The crystallization and preliminary X-ray characterization of the polysaccharide lyase family 11 rhamnogalacturonan lyase are presented.
Keywords: rhamnogalacturonan lyases, YesW
Abstract
Rhamnogalacturonan lyases degrade rhamnogalacturonan I, a major component of pectin, through a β-elimination reaction. YesW from Bacillus subtilis strain 168 is a novel rhamnogalacturonan lyase classified into polysaccharide lyase family 11 (PL-11). The enzyme was crystallized at 293 K using the sitting-drop vapour-diffusion method with 2-methyl-2,4-pentanediol (MPD) as a precipitant. Preliminary X-ray analysis revealed that the YesW crystals belong to space group P21 and diffract to 2.40 Å resolution, with unit-cell parameters a = 56.7, b = 105.6, c = 101.4 Å, β = 94.9°. This is the first report on the crystallization and preliminary X-ray analysis of a family PL-11 rhamnogalacturonan lyase.
1. Introduction
The plant cell wall consists of several macromolecular polysaccharides such as pectin, cellulose and hemicellulose (Carpita & Gibeaut, 1993 ▶). Pectin contains polygalacturonan as a linear component and rhamnogalacturonan I and rhamnogalacturonan II as branched chains (Darvill et al., 1978 ▶; McNeil et al., 1984 ▶; Thakur et al., 1997 ▶). Rhamnogalacturonan I is composed of alternating l-rhamnopyranose and d-galactopyranouronic acid as a main chain, with arabinans and galactans as side chains (McNeil et al., 1980 ▶). Rhamnogalacturonan II contains polygalacturonan as a main chain and a complex of approximately 30 monosaccharides including rare sugars such as apiose and aceric acid as a side chain (O’Neill et al., 1996 ▶).
Many plant-pathogenic fungi and bacteria produce enzymes that are responsible for degradation of the plant cell wall. For example, glycosidases and polysaccharide lyases cleave glycosidic bonds in pectin via hydrolysis and β-elimination reactions, respectively (Davies & Henrissat, 1995 ▶; Linhardt et al., 1986 ▶). In the case of the degradation of the rhamnogalacturonan I main chain, its α-1,4 glycosidic bonds are cleaved by the actions of rhamnogalacturonan rhamnohydrolase and rhamnogalacturonan lyase and its α-1,2 bonds by the actions of rhamnogalacturonan galacturonohydrolase and rhamnogalacturonan hydrolase (Mutter et al., 1994 ▶, 1998 ▶; Schols et al., 1990 ▶). In the Carbohydrate-Active enZYmes (CAZY) database (http://afmb.cnrs-mrs.fr), rhamnogalacturonan lyases are classified into two polysaccharide lyase (PL) families (4 and 11) based on primary sequence similarities. The crystal structure of the family PL-4 rhamnogalacturonan lyase RhgB from Aspergillus aculeatus KSM 510 has previously been determined (McDonough et al., 2004 ▶), although its mechanisms for catalytic reaction and substrate specificity remain to be clarified. In the case of the family PL-11 rhamnogalacturonan lyases, there is no report to date of structural analysis of the enzymes. Rgl11A from Pseudomonas cellulosa and Rgl11Y from Clostridium cellulolyticum have been identified as rhamnogalacturonan lyases belonging to family PL-11 (McKie et al., 2001 ▶; Pages et al., 2003 ▶). The microbes producing these family PL-11 lyases are saprophytic and activities of other plant cell-wall-degrading enzymes (i.e. pectinases, cellulases and hemicellulases) are also found in such bacteria (McKie et al., 2001 ▶; Pages et al., 2003 ▶). We recently discovered that YesW from Bacillus subtilis strain 168 is a family PL-11 rhamnogalacturonan lyase (Murata, unpublished results). The gene for YesW encodes a protein with 620 amino-acid residues. The recombinant YesW expressed in Escherichia coli is processed by the excision of 37 N-terminal amino-acid residues as a signal peptide (Murata, unpublished results). Owing to the lack of sequence similarity between family PL-11 and the other lyases, family PL-11 lyases may constitute a fold that has not been observed in polysaccharide lyases thus far analyzed. Structural analysis of family PL-11 lyases will also contribute to the clarification of their structure–function relationships such as the mechanisms for catalytic reaction and substrate specificity in rhamnogalacturonan lyase and of their physiological functions in the bacterial saprophytic process.
This article focuses on the crystallization and preliminary X-ray crystallographic analysis of YesW.
2. Materials and results
2.1. Rhamnogalacturonan lyase activity assays
YesW was incubated at 303 K for 5 min in a reaction mixture (1 ml) consisting of 0.05% rhamnogalacturonan I from potato (Megazyme International Ireland Ltd, Wicklow, Ireland), 50 mM Tris–HCl pH 7.5 and 2 mM CaCl2. Calcium ion was required for activation of YesW as found in the case of Rgl11A (McKie et al., 2001 ▶). The activity was determined by monitoring the increase in the absorbance at 235 nm arising from the double bond formed in the reaction products. One unit (U) of enzyme activity was defined as the amount of enzyme required to produce an increase of 1.0 in absorbance at 235 nm per minute using a 1 cm cuvette. Protein content was determined by the method of Bradford (1976 ▶), with bovine serum albumin as the standard.
