Abstract
Background
Grape berry development is a dynamic process that involves a complex series of molecular genetic and biochemical changes divided into three major phases. During initial berry growth (Phase I), berry size increases along a sigmoidal growth curve due to cell division and subsequent cell expansion, and organic acids (mainly malate and tartrate), tannins, and hydroxycinnamates accumulate to peak levels. The second major phase (Phase II) is defined as a lag phase in which cell expansion ceases and sugars begin to accumulate. Véraison (the onset of ripening) marks the beginning of the third major phase (Phase III) in which berries undergo a second period of sigmoidal growth due to additional mesocarp cell expansion, accumulation of anthocyanin pigments for berry color, accumulation of volatile compounds for aroma, softening, peak accumulation of sugars (mainly glucose and fructose), and a decline in organic acid accumulation. In order to understand the transcriptional network responsible for controlling berry development, mRNA expression profiling was conducted on berries of V. vinifera Cabernet Sauvignon using the Affymetrix GeneChip® Vitis oligonucleotide microarray ver. 1.0 spanning seven stages of berry development from small pea size berries (E-L stages 31 to 33 as defined by the modified E-L system), through véraison (E-L stages 34 and 35), to mature berries (E-L stages 36 and 38). Selected metabolites were profiled in parallel with mRNA expression profiling to understand the effect of transcriptional regulatory processes on specific metabolite production that ultimately influence the organoleptic properties of wine.
Results
Over the course of berry development whole fruit tissues were found to express an average of 74.5% of probes represented on the Vitis microarray, which has 14,470 Unigenes. Approximately 60% of the expressed transcripts were differentially expressed between at least two out of the seven stages of berry development (28% of transcripts, 4,151 Unigenes, had pronounced (≥2 fold) differences in mRNA expression) illustrating the dynamic nature of the developmental process. The subset of 4,151 Unigenes was split into twenty well-correlated expression profiles. Expression profile patterns included those with declining or increasing mRNA expression over the course of berry development as well as transient peak or trough patterns across various developmental stages as defined by the modified E-L system. These detailed surveys revealed the expression patterns for genes that play key functional roles in phytohormone biosynthesis and response, calcium sequestration, transport and signaling, cell wall metabolism mediating expansion, ripening, and softening, flavonoid metabolism and transport, organic and amino acid metabolism, hexose sugar and triose phosphate metabolism and transport, starch metabolism, photosynthesis, circadian cycles and pathogen resistance. In particular, mRNA expression patterns of transcription factors, abscisic acid (ABA) biosynthesis, and calcium signaling genes identified candidate factors likely to participate in the progression of key developmental events such as véraison and potential candidate genes associated with such processes as auxin partitioning within berry cells, aroma compound production, and pathway regulation and sequestration of flavonoid compounds. Finally, analysis of sugar metabolism gene expression patterns indicated the existence of an alternative pathway for glucose and triose phosphate production that is invoked from véraison to mature berries.
Conclusion
These results reveal the first high-resolution picture of the transcriptome dynamics that occur during seven stages of grape berry development. This work also establishes an extensive catalog of gene expression patterns for future investigations aimed at the dissection of the transcriptional regulatory hierarchies that govern berry development in a widely grown cultivar of wine grape. More importantly, this analysis identified a set of previously unknown genes potentially involved in critical steps associated with fruit development that can now be subjected to functional testing.
Background
Grapes have been cultivated and fermented into wine for more than 7,000 years. Worldwide, grapes are one of the most widely cultivated fruit crops, encompassing 7.4 million hectares of arable land in 2006 [1] and with 68.9 million metric tons produced in 2006, ranks second among bananas, oranges, and apples with 69.7, 63.8 and 62.1 million metric tons respectively, produced during this same period. However, because the majority of the grapes that are harvested are fermented into wine, the economic impact for this commodity is far greater than the value of the grapes. For example, wine sales from California alone in 2006 was at an all-time high and growing with approximately $18 billion dollar in sales [2]. According to 2005 statistics, the California wine industry has a $52 and $125 billion economic impact on the state and U.S. economies, respectively [3].
In addition to their economic importance, consumption of grapes and wine has numerous nutritional and health benefits for humans [4,5]. For example, there are more than 200 polyphenolic compounds in red wines that are thought to act as antioxidants. In particular, one antioxidant compound, trans-resveratrol, has been shown to play a role in the prevention of heart disease (atherosclerosis) [6] and cancer [7]. Resveratrol slows the aging process in animals [8], acts as a signaling molecule in the brain [9], and down-regulates the expression of genes that are involved in cell cycle and cell proliferation in human prostate cells [10]. Therefore, for a variety of reasons, there is great interest in manipulating grape berry development and quality for both economic and health reasons.
In contrast to the well studied climacteric fruits such as tomato and apple, very little is known about the development and ripening processes of non-climacteric fruits such as grape or strawberry [11,12]. In 1992, Coombe, one of the leaders in the field, described our knowledge of grape berry development and the regulation of ripening as "embryonic [13]."
Grape berries, like other berry fruits, undergo a complex series of physical and biochemical changes during development, which can be divided into three major phases [13] with more detailed descriptive designations, known as the modified E-L system, being used to define more precise growth stages over the entire grapevine lifecycle [14]. During the initial stage of berry growth (Phase I) cell division is rapid and all cells are established in the developing fruit in the first two weeks after flowering followed by a subsequent sigmoidal increase in berry size over approximately 60 days due to cell expansion. Two important organic acids, tartrate and malate, are synthesized and reach maximal concentrations by the end of Phase I. Biosynthesis of tannins and hydroxycinnamates, which are major precursors for phenolic volatiles, also occurs, primarily during Phase I. Tannins are located primarily in the skin and seeds of the berry, and are perceived as astringent compounds important for color stability and the body of red wine.
Phase II is characterized as a lag phase during which there is no increase in berry size. Biosynthetic processes are not well characterized for this stage, but it is known that sugar accumulation begins during this phase just prior to véraison (the onset of ripening) [13]. Véraison marks the start of Phase III of berry growth, which is characterized by the initiation of color development (anthocyanin accumulation in red grapes) and berry softening. Berry growth is sigmoidal during Phase III, as the berries double in size. At the onset of this stage, sugars (largely glucose and fructose) continue to accumulate, and organic acid concentrations decline. The acid:sugar balance at harvest is critical for high quality wines, as it affects important sensory attributes [15]. A large number of the flavor compounds and volatile aromas are synthesized at the end of Stage III. Many of these aromas are derived from terpenoids. However, the availability of seed tannins declines through oxidative processes during Phase III, causing the tannins to bind to the seed coat, reducing the astringent components within the berry. Skin tannins begin to interact and bind with anthocyanins and each other, increasing tannin polymer size and complexity.
Two major objectives of modern viticultural practices include the ability to produce a uniformly ripe crop and to harvest at optimal grape maturity. Large variations in ripening among berries within a cluster and within a vineyard make it difficult to determine when a crop is at its best possible ripeness. The start of véraison is recognized to be a critical determinant for berry harvest dates, yet little is known about what initiates this important stage. A more detailed understanding of the complex changes in gene expression that orchestrate berry developmental processes is needed.
Several mRNA expression-profiling studies have been completed for Vitis berries. Differential screening of cDNA libraries from (Vitis vinifera cv. Shiraz) and northern blot analysis revealed that large differences in gene expression occur during berry ripening and led to the isolation of a large number of grape ripening-induced protein (GRIP) genes [16]. Monitoring of gene expression profiles in flowers and across six time points during grape (Vitis vinifera cv. Shiraz) berry skin development to 13 weeks post-flowering resolved four sets of genes with distinctive and similar expression patterns using spotted cDNA microarrays containing 4,608 elements [17]. mRNA expression was also studied across nine stages of wildtype cv. Shiraz berry development (green "pea" to overripe) [18] and in a fleshless berry mutant cv. Ugni Blanc using oligonucleotide microarrays containing 3,200 elements [19]. Differences in transcript expression profiles in the skin of ripening fruit (12 to 13 weeks after flowering) of seven different cultivars were also examined using a 9,200 feature cDNA microarray [20]. In this study, we conducted mRNA expression profiling on one of the widely grown varieties of V. vinifera (cv Cabernet Sauvignon) using the Vitis Affymetrix GeneChip® oligonucleotide microarray ver. 1.0, which contains 14,470 Unigenes, over seven temporal stages (green "pea" to ripe) of berry development. We also correlated specific transcript profiles with specific metabolite profiles to gain deeper insights into discrete aspects of grape berry developmental dynamics.
