Abstract
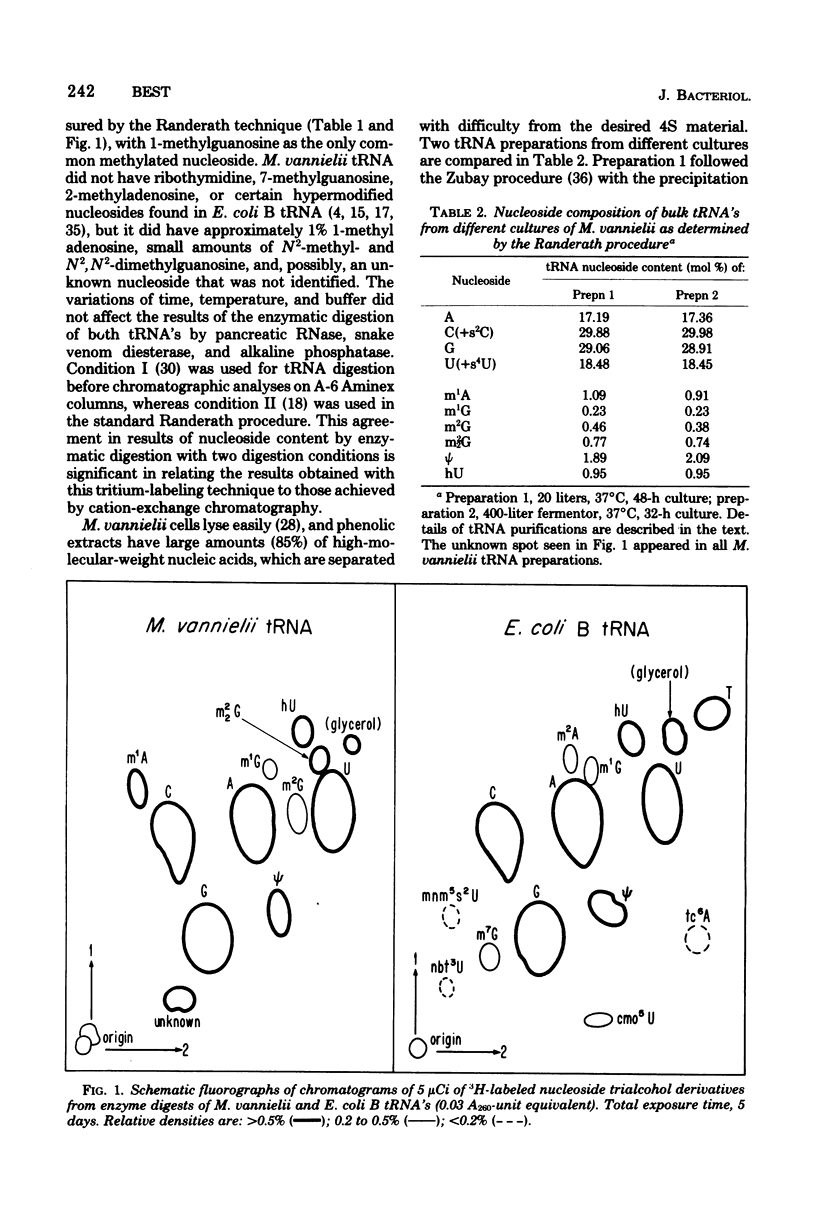
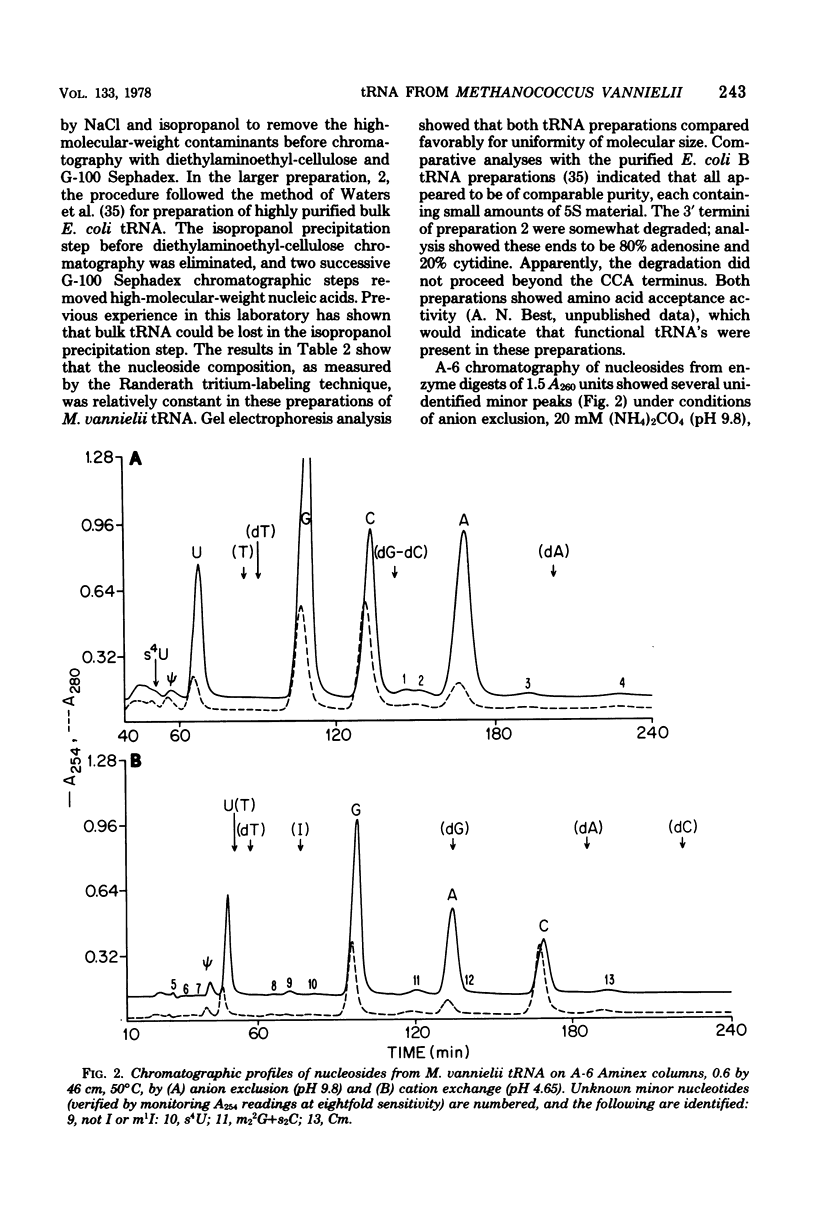
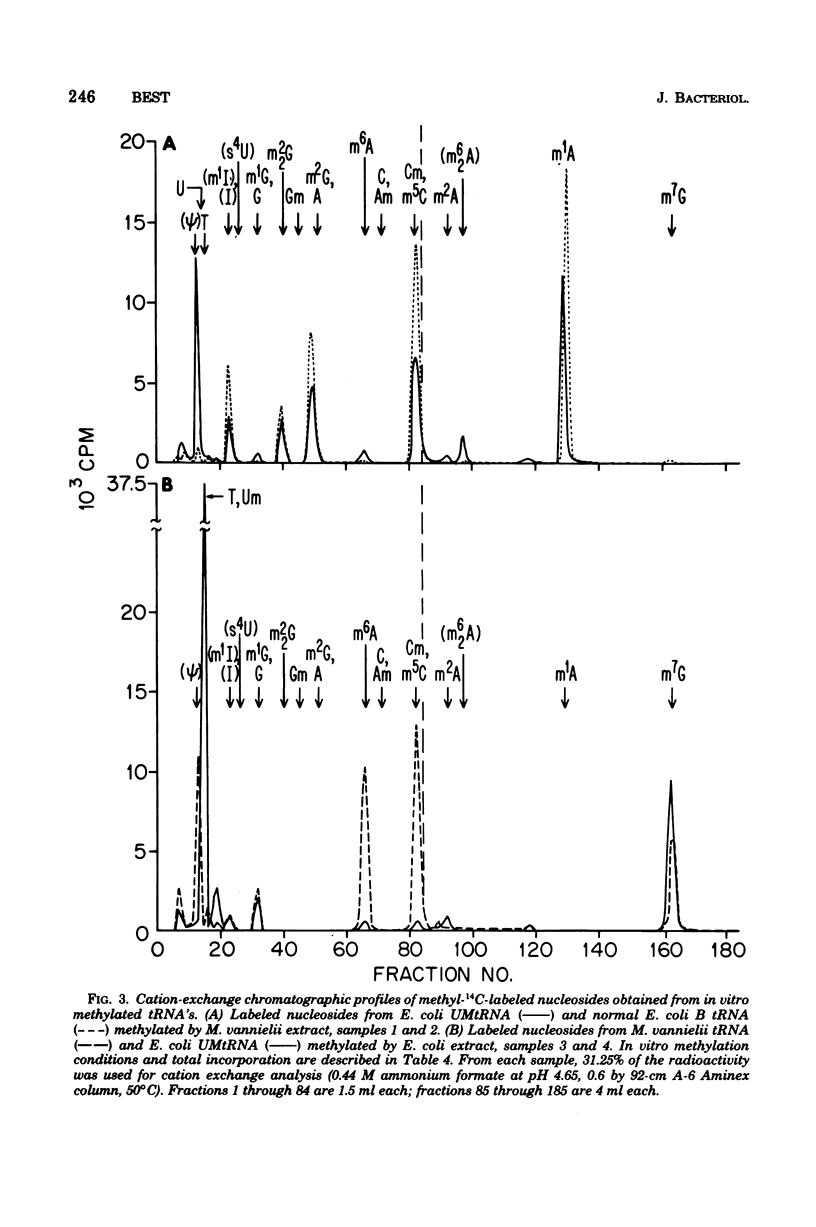
Purified bulk tRNA from Methanococcus vanielii (carbon source, formate) showed variation in the modified nucleoside pattern reported for Escherichia coli as analyzed by both ion-exchange and thin-layer chromatography. Ribothymidine and 7-methylguanosine were absent; 1-methyladenosine, 1-methylguanosine, N2-methylguanosine, N2,N2-dimethylguanosine, thiolated nucleosides, pseudouridine, dihydrouridine, and O2'-methylcytidine were quantitated. In vitro methylation by M. Vannielii extracts with S-adenosylmethionine and undermethylated E. coli tRNA revealed active tRNA methyltransferases for formation of methylated residues found in native M. vannielii tRNA, but none for the formation of 7-methylguanosine or ribothymidine. The native M. vannielii tRNA became methylated in the 7-methylguanosine position by E. Coli extracts, but ribothymidine was not formed. Both M. vannielii and E. coli tRNA methyltransferases produced unidentified methylated residues in tRNA's lacking or deficient in ribothymidine.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agris P. F., Koh H., Söll D. The effect of growth temperatures on the in vivo ribose methylation of Bacillus stearothermophilus transfer RNA. Arch Biochem Biophys. 1973 Jan;154(1):277–282. doi: 10.1016/0003-9861(73)90058-1. [DOI] [PubMed] [Google Scholar]
- Baumstark B. R., Spremulli L. L., RajBhandary U. L., Brown G. M. Initiation of protein synthesis without formylation in a mutant of Escherichia coli that grows in the absence of tetrahydrofolate. J Bacteriol. 1977 Jan;129(1):457–471. doi: 10.1128/jb.129.1.457-471.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia L. L., Morris H. P., Randerath K., Randerath E. Base composition studies on mitochondrial 4 S RNA from rat liver and Morris hepatomas 5123D and 7777. Biochim Biophys Acta. 1976 Feb 18;425(1):49–62. doi: 10.1016/0005-2787(76)90215-x. [DOI] [PubMed] [Google Scholar]
