Abstract
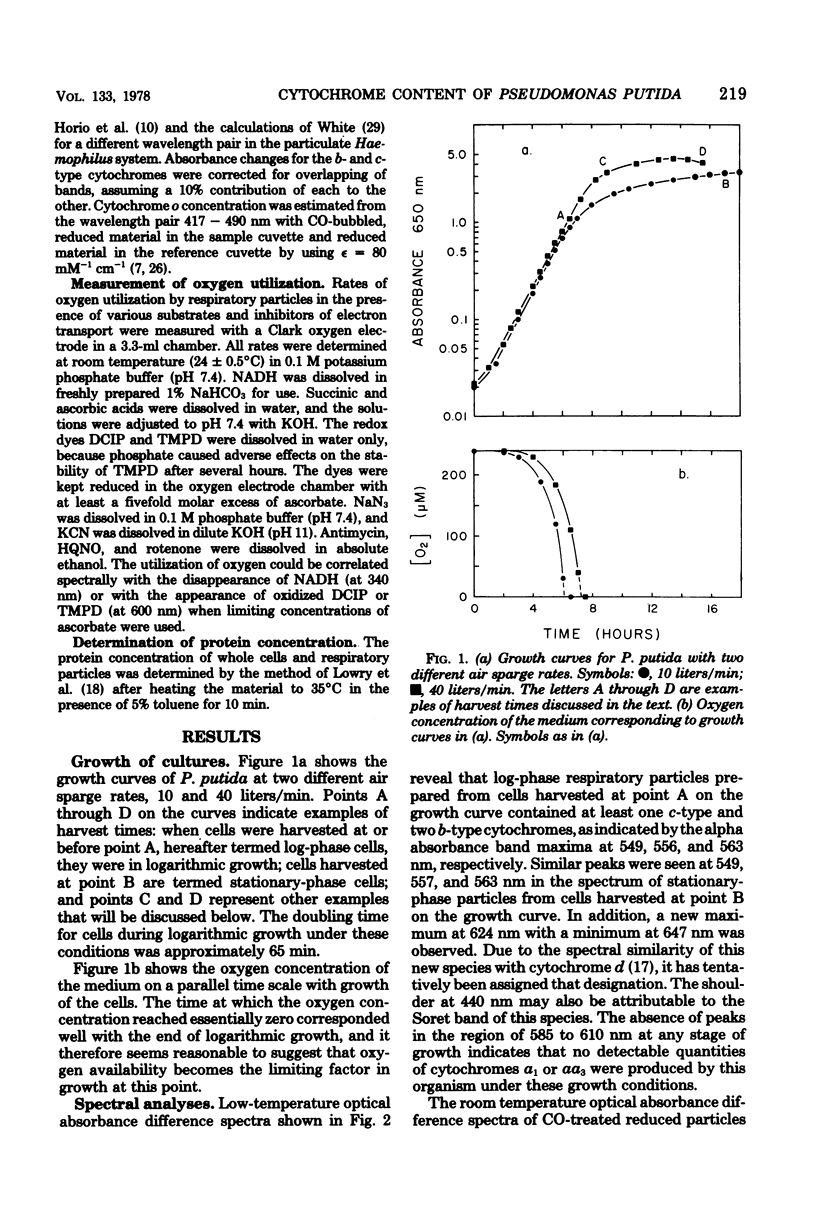
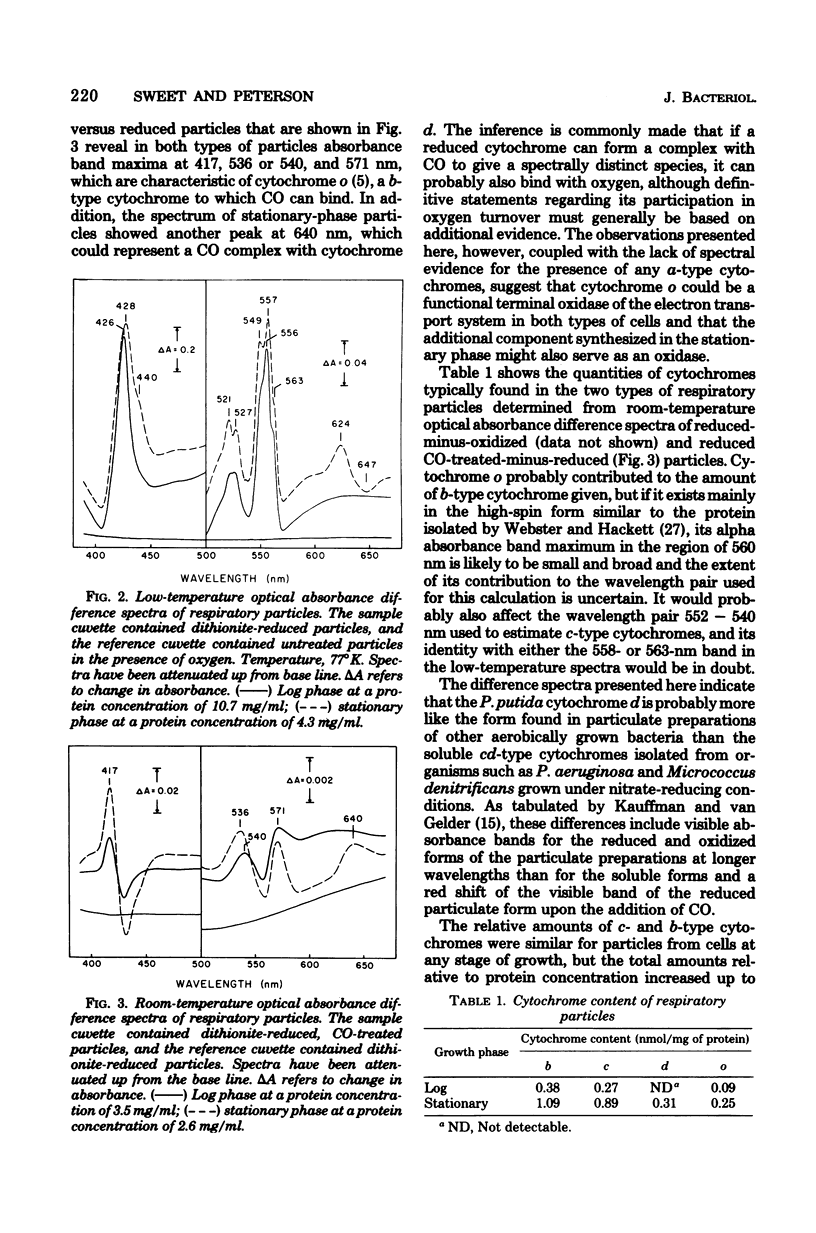
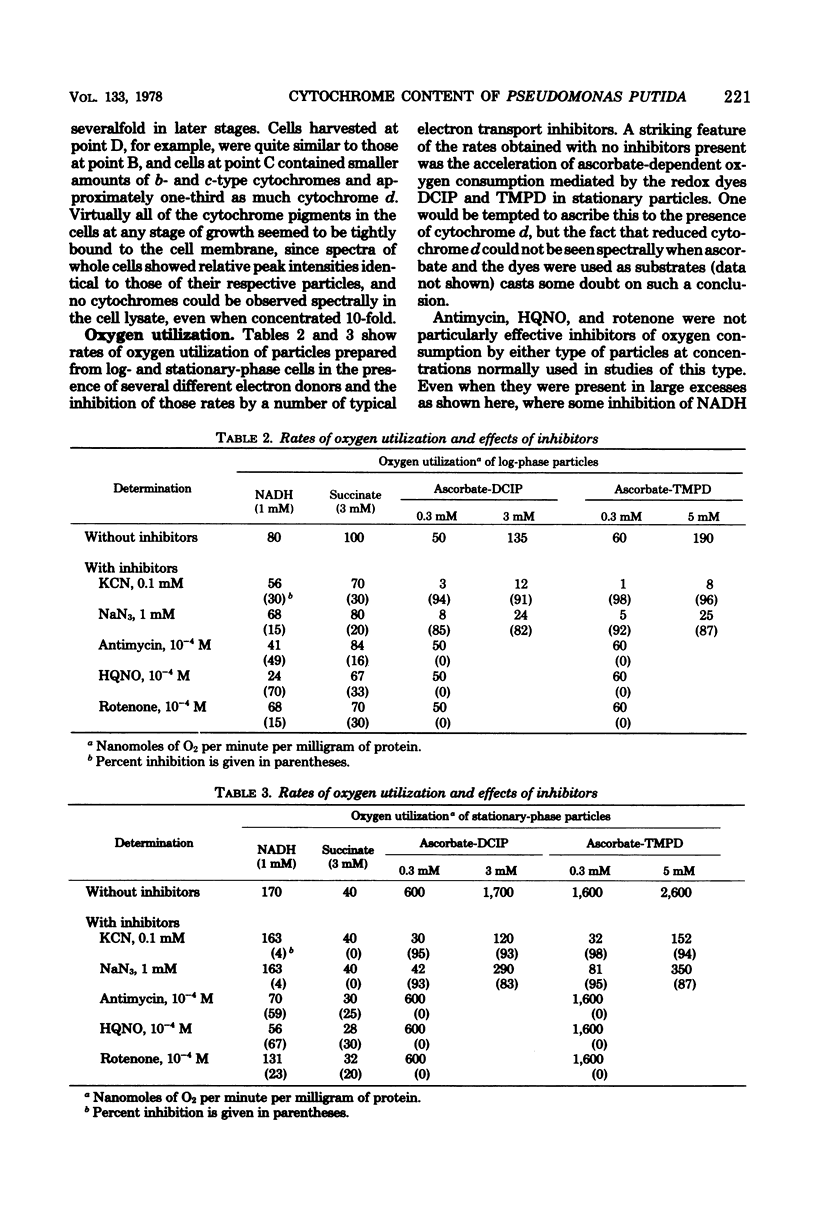
Optical absorbance difference spectra of membrane vesicles prepared from aerobically grown Pseudomonas putida indicated that, when harvested in logarithmic phase, the cells contained one c-type cytochrome and two or three b-type cytochromes, one of which was cytochrome o. As the cells grew into stationary phase and the oxygen concentration of the medium dropped to essentially zero, an additional component believed to be cytochrome d was produced. Both the o- and d-type cytochromes might function as terminal oxidases. No a-type cytochromes could be detected at any stage of growth. Polarographic measurement of oxygen utilization revealed that cyanide and azide are effective inhibitors of the oxidation of ascorbate coupled with 2,6-dichlorophenolindophenol or N,N,N',N'-tetramethyl-p-phenylenediamine in respiratory particles from either log-phase or stationary-phase cells. Reduced nicotinamide adenine dinucleotide- or succinate-dependent oxygen utilization, however, was sensitive to these inhibitors only in log-phase particles. These results indicate that an alternate terminal oxidase may be synthesized by this organism in response to restricted oxygen availability and that branching of the respiratory system may result.