Results and discussion
Grape berry development
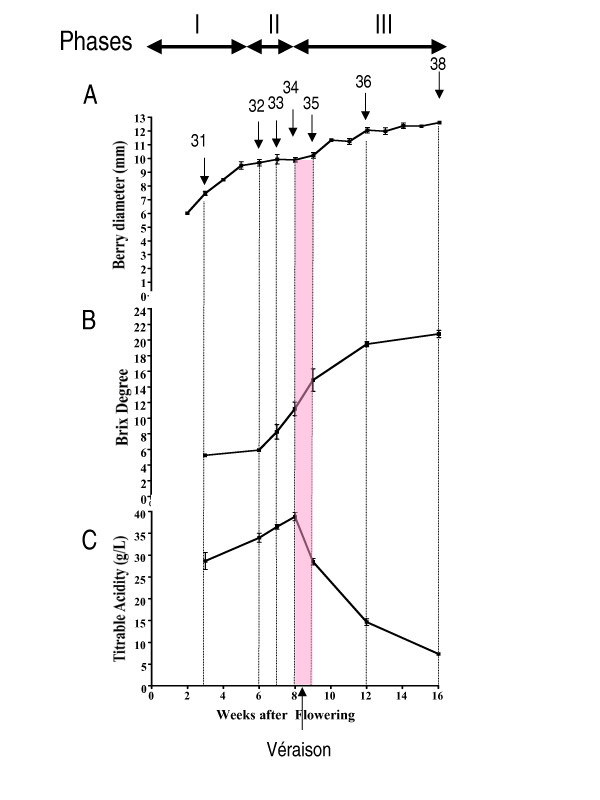
Vitis vinifera cv. Cabernet Sauvignon grapes were harvested on a weekly basis over the course of berry development from the Shenandoah Vineyard, Plymouth, California during the summer of 2004. Samples corresponding to stages 31 to 38 of the modified E-L system [14] were measured for berry diameter, °Brix (an approximate measure of the mass ratio of dissolved solids, mostly sucrose, to water in fruit juices) and titratable acidity (Figure 1). Berry diameter increased over time with a classical double sigmoid pattern (Figure 1A). Average berry diameter increased during the first 7 weeks of development (E-L stage 31), followed by a cessation of berry expansion at 7 to 8 weeks post-anthesis (E-L stages 32 to 34), and then the increase in berry diameter resumed until maturity (E-L stages 35 to 38). °Brix increased 6 weeks post-anthesis to a peak value of 22 °Brix at 16 weeks post-anthesis (Figure 1B). In contrast, titratable acidity (g/L), which reflects acid accumulation (mainly tartaric and malic acid), increased steadily up to 8 weeks post-anthesis and then sharply declined at the start of véraison between E-L stages 34 and 35 reaching approximately 7 g/L of titratable acids at harvest (Figure 1C).
Figure 1.
Physiological data at different stages of berry development. Changes in physiological parameters measured during the major phases (I to III) of berry development and ripening of Cabernet Sauvignon grape berries. A, Berry Diameter (n = 6); B, Brix degree (°) or total soluble solids in the berry juice (n = 6); C, Titratable Acidity (g/L) (n = 6). The stage at which véraison occurs is indicated in pink. Numbers with arrows point to the individual developmental stages defined by the E-L system Coombe [14] used for transcriptome profiling.
Microarray analysis
The mRNA expression profiles of seven time points spanning E-L stages 31 to 38 as indicated in Figure 1 were compared using the Affymetrix GeneChip® Vitis genome array ver. 1.0. Testing was performed using biological triplicates for each time point. Multiple time points within Stage II (E-L stages 32 to 35) were sampled due to the large number of biochemical changes expected to occur around véraison that affect berry ripening and fruit quality. A visual inspection of the distributions of raw perfect match (PM) probe-level intensities for all 21 arrays showed that the pre-processed and normalized PM intensities using Robust Multi-Array Average (RMA) [21] were consistent across all arrays. Digestion curves describing trends in RNA degradation between the 5' end and the 3' end in each probe set were examined and all 21 proved very similar [Additional File 1A,B]. Correlations among biological replicates were good: Spearman coefficients ranged from 0.977 to 0.997; Pearson coefficients ranged between 0.977 and 0.996.
From the Vitis 16,602 probesets represented on the array [Additional File 1C], an overall mean call rate of 74.5% per array (range 73.5% to 76.2%) was obtained. Data from the 12,596 probe sets that were found to be present in at least two out of the three biological triplicates were retained for further analyses. After performing an ANOVA and a multiple testing correction (Benjamini and Hochberg) [22], we found that 10,068 probesets (60.6%) were differentially expressed (p ≤ 0.05) between two or more E-L stages of berry development [Additional File 2: Table 1]. Because one Unigene can be related to several probesets, the number of Unigenes decreased to 9,143 Unigenes [Additional File 2: Table 2]. These probesets will be hereby referred to as those passing the ANOVA filter. From this set of genes, we extracted a subset of 4,510 probesets that displayed a two-fold or greater change in steady-state transcript abundance over the course of development (i.e., across any two of the seven developmental stages) [Additional File 2: Table 3] representing 4,151 Unigenes (28.3%) in the DFCI Grape Gene Index database VvGI5 [23]. We refer to this subset of genes as the two-fold ratio (TFR) set [Additional File 2: Table 4].
Principal component analysis (PCA), was used to simplify and define associations between different developmental stages within the global transcriptomic data (Additional File 3). Two principal components explaining 97.4% of the overall variance of transcription profiles (86.8% and 7.6% for axes 1 and 2, respectively) allowed us to clearly differentiate E-L stages 31 and 35 from the other developmental stages analyzed (Additional File 3). It was not possible to clearly separate E-L stages 32 to 34 or 36 to 38 indicating that the transcription patterns occurring at these stages were similar to one another. However, stage 35, which corresponds to early post-véraison, could be distinguished suggesting that transcription patterns at this point in berry ripening are unique to this critical stage in berry development. Further analysis using a third axis explaining 2.7% of the overall variance, confirmed the previous results and slightly improved the resolution among stages 31, 35, and 36 to 38.
Clustering of significant genes
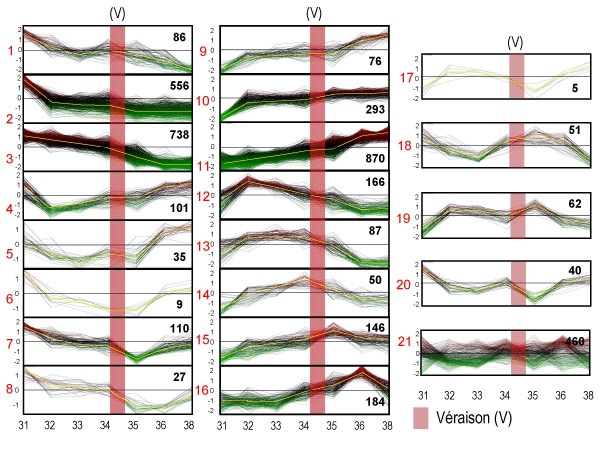
We used the Pavlidis Template Matching (PTM) algorithm [24], to divide the 4,151 TFR Unigenes into twenty gene groups or clusters. Specifically, twenty gene profiles of interest were selected [Additional File 4] to reflect major transcriptional patterns of development across E-L stages 31 to 38 (Figure 2). The PTM algorithm then classified the gene profiles into twenty groups via measurements of Pearson correlation: a correlation coefficient of greater than 0.75 was used to determine cluster membership. Six profiles showed a steady decline (profile groups 1 to 3) or increase (profile groups 9 to 11) in steady-state transcript abundance over time with distinctly different slopes. These six profile groups encompassed 63% of the Unigenes with a majority expressed in profiles 2 and 3 (31.9%) and profiles 9 and 11 (28%; Figure 2). Eight profiles had transient peak increases (profile groups 4 to 8) or decreases (profile groups 12 to 16) in transcript abundance at each of E-L stages 32 to 36. These transient profiles accounted for 22% of the Unigenes. A majority (68.2%) of these transiently expressed genes (profile groups 4 to 8 and 12 to 16) exhibited increased transcript abundance with the highest proportion within profile group 16 (E-L stage 36), followed closely by profile group 15 (E-L stage 35 around véraison), and profile group 12 (E-L stage 32) (Figure 2). Interestingly, genes with transient decreases early in berry development (profile groups 4 and 5) also exhibited large increases in transcript abundance during the later stages (E-L stages 36 to 38). The last four profiles (profile groups 17–20) were selected as having two peaks of expression between E-L stages 32 and 36 (Figure 2). Approximately 4.3% of transcripts had such "up and down" expression patterns (profile groups 17–20). Finally, Unigenes that did not match one of these profiles were grouped into a 21st cluster (Figure 2), accounting for 11% of the total transcripts considered (profile group 21). Taken together, this analysis revealed that berry development is not only a progressive process, wherein the majority of genes exhibit a steady increase or decrease in expression across all stages of development (profile groups 1 to 3 and 9 to 11), but also a dynamic process, wherein a large number of genes exhibit large, transient changes in transcript abundance at specific times of development. Most notably, the last phase of berry development (Phase III, profile groups 14, 15 and 16) was the time when the largest number of genes (380 transcripts or 9.1%) exhibited transient increases in steady-state transcript abundance.