- HURWITZ J., GOLD M., ANDERS M. THE ENZYMATIC METHYLATION OF RIBONUCLEIC ACID AND DEOXYRIBONUCLEIC ACID. IV. THE PROPERTIES OF THE SOLUBLE RIBONUCLEIC ACID-METHYLATING ENZYMES. J Biol Chem. 1964 Oct;239:3474–3482. [PubMed] [Google Scholar]
- Harada F., Kimura F., Nishimura S. Primary sequence of tRNA val from Escherichia coli B. I. Oligonucleotide sequences of digests of Escherichia coli tRNA val with RNase T and pancreatic RNase. Biochemistry. 1971 Aug 17;10(17):3269–3277. doi: 10.1021/bi00793a017. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Sakai M., Muramatsu M. 2'-O-methylated oligonucleotides in ribosomal 18S and 28S RNA of a mouse hepatoma, MH 134. Biochemistry. 1975 May 6;14(9):1956–1964. doi: 10.1021/bi00680a024. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murao K., Hasegawa T., Ishikura H. 5-methoxyuridine: a new minor constituent located in the first position of the anticodon of tRNAAla, tRNAThr, and tRNAVal from Bacillus subtilis. Nucleic Acids Res. 1976 Oct;3(10):2851–2860. doi: 10.1093/nar/3.10.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S., Harada F., Narushima U., Seno T. Purification of methionine-, valine-, phenylalanine- and tyrosine-specific tRNA from Escherichia coli. Biochim Biophys Acta. 1967 Jun 20;142(1):133–148. doi: 10.1016/0005-2787(67)90522-9. [DOI] [PubMed] [Google Scholar]
- Nishimura S. Minor components in transfer RNA: their characterization, location, and function. Prog Nucleic Acid Res Mol Biol. 1972;12:49–85. [PubMed] [Google Scholar]
- Randerath E., Chia L. L., Morris H. P., Randerath K. Base analysis of RNA by 3H postlabeling--a study of ribothymidine content and degree of base methylation of 4 S RNA. Biochim Biophys Acta. 1974 Oct 11;366(2):159–167. doi: 10.1016/0005-2787(74)90330-x. [DOI] [PubMed] [Google Scholar]