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackrell B. A., Jones C. W. The respiratory system of Azotobacter vinelandii. 2. Oxygen effects. Eur J Biochem. 1971 May 11;20(1):29–35. doi: 10.1111/j.1432-1033.1971.tb01358.x. [DOI] [PubMed] [Google Scholar]
- Arima K., Oka T. Cyanide Resistance in Achromobacter I. Induced Formation of Cytochrome a(2) and Its Role in Cyanide-Resistant Respiration. J Bacteriol. 1965 Sep;90(3):734–743. doi: 10.1128/jb.90.3.734-743.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft J. R., Haddock B. A. Synthesis of alternative membrane-bound redox carriers during aerobic growth of Escherichia coli in the presence of potassium cyanide. Biochem J. 1975 May;148(2):349–352. doi: 10.1042/bj1480349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch R. G. Bacterial cytochromes. Annu Rev Microbiol. 1968;22:181–200. doi: 10.1146/annurev.mi.22.100168.001145. [DOI] [PubMed] [Google Scholar]
- CASTOR L. N., CHANCE B. Photochemical determinations of the oxidases of bacteria. J Biol Chem. 1959 Jun;234(6):1587–1592. [PubMed] [Google Scholar]
- HORIO T., HIGASHI T., YAMANAKA T., MATSUBARA H., OKUNUKI K. Purification and properties of cytochrome oxidase from Pseudomonas aeruginosa. J Biol Chem. 1961 Mar;236:944–951. [PubMed] [Google Scholar]