Figure 2.
Twenty-one profiles of steady-state transcripts exhibiting a two-fold or greater expression across berry development. Profiles are plotted as RMA data values plotted on the log2 scale centered by the mean of all values (Stage 31 to stage 38). E-L Stages are indicated along the X-axis. Profiles numbers are indicated with red numbers with the number of transcripts within each profile indicated with black numbers: Véraison (V) is indicated with a pink stripe. The gradient red to green coloration of individual gene plots indicates values above or below the mean of the cluster, respectively. The cluster template profile is designated by a yellow line.
Functional categorization of Unigenes across different stages of development
Functional categories were assigned to Unigenes with two-fold or greater changes in steady-state transcript abundance over the course of the seven developmental stages using the Munich Information Center for Protein Sequences (MIPS, ver. 2.0) catalog with annotations of the top Arabidopsis BLAST hits [25]. Because we detected some errors in the functional annotation for some Unigenes, functional categorization of each Unigene were verified manually and corrected if necessary. Corrections were only performed for the 4,151 Unigenes that displayed a two-fold or greater change in expression [See Additional File 2: Table 4]. Functional annotations could be assigned to approximately 64% of transcripts (Figure 3A). An additional 23% of Unigenes had matches to genes with unknown functions or unclear classifications (unclassified), and 13% did not have a BLAST hit (no hit) in public, non-redundant (NR) databases. The relative distribution of Unigenes within each of nineteen functional categories was determined (Figure 3B). To facilitate a functional comparison of the three major stages of berry development, Unigenes from each of the profile groups were regrouped into the three major developmental phases to reflect the greatest degree of transcript abundance changes at each phase: Phase I (profiles 1, 2, and 3), Phase II (profiles 4, 5, 6, 12, 13, and 14), and Phase III (profiles 7, 8, 9, 10, 11, 15, and 16). Statistically significant differences in the distribution of genes within functional categories amongst these developmental stages were observed (Figure 3B; see Additional File 5: Tables 1 and 2). Functional categories that had a large number of transcripts in Phase I followed by a decrease in Phase III included biogenesis of cellular component (42), transport regulation (20), energy (2), and metabolism (1). This is consistent with the developmental aspects of this phase, which are characterized by cell division and expansion, which require a high level of metabolic activity. The process of cell division requires large quantities of structural materials and consumes energy, while cell expansion requires large quantities of solutes and water.
Figure 3.
Functional analyses of steady-state transcripts with a two-fold or greater change in abundance over the course of berry development. A) Percentage of annotated unigenes with a two-fold or greater change in transcript abundance. B) Distribution of Unigenes according to their MIPS functional categories (MIPS 2.0) within the three main phases of berry development. Phase I (E-L stage 31), herbaceous phase; Phase II (E-L stages 32 to 34), lag phase; Phase III (E-L stages 35 to 38), ripening phase. Statistically significant differences between Phase I against II are indicated with white squares. Statistically significant differences between Phase II against III are indicated with black squares. Statistically significant differences between Phase I against III are indicated with asterisks. Percentages are based upon the number of Unigenes in each set. Numbers in parentheses following category names indicates the MIPS number for each category.
The opposite trend of increasing transcript abundance from Phase I to Phase III was observed for functional groups that included transcription (11), protein synthesis (12), protein fate (14), protein with binding function (16), and to a lesser extent with interaction with cellular environment (34). These trends served to further indicate the complexity of the transcriptional, translational, and interaction-based regulatory processes necessary for berry development.
Exemplar Unigenes associated with important molecular events of berry development
In order to identify genes with potentially important roles in specific stages of berry development, transcripts with a dynamic pattern were identified from within the first 20 PTM algorithm-defined profile groups. The transcript profiles were examined in further detail (Figure 4).
Figure 4.
Transcripts displaying transient expression patterns. Each value plotted is the mean normalized intensity values obtained for the three biological replicates. The three key phases of the berry development (I, II, III) were applied as reference. A) Black solid round (1618814_at, NP864096)-ornithine decarboxylase, red solid triangle (1616399_s_at, CB005833)-arginine decarboxylase, green solid triangle (1611257_a_at, TC51832)-L-asparaginase, blue solid diamond (1618848_at, TC52577)-xyloglucan endotransglycosylase transferase. B) Black solid round (1608074_s_at, TC62965)-α-expansin, red solid triangle (1608191_at, TC64448) α-expansin, green solid triangle (1613161_at, TC69794)-limonene cyclase, blue solid diamond (1618595_at, TC53841)-(-)-isopiperitenol dehydrogenase.
Polyamines (PAs) are a class of compounds that have plant growth regulator activity. Their roles in cell division [26] and fruit set [27] have been widely investigated. Free, conjugated and wall-bound forms of polyamines accumulate mostly at anthesis before decreasing at fruit set in grapes [28]. Two transcripts were detected that belong to profile 4, which are strongly down-regulated at E-L stage 32 (1618814_at, 1616399_s_at; AY174164, TC68466). Both are related to ornithine decarboxylase and arginine decarboxylase, which are involved in polyamine metabolism [29]. These two genes located at the start of the PA pathway might play a role in providing precursors that would be used during Phase I of berry development.
In higher plants, the catabolism of asparagine (Asn) occurs by two routes. The first pathway involves the hydrolysis of Asn, releasing ammonia and aspartate by asparaginase activity. L-asparaginase is one of the enzymes for Asn utilization by plants that plays an important role in the nitrogen metabolism of developing plant tissues [30]. One Unigene encoding L-asparaginase (1611257_a_at; TC51832) displayed a specific peak during E-L stages 32 (Figure 4A). This last result indicates that this enzyme could play a role during the first phase of berry development as a provider of ammonia for de novo protein synthesis in grape. This result is also supported by the significant transcript abundance of Unigenes encoding glutamate dehydrogenase or glutamine synthetase (data not shown, see Additional File 2: Table 4, 1607579_at, 1613697_at, 1609819_s_at) during the first phase of berry development. These enzymes participate in nitrogen assimilation in plants [31].
In grape berries, fruit softening occurs during Phase III and is largely affected by cell-wall loosening [32] and turgor [33]. Xyloglucans account for about 10% of the cell wall composition in berries [32]. In fruit, xyloglucan depolymerization is associated with fruit softening [34]. Xyloglucan endotransglycosylases, which hydrolyze and transglycosylate xyloglucans, were encoded by multiple isogenes, the majority of which were expressed highly during Phase I in berry development (E-L stage 31), but then declined (data not shown; see Table 1). One Unigene (1618848_at; TC52577), however, which is a xyloglucan endotransglucosylase/hydrolase, displayed a 185-fold increase in expression during Phase II, peaking at E-L stage 33 (Figure 4A). This xyloglucan endotransglucosylase Unigene is closely related to a xyloglucan endotransglucosylase/hydrolase (SIXTH5) that can act reversibly. It has been characterized recently as a tomato xyloglucan depolymerase in vitro in the presence of xyloglucan oligosaccharides (XGOs) [35].
