robust evidence of persistent activation throughout the delay period in the superior and inferior PCS and the IPS. But again, we did not observe significant differences between the MGS and SIR tasks during the delay period.
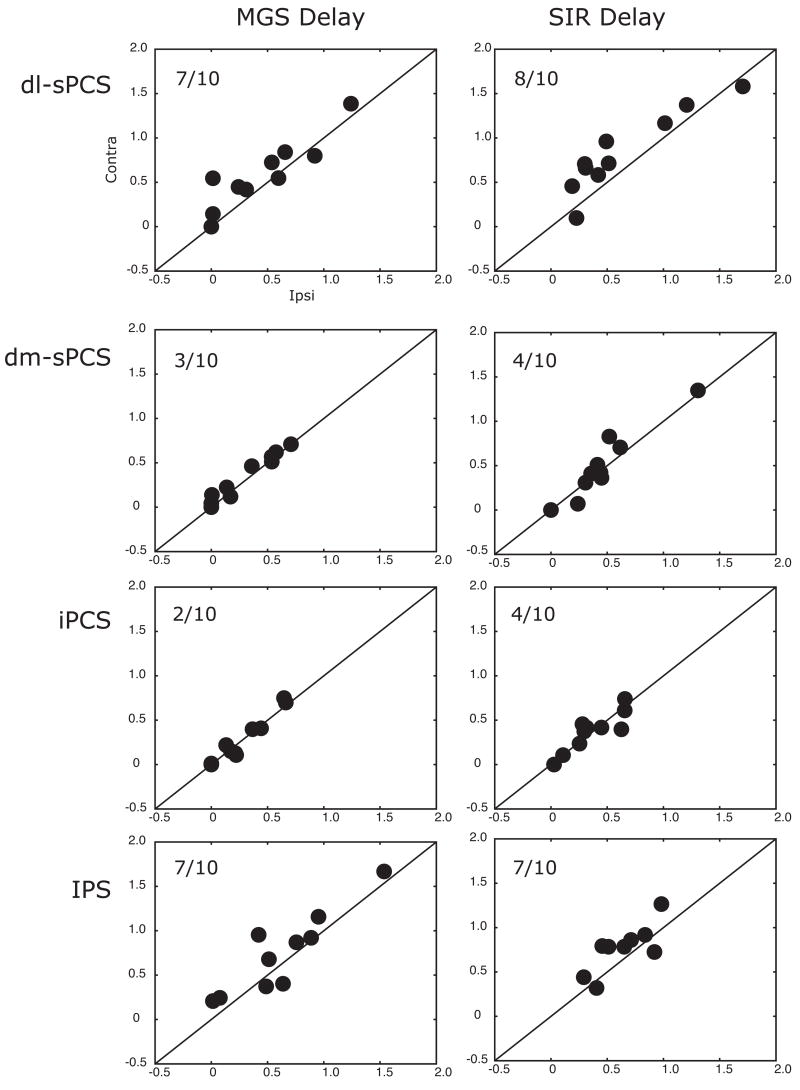
Third, we tested the hypothesis that ROIs would show a bias for the maintenance of spatial positions presented in the contralateral compared to ipsilateral visual field. As can be seen in Figure 6, the dl-sPCS, and IPS seem to have a slightly larger response during the delay for contralateral (solid lines) compared to ipsilateral (dashed lines) sample cues during both the MGS and SIR tasks. To address this carefully, for each subject we created laterality bias indices (see Methods) that quantified the degree to which activity showed a differential response during the delay period and we tested the significance of these with one-tailed t-tests. We plotted these indices in Figure 7 for each of the ROIs that showed some evidence of delay period activity. In the dl-sPCS 8/10 subjects and in the IPS 7/10 subjects showed a bias for contralateral cues, where delay period activity was greater in the hemisphere that was contralateral to the visual field within which the sample cue was presented, dl-sPCS t[9]=1.93, p<0.05; IPS t[9]=2.97, p<0.01. No other ROI showed a significant laterality index. Generally, the contralateral, compared to ipslateral, hemisphere not only shows a greater magnitude but a larger area of activity (Supplementary Figure S4).
Figure 6.
Trial-averaged BOLD time courses time-locked to the presentation of the sample cue and the generation of the memory-guided response for the MGS and SIR tasks. Data from the hemisphere contralateral and ipsilateral to the visual field within which the sample cue was presented are plotted separately. See Figure 2 for abbreviated regions.
Figure 7.
Indices of laterality bias during the delay periods of the MGS (left column) and SIR (right column) tasks. Each dot represents a subject’s laterality index (see methods for computation), where dots above the line of equality denote subjects whose delay period activity was greater when maintaining spatial positions in the contralateral compared to ipsilateral visual field. Only the dl-sPCS and IPS showed a contra-lateral bias. The ratio in the upper left corner is the fraction of subjects who showed a contralateral bias. See Figure 2 for a list of the abbreviated regions.