- Haddock B. A., Downie J. A., Garland P. B. Kinetic characterization of the membrane-bound cytochromes of Escherichia coli grown under a variety of conditions by using a stopped-flow dual-wavelength spectrophotometer. Biochem J. 1976 Feb 15;154(2):285–294. doi: 10.1042/bj1540285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D. E. A study of the effect of growth conditions on chemostat-grown Klebsiella aerogenes and kinetic changes of A 500-nm absorption band. Biochim Biophys Acta. 1972 Jul 12;275(1):83–92. doi: 10.1016/0005-2728(72)90026-6. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Redfearn E. R. The cytochrome system of Azotobacter vinelandii. Biochim Biophys Acta. 1967 Sep 6;143(2):340–353. doi: 10.1016/0005-2728(67)90088-6. [DOI] [PubMed] [Google Scholar]
- Jurtshuk P., Jr, Mueller T. J., Acord W. C. Bacterial terminal oxidases. CRC Crit Rev Microbiol. 1975 May;3(4):399–468. doi: 10.3109/10408417509108757. [DOI] [PubMed] [Google Scholar]
- Kamen M. D., Horio T. Bacterial cytochromes. I. Structural aspects. Annu Rev Biochem. 1970;39:673–700. doi: 10.1146/annurev.bi.39.070170.003325. [DOI] [PubMed] [Google Scholar]