Table 1.
Transcripts (TFR pool) related cell wall metabolism categorized by the first hit in the MIPS2 catalog
| Probeset ID | GenBank Annotation | VvGI5 | UniProt ID | Gene Name Description | Function | Profile | Fold Change |
| 1622791_at | CB973455 | TC56114 | Q6J8X2 | Cellulose synthase | Cell Wall Biosynthesis | 2 | 112.68 |
| 1619280_at | CF211860 | TC59569 | Q6J8W9 | Cellulose synthase | Cell Wall Biosynthesis | 2 | 87.25 |
| 1613018_at | CB971117 | TC61561 | Q851L8 | Cellulose synthase | Cell Wall Biosynthesis | 2 | 6.92 |
| 1606646_at | CA812296 | TC56773 | Q6XZC2 | Cellulose synthase | Cell Wall Biosynthesis | 2 | 4.34 |
| 1615577_at | CB340193 | TC52068 | Q6XP46 | Cellulose synthase | Cell Wall Biosynthesis | 3 | 3.5 |
| 1607069_at | CB982496 | TC53451 | Q45KQ0 | Cellulose synthase | Cell Wall Biosynthesis | 10 | 3.18 |
| 1611149_at | BM437543 | TC56091 | Q3Y6V1 | Cellulose synthase | Cell Wall Biosynthesis | 21 | 2.85 |
| 1612999_at | CF513786 | - | O80890 | Cellulose synthase | Cell Wall Biosynthesis | 7 | 2.76 |
| 1620206_at | CF515519 | TC66132 | Q6FD0 | β 1,4-Mannan synthase | Cell Wall Biosynthesis | 15 | 2.69 |
| 1616808_at | CF207742 | TC57597 | Q45KQ0 | Cellulose synthase | Cell Wall Biosynthesis | 21 | 2.21 |
| 1619938_at | CF514664 | TC63356 | Q6YBV2 | Cellulose synthase | Cell Wall Biosynthesis | 3 | 2.19 |
| 1620840_at | CB968965 | TC53122 | Q4F8K2 | α-expansin | Cell Wall Expansion | 2 | 20.26 |
| 1619010_s_at | BQ794765 | TC54832 | Q84US9 | Expansin | Cell Wall Expansion | 10 | 18.54 |
| 1608191_at | CD798831 | TC64448 | Q49QW6 | Expansin | Cell Wall Expansion | 21 | 13.18 |
| 1612253_at | CB970527 | TC62108 | Q6RX68 | Expansin | Cell Wall Expansion | 3 | 9.23 |
| 1608074_s_at | CF215793 | TC62965 | Q84UT0 | Expansin | Cell Wall Expansion | 21 | 6.28 |
| 1610418_at | BQ798078 | TC67284 | Q8GZD3 | Expansin | Cell Wall Expansion | 10 | 4.9 |
| 1613527_at | CB978490 | TC53065 | Q6T5H5 | Expansin | Cell Wall Expansion | 15 | 4.6 |
| 1618121_at | CF213691 | - | Q9LUI1 | Extensin | Cell Wall Expansion | 2 | 4.1 |
| 1608504_at | BQ797231 | TC52168 | Q6K4C6 | Expansin | Cell Wall Expansion | 4 | 2.56 |
| 1612154_at | CB970048 | TC61667 | O50044 | KDO 8-P synthase | Cell Wall Expansion | 3 | 2.31 |
| 1609651_at | CF404678 | TC55463 | Q9LJX2 | Pectinesterase inhibitor | Cell Wall Modification | 2 | 690.87 |
| 1618848_at | CB977336 | TC52577 | Q9ZRV1 | Xyloglucan endotransglycosylase 1 | Cell Wall Modification | 13 | 184.42 |
| 1622288_at | CB974798 | TC59058 | Q9M660 | Cell-Wall P4 | Cell Wall Modification | 2 | 124.19 |
| 1617556_s_at | BQ797260 | TC67257 | Q9M4I1 | Proline-rich cell wall protein | Cell Wall Modification | 10 | 105.38 |
| 1619762_at | CF214586 | TC67718 | Q7Y250 | Arabinogalactan protein | Cell Wall Modification | 2 | 61.79 |
| 1620201_at | CB972625 | TC70982 | Q53WM8 | Pectinesterase | Cell Wall Modification | 2 | 42.57 |
| 1619519_at | CB971445 | TC65487 | Q7Y250 | Arabinogalactan protein | Cell Wall Modification | 2 | 39.53 |
| 1616045_a_at | AJ237983 | - | Q9M4I0 | Proline-rich cell wall protein | Cell Wall Modification | 11 | 38.03 |
| 1617023_at | CF210510 | TC53552 | FLA1 | Arabinogalactan protein | Cell Wall Modification | 3 | 37.53 |
| 1611601_at | CB977009 | TC57247 | Q6ZDX2 | Pectinesterase | Cell Wall Modification | 2 | 34.83 |
| 1619613_at | CD801720 | TC68597 | Q9SAP3 | Proline-rich protein | Cell Wall Modification | 2 | 34.56 |
| 1616528_s_at | CD801342 | TC55188 | Q1SAY6 | Proline-rich protein | Cell Wall Modification | 2 | 33.73 |
| 1621880_s_at | CK138206 | TC66098 | Q8VZG5 | β-xylosidase | Cell Wall Modification | 3 | 31.38 |
| 1608727_at | CB973483 | TC56396 | Q9LZX4 | Fasciclin arabinogalactan protein 10 | Cell Wall Modification | 3 | 30.04 |
| 1615533_s_at | CF415374 | TC51824 | Q7Y252 | Endo-xyloglucan transferase | Cell Wall Modification | 3 | 27.95 |
| 1622481_x_at | CF568921 | TC67150 | Q39763 | Proline-rich protein | Cell Wall Modification | 1 | 27.63 |
| 1614426_at | CD801116 | TC64184 | Q4F8J3 | Xyloglucan endotransglycosylase | Cell Wall Modification | 3 | 25.94 |
| 1619522_at | AY043231 | TC56838 | Q94B17 | β-galactosidase | Cell Wall Modification | 3 | 24.53 |
| 1622292_at | CF403386 | TC69174 | Q949Z1 | polygalacturonase | Cell Wall Modification | 2 | 24.24 |
| 1622295_at | CF215662 | TC68541 | Q5CCP8 | β-galactosidase | Cell Wall Modification | 3 | 24.1 |
| 1621477_s_at | CF215974 | TC67884 | Q9LYT5 | Pectinesterase | Cell Wall Modification | 1 | 23.62 |
| 1622121_at | BQ799039 | TC58094 | Q4F8J0 | Cellulase | Cell Wall Modification | 3 | 22.85 |
| 1615201_at | CF512517 | TC63907 | Q96232 | Proline-rich-like protein | Cell Wall Modification | 3 | 22.42 |
| 1618657_at | CF211626 | TC56055 | Q84LI7 | Exopolygalacturonase | Cell Wall Modification | 2 | 20.99 |
| 1616158_at | CD801717 | TC53176 | Q4JLV6 | Pectate lyase | Cell Wall Modification | 21 | 20.15 |
| 1612239_at | CF610039 | TC55421 | Q8VZG5 | β-xylosidase | Cell Wall Modification | 2 | 19.96 |
| 1620140_at | CF208989 | TC53499 | Q40161 | Polygalacturonase | Cell Wall Modification | 2 | 19.6 |
| 1611747_at | CF608890 | TC65113 | Q7XAS3 | β-D-glucosidase | Cell Wall Modification | 3 | 17.94 |
| 1609909_s_at | CF206328 | TC64184 | Q4F8J3 | Xyloglucan Endotransglycosylase | Cell Wall Modification | 3 | 15.14 |
| 1608313_at | CF209144 | TC52275 | Q76MS4 | β-xylosidase | Cell Wall Modification | 2 | 14.41 |
| 1615574_at | CB977067 | TC56317 | Q9M5J0 | Pectinesterase | Cell Wall Modification | 1 | 14.03 |
| 1619612_at | CF211611 | TC67414 | Q94KD8 | β-xylosidase | Cell Wall Modification | 2 | 13.83 |
| 1610073_at | CF206157 | TC51796 | Q8S902 | Xyloglucan Endotransglycosylase | Cell Wall Modification | 3 | 13.77 |
| 1621251_s_at | BQ795002 | TC69305 | Q8W3L8 | Xyloglucan Endotransglycosylase 2 | Cell Wall Modification | 10 | 13.64 |
| 1622735_s_at | CB340122 | TC51796 | Q84JX3 | Xyloglucan Endotransglycosylase | Cell Wall Modification | 3 | 13.47 |
| 1613844_at | CF404099 | TC54968 | Q9LUG8 | Endo-1,3-1,4-β-D-glucanase | Cell Wall Modification | 3 | 13.27 |
| 1617755_at | CF213513 | TC52924 | Q8GSQ4 | Pectin-glucuronyltransferase | Cell Wall Modification | 2 | 12.86 |