DISCUSSION
We measured cortical activity in humans performing spatial working memory tasks with the aim of unambiguously identifying areas that show persistent neural activity during the retention interval. Consistent with past studies, we found several frontal and parietal cortical areas that showed robust delay period activity. To better understand the nature of persistent neural activity, we compared two spatial working memory tasks that differed in whether or not the memory-guided response could be prepared and thus maintained during the memory delay. We tested the idea that the specific type of information that was being maintained would affect persistent activity. In the memory-guided saccade (MGS) task, after a retention interval, subjects made saccades to the remembered location of the visual cue briefly presented before the delay. In the spatial item recognition (SIR) task, after an identical cue and retention interval, subjects indicated with a button press whether the location of a second target cue was different than that of the first cue. During the MGS task, subjects could simply remember the location of the cue in terms of the forthcoming saccade (i.e., maintain a prospective motor code). While during the SIR task, no saccades were ever made and such a prospective strategy would not be efficient. First, we demonstrated that neural activity persisted the entire long and variable delay period in various foci in the precentral, inferior frontal, and intraparietal sulcus. Second and much to our surprise, delay period activity did not differ between the MGS and SIR task, even in areas that are believed to contain saccade related neurons. Third, neural activity in the intraparietal sulcus and the dorsolateral precentral sulcus was greater in the hemisphere contralateral to the visual field of the remembered cue.
Persistent activity in the human frontal and parietal cortex
Persistent neural activity that spans memory delays may reflect an internal representation of information that is no longer in the current environment, but is needed to perform a future desired action. If this is indeed true, then the activity should persist during the entire length of a delay period no matter how long it must be maintained. To test this hypothesis we used relatively long and variable length delay periods, which also helped decorrelate the delay and cue events as well as keep the subject in a ready state.
Based on the surface-based statistical maps (Figure 4b), we found robust delay period activity in dorsal and ventral lateral precentral sulcus and the intraparietal sulcus, bilaterally. The spatial pattern of activity resembles to a remarkable degree the pattern of activity seen time-locked to the presentation of the visual sample cue (Figure 4a). Therefore, the regions that initially respond during the visual encoding of the sample’s location are the same regions that showed persistent delay period activity. In fact, the majority of neurons in the monkey frontal cortex that have an increased rate of firing during the delay period of a MGS task show a marked increase as soon as the visual cue is presented (Takeda & Funahashi, 2007). This early transient response might signify the representation of the external visual stimulus, the very initial stages of maintaining the internal representation of the extinguished stimulus’ location, and/or the shift in covert attention or the selection of the stimulus’ location.
Critically, we confirmed the validity of the surface based statistical maps of delay period activity by plotting the trial-averaged time-series data. If valid, then the signals should persist as long as working memory was maintained. As can be seen in Figures 5 and 6, BOLD activity persisted above baseline levels for the entire duration of the memory delay, until the memory-guided response was made. We detected particularly robust delay period activity in the dorsal precentral sulcus and the intraparietal sulcus, bilaterally. Activity in these regions persisted throughout delay periods as long as 14 seconds and therefore, cannot be explained as merely sluggish BOLD responses to a visual sample cue bleeding into the delay period. In fact, in the dorsal precentral sulcus, one can see in the time courses that activity begins to increase, not decrease, towards the end of the delay (Figure 5).
The focus of activity in dorsal precentral sulcus was very near the junction with the superior frontal sulculs, which is thought to house the human homologue of the monkey FEF (Amiez et al., 2006; Blanke et al., 2000; Luna et al., 1998; Ro et al., 2002; Rosano et al., 2002). Indeed, it was in this area that showed greater activity evoked by memory-guided saccades than button presses at the response interval (Figure 3c). Therefore, we believe this area is the human FEF. Electrophysiological studies of neurons in monkey FEF have repeatedly shown persistent neural firing during spatial working memory delays (Funahashi et al., 1989; Goldberg & Bruce, 1985; Sommer & Wurtz, 2000). Similarly, the robust activations we observed during the delay period in the intraparietal sulcus may include the human homologue of monkey area LIP, whose neurons also show a pattern of persistent neural firing and enhanced local field potentials during spatial working memory delays (Barash et al., 1991; Bisley & Goldberg, 2003; Gnadt & Andersen, 1988; Gnadt & Beyer, 1998; Pesaran et al., 2002; Snyder et al., 1997).
Although the current data clearly demonstrate persistent human neural activity during working memory delay periods, other past studies, including studies from our lab, have reported delay period activity during MGS tasks (Brown et al., 2004; Connolly et al., 2002; Curtis & D'Esposito, 2006; Curtis et al., 2004; Medendorp et al., 2005, 2006; Schluppeck et al., 2006). Brown et al., (2004) only used four positions (i.e., upper/lower right/left) repetitively during the experiment, making it likely that subjects began to learn and even verbally label the positions, changing the study from one of spatial to verbal working memory. Connolly et al., (2002) used delay periods that were too short to reliably separate the delay period from the bounding cue and response periods. Curtis et al., (2004) used long enough delay periods, but they were fixed in duration at 10s, which makes the delay period covariate completely predictable from the cue. Since the delay always followed the cue for the exact same amount of time, estimation of the two parameters from a general linear model can be unstable (e.g., the signal could be fit equally well by loading on the delay covariate or the surrounding cue/response covariates). Finally, both Medendorp et al., (2005, 2006) and Schluppeck et al., (2006) only focused on a priori regions of interest in the intraparietal sulcus that contained a lateralized (Medendorp et al., 2005, 2006) or retinotopic (Schluppeck et al., 2006) representation of the contralateral visual field. Nonetheless, focal portions of the precentral sulcus (putative FEF) and the intraparietal sulcus (putative LIP) show the greatest consistency across human fMRI studies of delay period activity during MGS tasks (Curtis, 2006).