- Rogg H., Brambilla R., Keith G., Staehelin M. An improved method for the separation and quantitation of the modified nucleosides of transfer RNA. Nucleic Acids Res. 1976 Jan;3(1):285–295. doi: 10.1093/nar/3.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogg H., Staehelin M. Nucleotide sequences of rat liver serine-tRNA. 1. Products of digestion with pancreatic ribonuclease. Eur J Biochem. 1971 Jul 29;21(2):235–242. doi: 10.1111/j.1432-1033.1971.tb01461.x. [DOI] [PubMed] [Google Scholar]
- STADTMAN T. C., BARKER H. A. Studies on the methane fermentation. X. A new formate-decomposing bacterium, Methanococcus vannielii. J Bacteriol. 1951 Sep;62(3):269–280. doi: 10.1128/jb.62.3.269-280.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel C. E., Rabinowitz J. C. Initiation of protein synthesis by folate-sufficient and folate-deficient Streptococcus faecalis R. Biochemical and biophysical properties of methionine transfer ribonucleic acid. J Biol Chem. 1974 Feb 25;249(4):1198–1206. [PubMed] [Google Scholar]
- Schmidt W., Arnold H. H., Kersten H. Tetrahydrofolate-dependent biosynthesis of ribothymidine in transfer ribonucleic acids of Gram-positive bacteria. J Bacteriol. 1977 Jan;129(1):15–21. doi: 10.1128/jb.129.1.15-21.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shugart L., Chastain B. H., Novelli G. D., Stulberg M. P. Restoration of aminoacylation activity of undermethylated transfer RNA by in vitro methylation. Biochem Biophys Res Commun. 1968 May 10;31(3):404–409. doi: 10.1016/0006-291x(68)90490-7. [DOI] [PubMed] [Google Scholar]
- Shugart L., Stulberg M. P. Isolation, purification, and methylation of undermethylated tRNA Phe from an RC rel mutant of Escherichia coli. Methods Enzymol. 1974;29:492–502. [PubMed] [Google Scholar]
- Singhal R. P. Ion-exlusion chromatography: analysis and isolation of nucleic acid components, and influence of separation parameters. Arch Biochem Biophys. 1972 Oct;152(2):800–810. doi: 10.1016/0003-9861(72)90276-7. [DOI] [PubMed] [Google Scholar]
- Stadtman T. C. Methane fermentation. Annu Rev Microbiol. 1967;21:121–142. doi: 10.1146/annurev.mi.21.100167.001005. [DOI] [PubMed] [Google Scholar]