| 1615746_at | CB970034 | TC53433 | Q9FXI9 | Endo-1,4-β-glucanase | Cell Wall Modification | 3 | 11.99 |
| 1617785_at | CD800122 | TC54681 | Q9LW90 | Pectinesterase | Cell Wall Modification | 3 | 11.97 |
| 1607374_at | CF404162 | TC69448 | Q7XAS3 | β-D-glucosidase | Cell Wall Modification | 3 | 11.77 |
| 1610311_at | CF373485 | TC52429 | Q41725 | Arabinogalactan protein | Cell Wall Modification | 3 | 10.99 |
| 1620096_at | CF372841 | TC57673 | Q4F986 | Xyloglucan endotransglycosylase | Cell Wall Modification | 2 | 10.81 |
| 1616093_at | CF404665 | TC69415 | Q7XA92 | Pectinesterase | Cell Wall Modification | 3 | 10.36 |
| 1613467_at | CF212805 | TC54247 | Q9FSW6 | Arabinogalactan protein | Cell Wall Modification | 15 | 10.08 |
| 1617875_at | CB971740 | TC61493 | O04477 | β-N-acetylhexosaminidase | Cell Wall Modification | 3 | 9.73 |
| 1614803_at | AY046416 | TC70108 | Q8LGR6 | Proline-rich protein | Cell Wall Modification | 3 | 9.22 |
| 1616822_at | AF220196 | TC70108 | Q8LGR6 | Proline rich protein | Cell Wall Modification | 3 | 9.04 |
| 1610756_at | CF604824 | TC55088 | Q9LT39 | Polygalacturonase inhibitor | Cell Wall Modification | 1 | 8.2 |
| 1622591_at | CB981129 | TC70200 | Q9FHN6 | Pectinesterase | Cell Wall Modification | 2 | 8.06 |
| 1612672_at | CF215975 | TC62593 | Q9SEE7 | Pectinesterase | Cell Wall Modification | 2 | 7.61 |
| 1616522_at | CF403905 | TC55346 | Q9LEB0 | Pectinesterase | Cell Wall Modification | 2 | 7.51 |
| 1615198_at | CF209943 | TC65883 | Q9LEC9 | β-xylosidase | Cell Wall Modification | 3 | 7.48 |
| 1608756_at | BQ798436 | TC59719 | Q84LI7 | Polygalacturonase | Cell Wall Modification | 2 | 7.15 |
| 1609790_at | CF207994 | TC55069 | Q4F8J3 | Xyloglucan endotransglycosylase | Cell Wall Modification | 2 | 6.87 |
| 1614877_at | CB002982 | TC66230 | Q9C8T5 | Proline-rich protein | Cell Wall Modification | 2 | 6.78 |
| 1613330_at | CF404655 | - | Q93Z77 | Pectate lyase | Cell Wall Modification | 3 | 6.64 |
| 1608120_at | CF603941 | TC70545 | Q6U6I9 | Pectate lyase | Cell Wall Modification | 2 | 6.6 |
| 1613677_at | CB969707 | TC51953 | Q6J192 | Arabinogalactan protein | Cell Wall Modification | 2 | 6.16 |
| 1619383_s_at | BQ794831 | TC66587 | Q5CCQ0 | β-galactosidase | Cell Wall Modification | 3 | 6.14 |
| 1615603_at | CB346190 | TC64570 | Q8VY93 | Proline-rich protein | Cell Wall Modification | 3 | 5.75 |
| 1608180_at | CF201469 | TC68224 | O23950 | Endo-xyloglucan transferase | Cell Wall Modification | 2 | 5.51 |
| 1609593_at | CB981468 | TC68226 | Q9LZV3 | (1-4)-β-mannan endohydrolase | Cell Wall Modification | 15 | 5.49 |
| 1621225_at | CB974537 | TC52140 | Q9SUP5 | Polygalacturonase | Cell Wall Modification | 21 | 5.12 |
| 1613415_at | AB074999 | TC45132 | Q8W3L8 | Xyloglucan endotransglycosylase 1 | Cell Wall Modification | 10 | 5.1 |
| 1615995_at | CF212592 | - | P24806 | Xyloglucan Endotransglucosylase 24 | Cell Wall Modification | 21 | 5.02 |
| 1615809_at | CB980277 | TC69342 | Q38908 | Xyloglucan endotransglucosylase 30 | Cell Wall Modification | 11 | 4.87 |
| 1613719_at | CF214562 | TC69710 | Q7Y250 | Arabinogalactan protein | Cell Wall Modification | 2 | 4.8 |
| 1613528_at | CF513262 | TC66769 | Q8LPS9 | Pectinesterase | Cell Wall Modification | 2 | 4.56 |
| 1612668_at | CF519076 | TC61610 | Q5CHL3 | Hydroxyproline-rich glycoprotein | Cell Wall Modification | 21 | 4.32 |
| 1620063_at | CB921343 | TC61082 | Q9M3U4 | β-1-3 glucanase | Cell Wall Modification | 11 | 4.3 |
| 1611233_at | CF605724 | TC66632 | Q4W7I3 | β-xylosidase | Cell Wall Modification | 3 | 4.18 |
| 1622770_at | CF209970 | TC66250 | O65186 | Cellulase | Cell Wall Modification | 13 | 4.15 |
| 1609653_at | BQ797078 | TC70494 | Q9SBM1 | Hydroxyproline-rich glycoprotein | Cell Wall Modification | 10 | 4.15 |
| 1620618_at | BQ794587 | TC55377 | Q8LAB2 | Proline-rich protein | Cell Wall Modification | 2 | 3.59 |
| 1608799_at | BQ800204 | TC58800 | Q4ABV3 | Pectinesterase | Cell Wall Modification | 3 | 3.55 |
| 1616523_s_at | CF512513 | TC63963 | Q8L9T8 | Arabinogalactan protein | Cell Wall Modification | 1 | 3.53 |
| 1606652_at | CB969544 | TC52628 | Q8H1N7 | Polygalacturonase | Cell Wall Modification | 2 | 3.52 |
| 1622353_at | BQ800489 | TC51768 | Q5TIN5 | β-6-xylosyltransferase | Cell Wall Modification | 3 | 3.41 |
| 1619659_s_at | CF405842 | TC68391 | A1IIA8 | Pectate lyase | Cell Wall Modification | 14 | 3.37 |
| 1617920_at | CF609275 | TC52380 | Q6QLN2 | Endo-1,4-β-glucanase | Cell Wall Modification | 2 | 3.31 |
| 1608896_at | BQ796455 | TC59657 | Q5BM98 | Secondary cell wall-related glycosyltransferase | Cell Wall Modification | 4 | 3.24 |
| 1618849_at | BQ799201 | TC63732 | Q9SUP5 | Polygalacturonase | Cell Wall Modification | 21 | 3.16 |
| 1610996_at | BQ794786 | TC63941 | Q43111 | Pectinesterase 3 | Cell Wall Modification | 14 | 3.15 |
| 1615125_at | CF372050 | TC67073 | Q5BM97 | Secondary cell wall-related glycosyltransferase family 14 | Cell Wall Modification | 2 | 3.09 |
| 1608945_at | BQ793580 | TC54729 | P35694 | Xyloglucan endotransglycosylase | Cell Wall Modification | 15 | 3.09 |
| 1607567_at | BQ795116 | TC54314 | Q564G6 | Galactomannan galactosyltransferase | Cell Wall Modification | 11 | 3.06 |
| 1619068_at | CF215954 | TC60314 | Q94B11 | Xyloglucan endotransglycosylase | Cell Wall Modification | 3 | 2.78 |
| 1612425_at | CF371700 | TC56348 | Q6EP64 | Hydroxyproline-rich glycoprotein | Cell Wall Modification | 11 | 2.77 |
| 1616826_at | CB976610 | TC54888 | Q599J2 | β-1,2 Xylosyltransferase | Cell Wall Modification | 11 | 2.76 |
| 1609138_at | CF519079 | TC66620 | Q16861 | Super cysteine rich protein | Cell Wall Modification | 11 | 2.74 |
| 1617487_at | CD720403 | TC54500 | Q9SFF6 | Pectinacetylesterase | Cell Wall Modification | 2 | 2.69 |
| 1617687_at | CB981123 | TC57577 | Q494P2 | Xyloglucan endotransglycosylase 2 | Cell Wall Modification | 21 | 2.67 |
| 1606832_at | CF214798 | TC51861 | Q7Y223 | (1-4)-β-mannan endohydrolase | Cell Wall Modification | 2 | 2.58 |
| 1617712_at | CF607664 | TC67150 | Q39789 | Proline-rich cell wall protein | Cell Wall Modification | 2 | 2.52 |
| 1617919_at | CF605842 | TC55276 | Q9SHZ2 | β-1,3-glucanase | Cell Wall Modification | 18 | 2.4 |