- Kauffman H. F., van Gelder B. F. The respiratory chain of Azotobacter vinelandii. II. The effect of cyanide on cytochrome d. Biochim Biophys Acta. 1973 Sep 26;314(3):276–283. doi: 10.1016/0005-2728(73)90112-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MOSS F. Adaptation of the cytochromes of Aerobacter aerogenes in response to environmental oxygen tension. Aust J Exp Biol Med Sci. 1956 Oct;34(5):395–405. doi: 10.1038/icb.1956.48. [DOI] [PubMed] [Google Scholar]
- MOYED H. S., O'KANE D. J. Enzymes and coenzymes of the pyruvate oxidase of Proteus. J Biol Chem. 1956 Feb;218(2):831–840. [PubMed] [Google Scholar]
- Pudek M. R., Bragg P. D. Inhibition by cyanide of the respiratory chain oxidases of Escherichia coli. Arch Biochem Biophys. 1974 Oct;164(2):682–693. doi: 10.1016/0003-9861(74)90081-2. [DOI] [PubMed] [Google Scholar]
- WHITE D. C. SYNTHESIS OF 2-DEMETHYL VITAMIN K2 AND THE CYTOCHROME SYSTEM IN HAEMOPHILUS. J Bacteriol. 1965 Feb;89:299–305. doi: 10.1128/jb.89.2.299-305.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster D. A., Hackett D. P. The purification and properties of cytochrome o from Vitreoscilla. J Biol Chem. 1966 Jul 25;241(14):3308–3315. [PubMed] [Google Scholar]
- White D. C., Sinclair P. R. Branched electron-transport systems in bacteria. Adv Microb Physiol. 1971;5:173–211. doi: 10.1016/s0065-2911(08)60407-5. [DOI] [PubMed] [Google Scholar]