Contralateral bias
In the frontal and parietal cortex, neurons represent space in what appears to be eye-centered coordinates. The receptive fields or response fields of neurons in the monkey FEF and LIP are large in size; they are not sharply tuned like neurons in early visual cortical areas. However, they do tend to represent contralateral space (Ben Hamed et al., 2001; Bruce & Goldberg, 1985; Schall, 1991). The human precentral sulcus and intraparietal sulcus also appears to be similarly organized. Performing memory guided saccades on successive trials in which the target’s position systematically progresses in angle, like hands on a clock, evokes a traveling wave of activity in portions of the human intraparietal sulcus (Schluppeck et al., 2005; Sereno et al., 2001). The waves travel at the task’s frequency and can be seen in the hemisphere contralateral to the visual field within which contain the targets. Similar results have been recently reported in the human dorsal and ventral precentral sulcus (Hagler et al., 2007; Hagler & Sereno, 2006; Kastner et al., 2007). Since these phase-encoding experiments could not specify which portion of the task is driving the responses (e.g., visual cues, memory delays, or motor responses), the current results nicely compliment these data by providing compelling evidence that persistent activity during the memory delay period is topographically biased towards the contralateral visual field.
Event-related fMRI studies have not generally reported lateralized activity during spatial working memory tasks, but see (Curtis & D'Esposito, 2006; Medendorp et al., 2006; Schluppeck et al., 2006). Comparing the time courses of BOLD activity from the dorsal precentral and intraparietal sulci in the hemispheres that were contralateral and ipsilateral to the position of the sample cues, one can see that the differences are small. Delay activity is roughly about 10% of the overall evoked response greater in the contralateral compared to ipsilateral activity. The lateralized difference is small and rides on top of the large task evoked activity. Therefore, one needs a lot of power to detect such a difference and this may account for why most studies have not reported a lateralized effect. Nevertheless, here we find clear evidence of contralateralized delay period activity in the frontal cortex (i.e., dorsolateral precentral sulcus) and parietal cortex (i.e., mid intraparietal sulcus). Therefore, these areas are strong candidates for the human homologues of the monkey FEF and LIP.
Nature of persistent delay period activity
One aim of our recent work, including this study, was to better understand the nature of the information encoded within the persistent neural activity during spatial working memory delays in humans (Curtis & D'Esposito, 2006; Curtis et al., 2004; Curtis, Sun et al., 2005). Mainly, we have focused on testing the hypothesis that persistent activity arises from a sustained representation of the memory cue’s location, a retrospective memory code, versus the alternative hypothesis that activity arises from a sustained representation of the planned contingent motor response, a prospective memory code. In the current study, we did not find compelling evidence that could distinguish between these two hypotheses. Although we found robust evidence of persistent activity during the spatial working memory delay period, we did not find any areas whose level of activity was different in the MGS versus SIR tasks. We had hypothesized that the FEF would show greater activity during the MGS delay than during the SIR delay because precise metrics for the memory-guided saccade were known throughout the entire delay period. Many studies have reported robust persistent activity of FEF neurons during MGS delay periods (Funahashi et al., 1989; Goldberg & Bruce, 1985; Snyder et al., 1997; Sommer & Wurtz, 2000). This activity is spatially selective in that it is greater during trials in which the position of the remembered stimulus matches the neuron’s response field. Therefore, the persistent activity in the human FEF could reflect the activity of neurons that are responsible for moving the eyes to the remembered location after the delay.
In a past study, we compared the delay period activity during a MGS task with a non-match-to-sample MGS task (Curtis et al., 2004). The MGS task was essentially identical to the current MGS task. In the non-matching MGS task, subjects made saccades to one of two targets presented after the delay period, in this case the target that did not match the position of the sample cue. Importantly, only in the MGS, not non-matching MGS, task was the metrics of the memory-guided saccade known through the delay. Although we found robust delay period activity in the FEF during both the MGS and the non-matching MGS it was greater in the MGS delay, supporting the hypothesis that the activity is an index of prospective motor plans. In the current study we found no such differences between MGS and SIR delay period activity. We think that the main reason for the difference had to do with matching the two tasks for difficulty. The precision with which the sample cue had to be represented was greater in the MGS than non-matching MGS task in the Curtis et al., (2004) study. In the current study, this potential confound was controlled and under these conditions we do not see any differences evoked by knowing the metrics of the memory-guided saccade during the delay.