| 1617015_at | CF209172 | TC54616 | Q7XRM8 | Pectate lyase | Cell Wall Modification | 2 | 2.34 |
| 1618863_at | CF208339 | TC52953 | Q93Y12 | α-glucosidase | Cell Wall Modification | 3 | 2.28 |
| 1617939_s_at | CB910883 | TC52435 | Q41695 | Pectinacetylesterase | Cell Wall Modification | 1 | 2.28 |
| 1616734_at | CF405846 | TC52115 | Q6ZIF8 | Pectin-glucuronyltransferase | Cell Wall Modification | 3 | 2.28 |
| 1607945_at | AF243475 | - | Q9M505 | Pectate lyase | Cell Wall Modification | 2 | 2.27 |
| 1612551_at | CF605967 | TC63126 | Q9M3C5 | β-N-acetylhexosaminidase | Cell Wall Modification | 21 | 2.26 |
| 1622843_s_at | CF212102 | TC65557 | Q9LVC0 | Arabinogalactan protein | Cell Wall Modification | 4 | 2.25 |
| 1611230_at | AF159124 | - | Q9XGT3 | β-galactosidase | Cell Wall Modification | 2 | 2.25 |
| 1619468_at | AY043232 | TC38735 | Q94B16 | Pectin methylesterase PME1 | Cell Wall Modification | 12 | 2.24 |
| 1610118_at | CB974025 | TC60557 | O23562 | Glucanase | Cell Wall Modification | 18 | 2.21 |
| 1614868_at | CB920940 | TC64720 | Q9M0S4 | Arabinogalactan protein | Cell Wall Modification | 5 | 2.17 |
| 1607528_at | AY043236 | TC61627 | Q94B12 | Cellulase CEL1 | Cell Wall Modification | 21 | 2.11 |
| 1614814_s_at | CB345895 | TC57381 | O24136 | CP12 precursor | Cell Wall Modification | 13 | 2.07 |
Expansins play important roles in cell wall loosening via non-enzymatic mechanisms and are involved in cell expansion [36]. Most expansin genes displayed steadily increasing or decreasing patterns during berry development (see Table 1). Others showed peak expression around E-L stage 34 (α-expansin, 1608074_at, TC62965; α-expansin, 1608191_at, TC64448; Figure 4B). An expansin gene from strawberry, FaExp4, displays exactly the same peak transient expression pattern as these latter two genes at a comparable ripening stage as grape berries, called the White stage in strawberry fruits, just before red fruit color development [37]. Thus, these expansins in grape berry may be required during the Phase III of grape berry development, when the second phase of cell expansion occurs.
Terpenes, which are precursors for important aroma compounds [38], accumulate at véraison [39,40]. One Unigene encoding a limonene cyclase (1613161_at; TC69794; Figure 4B), which is in the monoterpene pathway, is involved in the conversion of geranyl diphosphate into limonene [41]. Limonene and some of its derived compounds such as menthol or 1,8 cineol are intimately associated with the "eucalyptus fragrance" of red wine [42]. Accumulation of 1,8-cineole in wines is derived from precursors in grape, like limonene. The strong induction of our Unigene related to limonene cyclase (~40 fold from E-L stages 32 to 34) correlates well with the beginning of accumulation of 1,8-cineole in red grape samples [43]. One Unigene (1618595_at, TC53841; Figure 4B) belonging to profile 15 and encoding alcohol dehydrogenase exhibited strong homology with an (-) isopiperitenol dehydrogenase, which is involved in the same monoterpene pathway [44]. This transcript abundance of this Unigene is correlated to the expression of the limonene cyclase previously discussed above indicating a possible activation of these enzymes in the same metabolic pathway [44].
Phytohormone biosynthesis and responses
A number of plant growth regulators including abscisic acid (ABA), auxin (indole-3-acetic acid [IAA], brassinosteroids (BR), ethylene, and gibberellic acid (GA) have been implicated in the control of berry development and ripening. Therefore, steady-state transcript accumulation patterns of Unigenes with functions related to hormone biosynthesis and response were tracked over the course of berry development (Figure 5, Table 2).
Figure 5.
Expression of phytohormone transcripts. A) Black solid round (1608022_at, TC57089)-NCED isoform 1, red solid triangle (1607029_at, TC55541)-NCED isoform 4, green solid triangle (1614892_at, TC54474)-ABI1 protein phosphatase type 2C, blue solid diamond (1619802_at, TC67323)-RD22, orange solid square (1621346_at, TC65114)-ABI3 transcription factor. B) Black solid round (1617012_at, TC68057)-ethylene responsive factor 1, red solid triangle (1619585_at, TC62897)-ethylene induced transcription factor, green solid triangle (1621552_at, TC66829)-ethylene co-activator, blue solid diamond (1615952_s_at, TC56709)-aminocyclopropane carboxylic acid synthase, orange solid square (1622402_at, TC62349)-ERS1 ethylene receptor, lavender open square (1618518_at, TC55908)-EIN4/ETR5 ethylene receptor. *: transcript that does not pass the two-fold ratio. C) Black solid round (1617572_at, TC66046)-BRH1 brassinosteroid-responsive protein, red solid triangle (1612516_at, TC56501)-BRI1 brassinosteroid-responsive protein, green solid triangle (1619068_at, TC60314)-brassinosteroid-responsive protein, blue solid diamond (1608945_at, TC54729)-BRU1 brassinosteroid-responsive protein. *: transcript that does not pass the two-fold ratio. Black solid round (1618181_at, TC67464)-GIDL1 receptor, red solid triangle (1620071_at, TC56624)-GIDL2 Receptor, green solid triangle (1606777_s_at, TC56894)-GA1a gibberellin oxidase, blue solid diamond (1610610_at, TC66284)-gibberellic acid β hydroxylase. *: transcript that does not pass the two-fold ratio. E) Black solid round (1614660_at, TC53887)-auxin responsive protein (Aux22), red solid triangle (1613813_a_at, TC65541)-auxin responsive factor 2, green solid triangle (1609591_at, TC63193)-small auxin up RNA protein, blue solid diamond (1606566_at, TC62299)-SAUR protein, orange solid square (1616225_at, TC52772)-auxin responsive factor 18, lavender open square (1619610_at, TC56575)-IAA-amino acid hydrolase, brown open triangle (1611479_at, CD799903)-auxin transporter, pink open triangle (1617179_at, CF414958)-auxin efflux carrier, purple open diamond (1610034_at, TC59892)-auxin binding protein. F) Black solid round (1607601_at, TC61395)-12-oxophytodienoate reductase, red solid triangle (1614324_at, CF213899)-constitutive pathogen response 5 (CPR5), green solid triangle (1620306_at, TC69712)-cytokinin oxidase, blue solid diamond (1612955_at, TC52530)-Type-A response regulator.