2.2. Protein expression and purification
Recombinant YesW was purified from E. coli cells (Murata, unpublished results). Unless specified otherwise, all procedures were performed at 273–277 K. E. coli cells harbouring pET21b/YesW were grown in 40.5 l LB medium (1.5 l per flask), collected by centrifugation at 6000g for 5 min at 277 K, washed with 20 mM Tris–HCl pH 7.5 containing 2 mM CaCl2 (buffer A) and resuspended in buffer A. The cells were ultrasonically disrupted at 273 K and 9 kHz for 20 min and the clear solution obtained on centrifugation at 20 000g for 20 min at 277 K was used as the cell extract. After overnight dialysis at 277 K against buffer A, the cell extract was applied onto an Ni2+-Chelating Sepharose Fast Flow column (2.5 × 5 cm; Amersham Biosciences, Uppsala, Sweden) previously equilibrated with buffer A containing 0.2 M NaCl and 40 mM imidazole. Histidine-tagged YesW was eluted with a linear gradient of imidazole (40–500 mM) in buffer A (200 ml) containing 0.2 M NaCl and 5 ml fractions were collected every 5 min. Fractions containing YesW, which were eluted between 100 and 200 mM imidazole, were combined and dialyzed overnight at 277 K against 20 mM Tris–HCl pH 7.5 containing 2 mM CaCl2 and 0.2 M NaCl (buffer B). After dialysis, the sample was divided into five fractions (5 ml each) and each fraction was applied onto a HiLoad 16/60 Superdex 200pg column (1.6 × 60 cm; Amersham Biosciences, Uppsala, Sweden) previously equilibrated with buffer B. YesW was eluted with buffer B (120 ml) and 2 ml fractions were collected every 2 min. Fractions containing YesW were combined and used as the purified YesW. The purified enzyme was shown to be homogeneous by SDS–PAGE and its molecular weight was determined to be approximately 64 kDa. The N-terminal amino-acid sequence of the protein was subsequently determined to be NH2-AARQMEALN, which corresponds to residues 38–46 of the N-terminus of the YesW amino-acid sequence published in the DNA/protein database (620 amino-acid residues; GenPept accession No. CAB12524), indicating that N-terminal 37 residues of YesW are probably cleaved as a signal peptide in E. coli cells. The molecular weight of the mature form of YesW including the C-terminal histidine tags (eight amino-acid residues, -LEHHHHHH) was calculated to be 64 444 Da (591 amino-acid residues), in agreement with the result from SDS–PAGE. The specific activity of the purified enzyme was 207.7 U mg−1. The purified enzyme was concentrated by ultrafiltration with a Centriprep (Millipore Co., Tokyo, Japan) to a final concentration of 24 mg ml−1. The final concentrated protein sample, containing 20 mM Tris–HCl pH 7.5, 2 mM CaCl2 and 0.2 M NaCl, was used in the crystallization step.
2.3. Crystallization
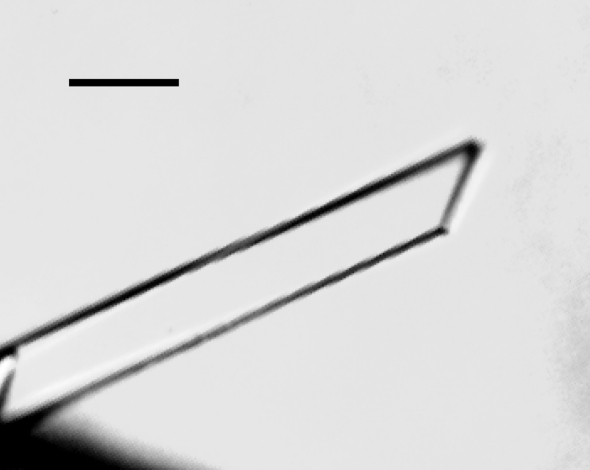
Purified YesW was crystallized at 293 K by the sitting-drop vapour-diffusion method. The droplet was prepared by mixing 3 µl protein solution with 3 µl precipitant solution. Long thin crystals of YesW appeared in a condition from the JBScreen 7 kit (Jena Bioscience, Jena, Germany) in about a month (Fig. 1 ▶). This condition was further optimized, with the best results obtained using 0.1 M Tris–HCl pH 8.5 and 40% 2-methyl-2,4-pentanediol (MPD).
Figure 1.
Crystal of YesW from B. subtilis strain 168. The scale bar is 0.1 mm in length.