- Uziel M., Koh C. K., Cohn W. E. Rapid ion-exchange chromatographic microanalysis of ultraviolet-absorbing materials and its application to nucleosides. Anal Biochem. 1968 Oct 24;25(1):77–98. doi: 10.1016/0003-2697(68)90083-3. [DOI] [PubMed] [Google Scholar]
- Uziel M., Koh C. Rapid and sensitive measurement of 7-methylguanosine and N 6 -isopentenyl derivatives of adenosine by cation-exchange chromatography. J Chromatogr. 1971 Jul 8;59(1):188–193. doi: 10.1016/s0021-9673(01)80025-x. [DOI] [PubMed] [Google Scholar]
- Uziel M., Smith L. H., Taylor S. A. Modified nucleosides in urine: selective removal and analysis. Clin Chem. 1976 Sep;22(9):1451–1455. [PubMed] [Google Scholar]
- Vold B. Modified nucleosides of Bacillus subtilis transfer ribonucleic acids. J Bacteriol. 1976 Jul;127(1):258–267. doi: 10.1128/jb.127.1.258-267.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. T., RajBhandary U. L. Formylatable methionine transfer RNA from Mycoplasma: purification and comparison of partial nucleotide sequences with those of other prokaryotic initiator tRNAs. Nucleic Acids Res. 1975 Jan;2(1):61–78. doi: 10.1093/nar/2.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Oshima T., Saneyoshi M., Nishimura S. Replacement of ribothymidine by 5-methyl-2-thiouridine in sequence GT psi C in tRNA of an extreme thermophile. FEBS Lett. 1974 Jul 1;43(1):59–63. doi: 10.1016/0014-5793(74)81105-1. [DOI] [PubMed] [Google Scholar]
- Waters L. C., Shugart L., Yang W. K., Best A. N. Some physical and biological properties of 4-thiouridine- and dihydrouridine-deficient tRNA from chloramphenicol-treated Escherichia coli. Arch Biochem Biophys. 1973 Jun;156(2):780–793. doi: 10.1016/0003-9861(73)90332-9. [DOI] [PubMed] [Google Scholar]
- al-Arif A., Sporn M. B. An analytical method for the separation of sugar-methylated ribonucleosides from base-methylated and nonmethylated ribonucleosides. Anal Biochem. 1972 Aug;48(2):386–393. doi: 10.1016/0003-2697(72)90091-7. [DOI] [PubMed] [Google Scholar]