These results are consistent with two theories. One is an extension of the premotor theory of attention (Rizzolatti et al., 1987), which states that covert spatial attention is simply the corollary of a planned eye movement. Visual perception is enhanced at the locus of a saccade goal and forcing subjects to attend one location but plan a saccade to another impairs either visual or saccade performance (Deubel & Schneider, 1996; Hoffman & Subramaniam, 1995; Kowler et al., 1995; Rizzolatti et al., 1987; Sheliga et al., 1994; Van der Stigchel et al., 2007). Moreover, neuroimaging studies of spatial attention (Corbetta et al., 1998; Corbetta & Shulman, 2002; Ikkai & Curtis, in press; Serences & Yantis, 2006) and saccade planning activate similar frontal and parietal areas (Connolly et al., 2002; Curtis, Cole et al., 2005; Curtis & D'Esposito, 2003b, 2006). Therefore, clear evidence exists linking spatial attention and saccade planning. We can extend this idea to the case of spatial working memory by stating that the maintenance of a location is simply the corollary of a planned eye movement to that location. Indeed, the trajectory of saccades are influenced by the location of a memorized cue; they curve away from its location (Theeuwes et al., 2005). Saccades made during the retention interval of a spatial working memory task can both disrupt performance and cause increased BOLD signal in the putative human FEF (Postle & Hamidi, in press). With regard to the current study’s findings, the mechanism by which subjects are maintaining the sample cue’s location could be a prospective saccade plan, but in the SIR task, this saccade plan is simply cancelled after the delay.
Another intriguing possibility is that the human FEF does not code for eye movements per se, but instead its activity forms a map of space that represents the most salient or prioritized locations in the visual field (Serences & Yantis, 2006; Thompson et al., 2005). This map could be read out by downstream areas like the superior colliculus and brain stem saccade generator, as mechanism for saccade selection. Additionally, it could be read-out by posterior visual areas as a mechanism by which competition for neural representation is biased (Desimone & Duncan, 1995; Moore et al., 2003). If for parsimony alone, we favor the latter hypothesis, that activity in the superior precentral sulcus represents a map of prioritized space. If activity in the FEF does not predict whether a saccade will be made, as in the case of the SIR task, and activity in downstream areas does, then we must reconsider the notion that the FEF is an “eye field” or reconsider our definitions of what constitutes an “eye field.” Clearly, more work is needed to distinguish between these two models.
Prefrontal cortex and spatial working memory
We did not find any delay period activity in the superior frontal sulcus, a region that has been argued to be specialized for spatial working memory and may represent the human functional homologue of the monkey principal sulcus (area 46, 9/46) (Courtney et al., 1998). Funahashi and colleagues have demonstrated that the posterior third of the principal sulcus is necessary for accurate MGS performance (Funahashi et al., 1993) and neurons in the principal sulcus code for the locations of memorized cues (Funahashi et al., 1989; Takeda & Funahashi, 2002, 2004). Interestingly, none of the human fMRI studies that used a MGS task to tap spatial working memory processes have reliably activated the superior frontal sulcus. Therefore, the superior frontal sulcus is neither the functional homologue of the principle sulcus nor does it seem to be specialized for spatial working memory. Humans do not have a principal sulcus, but evidence suggests that the mid-lateral middle frontal gyrus in and near the intermediate frontal sulcus is the most likely human homologue of the principal sulcus, areas 46 and 9/46 (Petrides & Pandya, 1999; Rajkowska & Goldman-Rakic, 1995). Studies that have activated portions of the dorsal frontal cortex anterior to the precentral sulcus have required subjects to remember more than one spatial position (Courtney et al., 1998; Leung et al., 2002; Rowe et al., 2000; Sala et al., 2003). Therefore, two possibilities exist that might explain the inconsistency. One, it may be that by increasing the memory load, activation then emerges in the superior frontal sulcus. This does not seem to be the case as Leung and colleagues (2004) did not find that activity in the superior frontal sulcus when the spatial memory load increased from one to four positions (Leung et al., 2004). Two, whenever one must store more than one position in memory, an additional cognitive process may be evoked. For instance, instead of remembering the position of a cue, one needs to remember the configuration of the cues, where the position of each cue, in relation to the other cues, can be used as a referent. This could be an efficient strategy to help compress or “chunk” the data as the load increases toward capacity (Bor et al., 2003). Moreover, during a MGS task activity in superior frontal sulcus did not sustain during the delay period, but did increase when the delay was extended (Geier et al., 2007), again suggesting that the prefrontal cortex may not play an important role in maintenance per se.
In the current study, we did find delay period activation in the right mid-dorsal lateral prefrontal cortex (dlPFC). The dlPFC plays an important role in spatial working memory (for reviews see (Curtis & D'Esposito, 2003a, 2004). Lesions to the human and monkey dlPFC and FEF cause decreased accuracy of memory-guided saccades (Dias & Segraves, 1999; Funahashi et al., 1993; Gaymard et al., 1999; Ploner et al., 1999; Rivaud et al., 1994a; Sawaguchi & Goldman-Rakic, 1991; Sommer & Tehovnik, 1997). Analysis of the types of errors following cortical damage suggest that the FEF tend to cause systematic memory-guided errors that are hypometric (i.e., saccades fall short of cued location), while dlPFC damage can result in memory-guided saccades that are variably scattered around the cues (Dias & Segraves, 1999; Funahashi et al., 1993; Ploner et al., 1999). It may be that the FEF and dlPFC make distinct contributions to the maintenance of positional information, presumably related to a caudal-rostral functional gradient in the dlPFC to FEF, where neurons may represent progressively greater degrees of visuospatial to visuomotor information (di Pellegrino & Wise, 1993). If true, systematic errors may result from errors in translating spatial information into a motor plan, a putative role of the FEF. Variable errors may result from difficulties selecting or maintaining the exact spatial position of cued locations, a putative role of the dlPFC.