Table 2.
Transcripts (TFR pool) related to phytohormone biosynthesis and response categorized by the first hit in the MIPS2 catalog
| Probeset ID | GenBank Annotation | VvGI5 | UniProt ID | Gene Name Description | Function | Profile | Fold Change |
| 1608022_at | BQ798105 | TC57089 | Q5SGD1 | 9-cis-epoxycarotenoid dioxygenase 1 | ABA biosynthesis | 15 | 6.46 |
| 1607029_at | CD716868 | TC55541 | Q8LP14 | 9-cis-epoxycarotenoid dioxygenase 4 | ABA biosynthesis | 15 | 3.85 |
| 1617541_s_at | CB342503 | TC54423 | O49814 | β-carotene hydroxylase 2 | ABA biosynthesis | 3 | 3.25 |
| 1618171_s_at | BQ792407 | TC55939 | Q5SGC9 | Zeaxanthin epoxidase | ABA metabolism | 3 | 2.36 |
| 1614788_at | BQ792954 | TC54112 | Q3ZNL4 | Dehydrin 1a | ABA response | 11 | 26.63 |
| 1609063_at | BQ799245 | TC63341 | Q4VT47 | RD-22 (ABA regulated) | ABA response | 3 | 11.28 |
| 1621346_at | CB978597 | TC65114 | O48620 | ABI3 (ABA regulated) | ABA response | 14 | 7.57 |
| 1614892_at | CF511230 | TC54474 | O82468 | Phosphatase 2C (ABA regulated) | ABA response | 11 | 5.87 |
| 1615970_at | CF405892 | TC65344 | Q7XAV5 | Dehydration responsive element binding protein | ABA response | 15 | 4.53 |
| 1616735_at | CF604749 | TC51916 | O82176 | Phosphate-induced protein (ABA regulated) | ABA response | 16 | 3.44 |
| 1607955_at | CB978189 | TC63879 | Q9ZST5 | PII protein (ABA regulated) | ABA response | 12 | 3.07 |
| 1621396_at | CF514715 | TC51970 | Q94IB2 | Phi-2 (ABA regulated) | ABA response | 16 | 2.9 |
| 1617417_s_at | CD798528 | TC61938 | Q9M3V0 | Phosphatase 2C (ABA regulated) | ABA response | 5 | 2.77 |
| 1617791_s_at | CB004910 | TC70554 | Q45W74 | Dehydration-induced protein (ABA regulated) | ABA response | 4 | 2.55 |
| 1609665_a_at | CB005515 | TC58443 | Q9M9W9 | Phosphatase-2C (ABA regulated) | ABA response | 20 | 2.25 |
| 1609419_at | CB982969 | CB982969 | Q9S7V4 | Abscisic acid-induced protein | ABA response | 15 | 2.23 |
| 1610937_at | BQ792881 | TC65459 | Q67WL5 | Abscisic acid-induced protein | ABA response | 18 | 2.2 |
| 1611714_at | BQ794807 | TC53528 | Q06009 | Phosphatase 2A (ABA regulated) | ABA response | 3 | 2.12 |
| 1616882_at | CD799018 | TC53254 | Q7Y0S8 | Phi-1 (ABA regulated) | ABA response | 3 | 2.1 |
| 1621041_at | BQ794656 | TC56829 | Q9FIE3 | Vernalization-insensitive protein 3 (ABA regulated) | ABA response | 21 | 2.08 |
| 1619261_s_at | CB982969 | TC68788 | Q5XWP1 | Abscisic acid-induced protein | ABA response | 21 | 2.05 |
| 1619272_at | CF373376 | TC51939 | Q94AL8 | Cold acclimation protein (ABA regulated) | ABA response | 3 | 2.02 |
| 1619610_at | CB008850 | TC56575 | Q84XG9 | IAA-amino acid hydrolase | Auxin metabolism | 11 | 5.86 |
| 1615645_at | CB969433 | TC62316 | Q8LCI6 | IAA-amino acid hydrolase | Auxin metabolism | 3 | 3.19 |
| 1606566_at | CF211641 | TC62299 | Q681Q1 | Auxin-induced protein | Auxin response | 2 | 12.05 |
| 1609591_at | CD799271 | TC63193 | O23089 | Auxin-regulated protein | Auxin response | 11 | 11.74 |
| 1614851_s_at | CB973279 | TC62879 | Q1RY17 | Auxin responsive Factor | Auxin response | 3 | 11 |
| 1620662_at | CB981820 | TC59676 | Q6QUQ3 | Auxin and ethylene responsive GH3 | Auxin response | 11 | 10.17 |
| 1614098_at | CF608417 | TC57853 | Q9FEL8 | AUX1 like protein (influx carrier) | Auxin response | 2 | 8.8 |
| 1606509_at | CB971327 | TC52521 | Q7XEJ9 | Auxin induced protein | Auxin response | 3 | 8.29 |
| 1616225_at | CB972698 | TC52772 | Q9C5W9 | Auxin response factor 2 | Auxin response | 1 | 7.3 |
| 1612060_at | CB346335 | TC53973 | Q76DT1 | AUX1 like protein (influx carrier) | Auxin response | 7 | 6.96 |
| 1616104_at | CB004955 | TC55019 | O65695 | Auxin-regulated protein | Auxin response | 15 | 6.11 |
| 1612001_s_at | CF604676 | TC69850 | Q9XEY0 | Nt-gh3 (auxin and ethylene) | Auxin response | 2 | 4.69 |
| 1617163_at | BQ800616 | TC56821 | Q9SHL8 | Auxin efflux carrier | Auxin response | 3 | 4.23 |
| 1617513_at | CF203551 | TC52262 | Q8LAL2 | Auxin-responsive protein IAA26 | Auxin response | 2 | 3.94 |
| 1613054_at | BQ794856 | TC53877 | O65695 | Auxin regulated factor | Auxin response | 3 | 3.79 |
| 1621946_at | CB975415 | TC70724 | Q8H0E0 | PIN1 like auxin transport | Auxin response | 7 | 3.7 |
| 1616015_at | CF607669 | TC67186 | Q2LAJ4 | Auxin response factor | Auxin response | 7 | 3.69 |
| 1611491_at | CB900901 | TC57901 | Q769J4 | AtPIN3 (auxin efflux carrier) | Auxin response | 20 | 3.5 |
| 1612180_at | CF608682 | TC66988 | Q6L8T9 | Auxin responsive factor 5 | Auxin response | 7 | 3.49 |
| 1614660_at | CF207466 | TC53887 | P13088 | Auxin-induced protein AUX22 | Auxin response | 11 | 3.04 |
| 1617179_at | CF414958 | - | Q6YZX7 | Auxin efflux carrier | Auxin response | 21 | 2.91 |
| 1615728_at | AY082522 | TC60981 | Q84V38 | CsIAA3 (Auxin regulated) | Auxin response | 7 | 2.66 |
| 1610034_at | CB97302 | TC59892 | Q49RB8 | Auxin receptor | Auxin response | 10 | 2.45 |
| 1617097_at | BQ797969 | TC67796 | Q8LER0 | Auxin efflux carrier | Auxin response | 3 | 2.41 |
| 1613857_at | CD715051 | TC55162 | O24408 | Auxin responsive factor | Auxin response | 2 | 2.32 |
| 1607503_s_at | CF515267 | TC53837 | Q52QX4 | Auxin-repressed protein | Auxin response | 12 | 2.3 |
| 1619658_at | CF371851 | TC66647 | Q6QUQ3 | Auxin and ethylene responsive GH3 | Auxin response | 15 | 2.25 |
| 1610591_at | CB923320 | TC57860 | Q3LFT5 | Auxin regulated protein | Auxin response | 10 | 2.21 |
| 1620726_at | CB339504 | TC56077 | Q6YZJ0 | Auxin-regulated protein | Auxin response | 3 | 2.19 |
| 1617694_at | CB972462 | TC62076 | Q93XP5 | Auxin responsive factor | Auxin response | 2 | 2.18 |
| 1618394_at | CF371644 | CF371644 | Q949J8 | Auxin growth promoter protein | Auxin response | 2 | 2.1 |
| 1617572_at | CB918599 | TC66046 | Q9XF92 | BRH1 RING finger protein (Brassinosteroid regulated) | Brassinosteroid response | 21 | 3.53 |