2.4. X-ray analysis
A crystal of YesW was picked up from a droplet in a mounted nylon loop (Hampton Research Aliso Viejo, CA, USA) and was placed directly in a cold nitrogen-gas stream at 100 K. X-ray diffraction images of the crystal were collected at 100 K in the nitrogen-gas stream with a Bruker Hi-Star multiwire area detector using Cu Kα radiation generated by a MacScience M18XHF rotating-anode generator. Diffraction data for the crystal were obtained to a resolution of 2.40 Å and were processed using the SADIE and SAINT programs according to the manuals provided by Bruker (Bruker, Karlsruhe, Germany). The space group of the crystal was determined as P21 (monoclinic), with unit-cell parameters a = 56.7, b = 105.6, c = 101.4 Å, β = 94.9°. The preliminary X-ray crystallographic statistics of YesW are summarized in Table 1 ▶.
Table 1. Data-collection statistics for YesW crystal.
Values in parentheses refer to data in the highest resolution shell.
| Wavelength (Å) | 1.54 |
| Resolution (Å) | ∞–2.40 (2.49–2.40) |
| Space group | P21 |
| Unit-cell parameters (Å, °) | a = 56.7, b = 105.6, c = 101.4, β = 94.9 |
| Total observations | 94115 |
| Independent reflections | 44609 (4238) |
| Completeness (%) | 95.7 (92.3) |
| Redundancy | 2.11 (1.89) |
| I/σ(I) | 9.53 (3.66) |
| Rsym† (%) | 9.5 (25.3) |
R
sym = 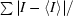
 , where I and 〈I〉 represent the diffraction intensity values of the individual measurements and the corresponding mean values, respectively. The summation is over all measurements.
, where I and 〈I〉 represent the diffraction intensity values of the individual measurements and the corresponding mean values, respectively. The summation is over all measurements.
The peak of the native Patterson test revealed that the crystal contains a dimer in the asymmetric unit and contained rotational NCS. When two molecules of the enzyme were present per asymmetric unit, the V M value (Matthews, 1968 ▶) and solvent content were calculated to be 2.39 Å3 Da−1 and 48.5%, respectively. A search for selenomethionyl derivatives for use in phasing by the multiple-wavelength anomalous dispersion (MAD) method is now in progress.
Acknowledgments
This work was supported in part by the Program for Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN) of Japan.
References
- Bradford, M. M. (1976). Anal. Biochem.72, 248–254. [DOI] [PubMed] [Google Scholar]
- Carpita, N. C. & Gibeaut, D. M. (1993). Plant J.3, 1–30. [DOI] [PubMed] [Google Scholar]
- Darvill, A. G., McNeil, M. & Albersheim, P. (1978). Plant Physiol.62, 418–422. [DOI] [PMC free article] [PubMed]
- Davies, G. & Henrissat, B. (1995). Structure, 3, 853–859. [DOI] [PubMed] [Google Scholar]
- Linhardt, R. J., Galliher, P. M. & Cooney, C. L. (1986). Appl. Biochem. Biotechnol.12, 135–176. [DOI] [PubMed] [Google Scholar]
- McDonough, M. A., Kadirvelraj, R., Harris, P., Poulsen, J. C. & Larsen, S. (2004). FEBS Lett.565, 188–194. [DOI] [PubMed] [Google Scholar]
- McKie, V. A., Vincken, J. P., Voragen, A. G., van den Broek, L. A., Stimson, E. & Gilbert, H. J. (2001). Biochem. J.355, 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil, M., Darvill, A. G. & Albersheim, P. (1980). Plant Physiol.66, 1128–1134. [DOI] [PMC free article] [PubMed]
- McNeil, M., Darvill, A. G., Fry, S. C. & Albersheim, P. (1984). Annu. Rev. Biochem.53, 625–663. [DOI] [PubMed] [Google Scholar]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed] [Google Scholar]
- Mutter, M., Beldman, G., Schols, H. A. & Voragen, A. G. (1994). Plant Physiol.106, 241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutter, M., Renard, C. M. G. C., Beldman, G., Schols, H. A. & Voragen, A. G. J. (1998). Carbohydr. Res.311, 155–164. [DOI] [PubMed] [Google Scholar]
- O’Neill, M. A., Warrenfeltz, D., Kates, K., Pellerin, P., Doco, T., Darvill, A. G. & Albersheim, P. (1996). J. Biol. Chem.271, 22923–22930. [DOI] [PubMed] [Google Scholar]
- Pages, S., Valette, O., Abdou, L., Belaich, A. & Belaich, J. P. (2003). J. Bacteriol.185, 4727–4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schols, H. A., Geraeds, C. C. J. M., Searle-van Leeuwen, M. F., Kormelink, F. J. M. & Voragen, A. G. J. (1990). Carbohydr. Res.206, 105–115.
- Thakur, B. R., Singh, R. K. & Handa, A. K. (1997). Crit. Rev. Food Sci. Nutr.37, 47–73. [DOI] [PubMed] [Google Scholar]