Summary
The mechanism for the short-term maintenance of information involves persistent neural activity during a retention interval, which is thought to provide a bridge in time between the cued memoranda and its later contingent response. Both in monkeys and humans, we observe persistent activity in several cortical and subcortical areas suggesting that maintenance is accomplished via a distributed network of brain areas. To understand the mechanisms of maintenance, however, we are faced with the daunting task of revealing what information is represented by persistent activity across the brain. The data from the current study suggests that persistent FEF activity might represent a prioritized map of space, rather than the metrics for saccades.
Supplementary Material
Acknowledgments
We thank Marcus Lauer, Souheil Inati, and Keith Sanzenbach for technical support. Funded by the Seaver Foundation & NIH R01 EY016407.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amiez C, Kostopoulos P, Champod AS, Petrides M. Local morphology predicts functional organization of the dorsal premotor region in the human brain. J Neurosci. 2006;26:2724–2731. doi: 10.1523/JNEUROSCI.4739-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barash S, Bracewell RM, Fogassi L, Gnadt JW, Andersen RA. Saccade-related activity in the lateral intraparietal area. II. Spatial properties. J Neurophysiol. 1991;66:1109–1124. doi: 10.1152/jn.1991.66.3.1109. [DOI] [PubMed] [Google Scholar]
- Ben Hamed S, Duhamel JR, Bremmer F, Graf W. Representation of the visual field in the lateral intraparietal area of macaque monkeys: a quantitative receptive field analysis. Exp Brain Res. 2001;140:127–144. doi: 10.1007/s002210100785. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Neuronal activity in the lateral intraparietal area and spatial attention. Science. 2003;299:81–86. doi: 10.1126/science.1077395. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Hyde JS. Contour-based registration technique to differentiate between task-activated and head motion-induced signal variations in fMRI. Magn Reson Med. 1997;38:470–476. doi: 10.1002/mrm.1910380315. [DOI] [PubMed] [Google Scholar]
- Blanke O, Spinelli L, Thut G, Michel CM, Perrig S, Landis T, et al. Location of the human frontal eye field as defined by electrical cortical stimulation: anatomical, functional and electrophysiological characteristics. Neuroreport. 2000;11:1907–1913. doi: 10.1097/00001756-200006260-00021. [DOI] [PubMed] [Google Scholar]
- Bor D, Duncan J, Wiseman RJ, Owen AM. Encoding strategies dissociate prefrontal activity from working memory demand. Neuron. 2003;37:361–367. doi: 10.1016/s0896-6273(02)01171-6. [DOI] [PubMed] [Google Scholar]
- Brown MR, DeSouza JF, Goltz HC, Ford K, Menon RS, Goodale MA, et al. Comparison of memory- and visually guided saccades using event-related fMRI. J Neurophysiol. 2004;91:873–889. doi: 10.1152/jn.00382.2003. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME. Primate frontal eye fields. I. Single neurons discharging before saccades. J Neurophysiol. 1985;53:603–635. doi: 10.1152/jn.1985.53.3.603. [DOI] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS. Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. J Neurophysiol. 1998;79:2919–2940. doi: 10.1152/jn.1998.79.6.2919. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, Menon RS, Munoz DP. Human fMRI evidence for the neural correlates of preparatory set. Nat Neurosci. 2002;5:1345–1352. doi: 10.1038/nn969. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, et al. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Petit L, Maisog JM, Ungerleider LG, Haxby JV. An area specialized for spatial working memory in human frontal cortex. Science. 1998;279:1347–1351. doi: 10.1126/science.279.5355.1347. [DOI] [PubMed] [Google Scholar]
- Curtis CE. Prefrontal and parietal contributions to spatial working memory. Neuroscience. 2006;139:173–180. doi: 10.1016/j.neuroscience.2005.04.070. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Cole MW, Rao VY, D'Esposito M. Canceling planned action: an FMRI study of countermanding saccades. Cereb Cortex. 2005;15:1281–1289. doi: 10.1093/cercor/bhi011. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci. 2003a;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M. Success and failure suppressing reflexive behavior. J Cogn Neurosci. 2003b;15:409–418. doi: 10.1162/089892903321593126. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M. The effects of prefrontal lesions on working memory performance and theory. Cogn Affect Behav Neurosci. 2004;4:528–539. doi: 10.3758/cabn.4.4.528. Srimal & Curtis - 34. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M. Selection and maintenance of saccade goals in the human frontal eye fields. J Neurophysiol. 2006;95:3923–3927. doi: 10.1152/jn.01120.2005. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Rao VY, D'Esposito M. Maintenance of spatial and motor codes during oculomotor delayed response tasks. J Neurosci. 2004;24:3944–3952. doi: 10.1523/JNEUROSCI.5640-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CE, Sun FT, Miller LM, D'Esposito M. Coherence between fMRI time-series distinguishes two spatial working memory networks. Neuroimage. 2005;26:177–183. doi: 10.1016/j.neuroimage.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Deubel H, Schneider WX. Saccade target selection and object recognition: evidence for a common attentional mechanism. Vision Res. 1996;36:1827–1837. doi: 10.1016/0042-6989(95)00294-4. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G, Wise SP. Visuospatial versus visuomotor activity in the premotor and prefrontal cortex of a primate. J Neurosci. 1993;13:1227–1243. doi: 10.1523/JNEUROSCI.13-03-01227.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias EC, Segraves MA. Muscimol-induced inactivation of monkey frontal eye field: effects on visually and memory-guided saccades. J Neurophysiol. 1999;81:2191–2214. doi: 10.1152/jn.1999.81.5.2191. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Dorsolateral prefrontal lesions and oculomotor delayed-response performance: evidence for mnemonic "scotomas". J Neurosci. 1993;13:1479–1497. doi: 10.1523/JNEUROSCI.13-04-01479.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;173:652–654. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- Gaymard B, Ploner CJ, Rivaud-Pechoux S, Pierrot-Deseilligny C. The frontal eye field is involved in spatial short-term memory but not in reflexive saccade inhibition. Experimental Brain Research. 1999;129:288–301. doi: 10.1007/s002210050899. [DOI] [PubMed] [Google Scholar]
- Geier CF, Garver KE, Luna B. Circuitry underlying temporally extended spatial working memory. Neuroimage. 2007;35:904–915. doi: 10.1016/j.neuroimage.2006.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnadt JW, Andersen RA. Memory related motor planning activity in posterior parietal cortex of macaque. Exp Brain Res. 1988;70:216–220. doi: 10.1007/BF00271862. [DOI] [PubMed] [Google Scholar]
- Gnadt JW, Beyer J. Eye movements in depth: What does the monkey's parietal cortex tell the superior colliculus? Neuroreport. 1998;9:233–238. doi: 10.1097/00001756-199801260-00011. [DOI] [PubMed] [Google Scholar]
- Goldberg ME, Bruce CJ. Cerebral cortical activity associated with the orientation of visual attention in the rhesus monkey. Vision Res. 1985;25:471–481. doi: 10.1016/0042-6989(85)90072-0. [DOI] [PubMed] [Google Scholar]
- Hagler DJ, Jr, Riecke L, Sereno MI. Parietal and superior frontal visuospatial maps activated by pointing and saccades. Neuroimage. 2007 doi: 10.1016/j.neuroimage.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ, Jr, Sereno MI. Spatial maps in frontal and prefrontal cortex. Neuroimage. 2006;29:567–577. doi: 10.1016/j.neuroimage.2005.08.058. [DOI] [PubMed] [Google Scholar]
- Hoffman JE, Subramaniam B. The role of visual attention in saccadic eye movements. Percept Psychophys. 1995;57:787–795. doi: 10.3758/bf03206794. [DOI] [PubMed] [Google Scholar]
- Holmes AP, Blair RC, Watson JD, Ford I. Nonparametric analysis of statistic images from functional mapping experiments. J Cereb Blood Flow Metab. 1996;16:7–22. doi: 10.1097/00004647-199601000-00002. [DOI] [PubMed] [Google Scholar]
- Ikkai A, Curtis CE. Cortical activity time-locked to the shift and maintenance of spatial attention. Cereb Cortex. doi: 10.1093/cercor/bhm171. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, Desimone K, Konen CS, Szczepanski SM, Weiner KS, Schneider KA. Topographic Maps in Human Frontal Cortex Revealed in Memory-Guided Saccade and Spatial Working Memory Tasks. J Neurophysiol. 2007 doi: 10.1152/jn.00010.2007. [DOI] [PubMed] [Google Scholar]
- Kowler E, Anderson E, Dosher B, Blaser E. The role of attention in the programming of saccades. Vision Res. 1995;35:1897–1916. doi: 10.1016/0042-6989(94)00279-u. [DOI] [PubMed] [Google Scholar]
- Kubota K, Niki H. Prefrontal cortical unit activity and delayed alternation performance in monkeys. J Neurophysiol. 1971;34:337–347. doi: 10.1152/jn.1971.34.3.337. [DOI] [PubMed] [Google Scholar]
- Leung HC, Gore JC, Goldman-Rakic PS. Sustained mnemonic response in the human middle frontal gyrus during on-line storage of spatial memoranda. J Cogn Neurosci. 2002;14:659–671. doi: 10.1162/08989290260045882. [DOI] [PubMed] [Google Scholar]
- Leung HC, Seelig D, Gore JC. The effect of memory load on cortical activity in the spatial working memory circuit. Cogn Affect Behav Neurosci. 2004;4:553–563. doi: 10.3758/cabn.4.4.553. [DOI] [PubMed] [Google Scholar]
- Li CS, Mazzoni P, Andersen RA. Effect of reversible inactivation of macaque lateral intraparietal area on visual and memory saccades. J Neurophysiol. 1999;81:1827–1838. doi: 10.1152/jn.1999.81.4.1827. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Strojwas MH, McCurtain BJ, Berman RA, Genovese CR, et al. Dorsal cortical regions subserving visually guided saccades in humans: an fMRI study. Cereb Cortex. 1998;8:40–47. doi: 10.1093/cercor/8.1.40. [DOI] [PubMed] [Google Scholar]
- Lynch JC. Saccade initiation and latency deficits after combined lesions of the frontal and posterior eye fields in monkeys. J Neurophysiol. 1992;68:1913–1916. doi: 10.1152/jn.1992.68.5.1913. [DOI] [PubMed] [Google Scholar]
- Machens CK, Romo R, Brody CD. Flexible control of mutual inhibition: a neural model of two-interval discrimination. Science. 2005;307:1121–1124. doi: 10.1126/science.1104171. [DOI] [PubMed] [Google Scholar]
- Medendorp WP, Goltz HC, Vilis T. Remapping the remembered target location for anti-saccades in human posterior parietal cortex. J Neurophysiol. 2005;94:734–740. doi: 10.1152/jn.01331.2004. [DOI] [PubMed] [Google Scholar]
- Medendorp WP, Goltz HC, Vilis T. Directional selectivity of BOLD activity in human posterior parietal cortex for memory-guided double-step saccades. J Neurophysiol. 2006;95:1645–1655. doi: 10.1152/jn.00905.2005. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM, Fallah M. Visuomotor origins of covert spatial attention. Neuron. 2003;40:671–683. doi: 10.1016/s0896-6273(03)00716-5. [DOI] [PubMed] [Google Scholar]
- Muri RM, Vermersch AI, Rivaud S, Gaymard B, Pierrot-Deseilligny C. Effects of single-pulse transcranial magnetic stimulation over the prefrontal and posterior parietal cortices during memory-guided saccades in humans. J Neurophysiol. 1996;76:2102–2106. doi: 10.1152/jn.1996.76.3.2102. [DOI] [PubMed] [Google Scholar]
- Nichols TEaHAP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Human Brain Mapping. 2002;15:1–15. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesaran B, Pezaris JS, Sahani M, Mitra PP, Andersen RA. Temporal structure in neuronal activity during working memory in macaque parietal cortex. Nat Neurosci. 2002;5:805–811. doi: 10.1038/nn890. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci. 1999;11:1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- Ploner CJ, Rivaud-Pechoux S, Gaymard BM, Agid Y, Pierrot-Deseilligny C. Errors of memory-guided saccades in humans with lesions of the frontal eye field and the dorsolateral prefrontal cortex. J Neurophysiol. 1999;82:1086–1090. doi: 10.1152/jn.1999.82.2.1086. [DOI] [PubMed] [Google Scholar]
- Polonsky A, Blake R, Braun J, Heeger DJ. Neuronal activity in human primary visual cortex correlates with perception during binocular rivalry. Nat Neurosci. 2000;3:1153–1159. doi: 10.1038/80676. [DOI] [PubMed] [Google Scholar]
- Postle BR, Berger JS, Taich AM, D'Esposito M. Activity in human frontal cortex associated with spatial working memory and saccadic behavior. J Cogn Neurosci. 2000;12(Suppl 2):2–14. doi: 10.1162/089892900564028. [DOI] [PubMed] [Google Scholar]
- Postle BR, Hamidi M. Nonvisual codes and nonvisual brain areas support visual working memory. Cereb Cortex. doi: 10.1093/cercor/bhl123. in press. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Remapping of areas 9 and 46 using quantitative criteria. Cereb Cortex. 1995;5:307–322. doi: 10.1093/cercor/5.4.307. [DOI] [PubMed] [Google Scholar]
- Rivaud S, Muri RM, Gaymard B, Vermersch AI, Pierrot-Deseilligny C. Eye movement disorders after frontal eye field lesions in humans. Experimental Brain Research. 1994a;102:110–120. doi: 10.1007/BF00232443. [DOI] [PubMed] [Google Scholar]
- Rivaud S, Muri RM, Gaymard B, Vermersch AI, Pierrot-Deseilligny C. Eye movement disorders after frontal eye field lesions in humans. Exp Brain Res. 1994b;102:110–120. doi: 10.1007/BF00232443. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Riggio L, Dascola I, Umilta C. Reorienting attention across the horizontal and vertical meridians: evidence in favor of a premotor theory of attention. Neuropsychologia. 1987;25:31–40. doi: 10.1016/0028-3932(87)90041-8. [DOI] [PubMed] [Google Scholar]
- Ro T, Farne A, Chang E. Locating the human frontal eye fields with transcranial magnetic stimulation. J Clin Exp Neuropsychol. 2002;24:930–940. doi: 10.1076/jcen.24.7.930.8385. [DOI] [PubMed] [Google Scholar]
- Rosano C, Krisky CM, Welling JS, Eddy WF, Luna B, Thulborn KR, et al. Pursuit and saccadic eye movement subregions in human frontal eye field: a high-resolution fMRI investigation. Cereb Cortex. 2002;12:107–115. doi: 10.1093/cercor/12.2.107. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RS, Passingham RE. The prefrontal cortex: response selection or maintenance within working memory? Science. 2000;288:1656–1660. doi: 10.1126/science.288.5471.1656. [DOI] [PubMed] [Google Scholar]
- Sala JB, Rama P, Courtney SM. Functional topography of a distributed neural system for spatial and nonspatial information maintenance in working memory. Neuropsychologia. 2003;41:341–356. doi: 10.1016/s0028-3932(02)00166-5. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Schall JD. Neuronal activity related to visually guided saccades in the frontal eye fields of rhesus monkeys: comparison with supplementary eye fields. J Neurophysiol. 1991;66:559–579. doi: 10.1152/jn.1991.66.2.559. [DOI] [PubMed] [Google Scholar]
- Schall JD, Thompson KG. Neural selection and control of visually guided eye movements. Annu Rev Neurosci. 1999;22:241–259. doi: 10.1146/annurev.neuro.22.1.241. [DOI] [PubMed] [Google Scholar]
- Schluppeck D, Curtis CE, Glimcher PW, Heeger DJ. Sustained activity in topographic areas of human posterior parietal cortex during memory-guided saccades. J Neurosci. 2006;26:5098–5108. doi: 10.1523/JNEUROSCI.5330-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluppeck D, Glimcher P, Heeger DJ. Topographic organization for delayed saccades in human posterior parietal cortex. J Neurophysiol. 2005;94:1372–1384. doi: 10.1152/jn.01290.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, Yantis S. Selective visual attention and perceptual coherence. Trends Cogn Sci. 2006;10:38–45. doi: 10.1016/j.tics.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Pitzalis S, Martinez A. Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science. 2001;294:1350–1354. doi: 10.1126/science.1063695. [DOI] [PubMed] [Google Scholar]
- Sheliga BM, Riggio L, Rizzolatti G. Orienting of attention and eye movements. Exp Brain Res. 1994;98:507–522. doi: 10.1007/BF00233988. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, Andersen RA. Coding of intention in the posterior parietal cortex. Nature. 1997;386:167–170. doi: 10.1038/386167a0. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Tehovnik EJ. Reversible inactivation of macaque frontal eye field. Exp Brain Res. 1997;116:229–249. doi: 10.1007/pl00005752. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. Composition and topographic organization of signals sent from the frontal eye field to the superior colliculus. J Neurophysiol. 2000;83:1979–2001. doi: 10.1152/jn.2000.83.4.1979. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. Frontal eye field sends delay activity related to movement, memory, and vision to the superior colliculus. J Neurophysiol. 2001;85:1673–1685. doi: 10.1152/jn.2001.85.4.1673. [DOI] [PubMed] [Google Scholar]
- Takeda K, Funahashi S. Prefrontal task-related activity representing visual cue location or saccade direction in spatial working memory tasks. J Neurophysiol. 2002;87:567–588. doi: 10.1152/jn.00249.2001. [DOI] [PubMed] [Google Scholar]
- Takeda K, Funahashi S. Population vector analysis of primate prefrontal activity during spatial working memory. Cereb Cortex. 2004;14:1328–1339. doi: 10.1093/cercor/bhh093. [DOI] [PubMed] [Google Scholar]
- Takeda K, Funahashi S. Relationship between prefrontal task-related activity and information flow during spatial working memory performance. Cortex. 2007;43:38–52. doi: 10.1016/s0010-9452(08)70444-1. [DOI] [PubMed] [Google Scholar]
- Theeuwes J, Olivers CN, Chizk CL. Remembering a location makes the eyes curve away. Psychol Sci. 2005;16:196–199. doi: 10.1111/j.0956-7976.2005.00803.x. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Biscoe KL, Sato TR. Neuronal basis of covert spatial attention in the frontal eye field. J Neurosci. 2005;25:9479–9487. doi: 10.1523/JNEUROSCI.0741-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeno MM, Goldberg ME. Spatial processing in the monkey frontal eye field. II. Memory responses. J Neurophysiol. 2001;86:2344–2352. doi: 10.1152/jn.2001.86.5.2344. [DOI] [PubMed] [Google Scholar]
- Van der Stigchel S, Meeter M, Theeuwes J. Top-down influences make saccades deviate away: The case of endogenous cues. Acta Psychol (Amst) 2007;125:279–290. doi: 10.1016/j.actpsy.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Wang XJ. Synaptic reverberation underlying mnemonic persistent activity. Trends Neurosci. 2001;24:455–463. doi: 10.1016/s0166-2236(00)01868-3. [DOI] [PubMed] [Google Scholar]
- Wardak C, Olivier E, Duhamel JR. Saccadic target selection deficits after lateral intraparietal area inactivation in monkeys. J Neurosci. 2002;22:9877–9884. doi: 10.1523/JNEUROSCI.22-22-09877.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited--again. Neuroimage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Aguirre G, D'Esposito M. A trial-based experimental design for fMRI. Neuroimage. 1997;6:122–138. doi: 10.1006/nimg.1997.0279. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Aguirre GK, D'Esposito M. Temporal isolation of the neural correlates of spatial mnemonic processing with fMRI. Brain Res Cogn Brain Res. 1999;7:255–268. doi: 10.1016/s0926-6410(98)00029-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.