| 1620306_at | CF404552 | TC69712 | Q5ZAY9 | Cytokinin oxidase | Cytokinin response | 3 | 57.36 |
| 1610071_at | BQ797708 | TC58750 | Q39802 | Cytokinin induced message | Cytokinin response | 11 | 13.16 |
| 1619945_at | CB345883 | TC61250 | Q84N27 | Cytokinin repressed protein | Cytokinin response | 19 | 3.05 |
| 1622308_at | CF210289 | TC63310 | Q8S933 | 1-aminocyclopropane-1-carboxylate synthase | Ethylene biosynthesis | 2 | 11.84 |
| 1615952_s_at | CF215641 | TC56709 | Q84X67 | 1-aminocyclopropane-1-carboxylic acid oxidase | Ethylene biosynthesis | 3 | 6.56 |
| 1609683_at | CF604955 | TC65735 | Q5U8L6 | Ethylene responsive factor 2 | Ethylene response | 12 | 22.64 |
| 1617012_at | CD802399 | TC68057 | P16146 | Ethylene-responsive element | Ethylene response | 11 | 14.05 |
| 1621552_at | BM437510 | TC66829 | Q9LV58 | Ethylene-responsive transcriptional co activator | Ethylene response | 21 | 9.08 |
| 1619585_at | CD800299 | TC62897 | Q75UJ4 | Ethylene responsive factor | Ethylene response | 13 | 4.21 |
| 1611910_s_at | AY395745 | TC63214 | Q6TKQ3 | Ethylene responsive factor 4 | Ethylene response | 3 | 4.13 |
| 1609990_at | CB009298 | TC63214 | Q6TKQ3 | Ethylene responsive factor 2 | Ethylene response | 3 | 4.12 |
| 1608511_at | CB342877 | TC62587 | Q6RZW7 | Ethylene responsive factor 5 | Ethylene response | 15 | 3.72 |
| 1609780_at | CA810742 | TC55438 | Q94E74 | Ethylene responsive factor 6 | Ethylene response | 3 | 3.22 |
| 1612699_at | BQ798614 | BQ798614 | Q9XIA5 | Ethylene-forming-enzyme-like dioxygenase | Ethylene response | 12 | 2.75 |
| 1619178_at | CB349106 | TC54200 | Q94E74 | Ethylene responsive 6 | Ethylene response | 19 | 2.62 |
| 1613799_at | CF517211 | TC55673 | Q6RZW8 | Ethylene responsive factor 4 | Ethylene response | 21 | 2.5 |
| 1622402_at | CD799344 | TC62349 | Q84PH6 | Ethylene receptor (EIN4) | Ethylene response | 20 | 2.4 |
| 1609559_at | CF215263 | TC58568 | Q94AW5 | Ethylene-responsive element | Ethylene response | 21 | 2.34 |
| 1606623_at | BQ797592 | TC70037 | Q9LVS8 | EREBP-4 | Ethylene response | 3 | 2.28 |
| 1612921_at | CF514773 | TC57403 | Q9LVS8 | EREBP-4 | Ethylene response | 3 | 2.26 |
| 1618213_at | CF203873 | CF203873 | Q9SWV2 | ER6 (Ethylene regulated) | Ethylene response | 3 | 2.16 |
| 1611657_at | CF208861 | TC67832 | O64588 | GH3 Root formation (gibberellin regulated) | GA response | 3 | 8.07 |
| 1610607_at | CF371650 | TC66111 | Q49RB3 | GASA | GA response | 3 | 6.98 |
| 1621228_at | BQ798029 | TC52322 | Q6S5L6 | GAI protein (Gibberellin regulated) | GA response | 7 | 2.52 |
| 1610610_at | CA810332 | TC66284 | Q9ZQA5 | Putative gibberellin β-hydroxylase | Gibberellin metabolism | 10 | 3.52 |
| 1620071_at | BQ800214 | TC56624 | Q9LYC1 | Gibberellin receptor | Gibberellin response | 11 | 5.3 |
| 1618181_at | CF512673 | TC67464 | Q9MAA7 | Gibberellin receptor 2 | Gibberellin response | 16 | 2.1 |
| 1622456_at | CF609276 | TC66424 | Q7PCB5 | Phytosulfokine | Growth factor | 3 | 9.3 |
| 1616312_at | CD720049 | TC61028 | Q7PCA0 | Phytosulfokine peptide precursor | Growth factor | 12 | 3.69 |
| 1607170_s_at | CB917184 | TC66717 | Q7PCA1 | Phytosulfokine | Growth factor | 21 | 2.07 |
| 1612021_at | CF213898 | CF213898 | Q7EYF8 | Phytosulfokine receptor | Growth factor response | 3 | 4.06 |
| 1607601_at | CF209956 | TC61395 | Q76DL0 | 12-oxophytodienoate reductase | JA metabolism | 2 | 4.2 |
| 1619407_s_at | CA809049 | TC67104 | Q76DL0 | 12-oxophytodienoate reductase | JA metabolism | 21 | 2.92 |
| 1620308_at | CF208037 | TC57918 | Q38944 | Steroid 5-alpha-reductase | Lipid, fatty acid and isoprenoid metabolism | 3 | 3.5 |
| 1613941_at | CA818531 | TC61611 | Q7X9G5 | Lipoxygenase | Lipid, fatty acid and isoprenoid metabolism | 1 | 2.99 |
| 1618940_at | CF212858 | TC64939 | Q8W250 | 1-deoxy-D-xylulose 5-phosphate reductoisomerase | Lipid, fatty acid and isoprenoid metabolism | 3 | 2.03 |
| 1613678_at | CB971023 | TC54495 | Q9M2G7 | Phosphatase | Phosphate metabolism | 3 | 2.07 |
| 1612552_at | CA818350 | CA818350 | Q9C9W8 | S-adenosyl-L-methionine:salicylic acid carboxyl methyltransferase | SA response | 11 | 6.99 |
| 1618457_at | CF205125 | CF205125 | Q9M6E7 | UDP-glucose:salicylic acid glucosyltransferase | SA response | 12 | 2.19 |
| 1619377_at | CF372632 | TC68498 | Q5Z825 | avrRpt2-induced AIG2 protein | SA response | 12 | 2.06 |
Abscisic acid
ABA amounts in berries decrease after anthesis, but then increase significantly at véraison [45]. External applications of ABA to ripening fruit can accelerate berry development (see [13] and references therein). The transcript abundance of 9-cis-epoxycarotenoid dioxygenase (NCED), which encodes the rate limiting step in ABA biosynthesis, increased during the lag phase and peaked at stage 35 around the start of véraison (Figure 5A). Both NCED1 (1608022_at, TC57089) and NCED4 (1607029_at, TC55541) had similar expression patterns, but differed significantly in their relative trancript abundance. A transcript (1614892_at, TC54474) encoding ABI1 (protein phosphatase 2C) showed an expression pattern like that of the NCED genes, but was more highly correlated with NCED4 than NCED1. The RD22 gene (1619802_at, TC67323), a dehydration-responsive protein, displayed a very large increase in abundance at véraison that continued to increase during berry maturation, whereas another transcript (1621346_at, TC65114) encoding an ABI3/VP1 (ABscisic acid Insensitive 3/ViviParous 1) transcription factor showed highest transcript abundance during the lag phase.
Ethylene
Traditionally, wine grape has been considered a non-climacteric fruit, however, there are studies that indicate that ethylene plays an important role in berry development and ripening [13] and is required for increased berry diameter and ripening processes, such as anthocyanin biosynthetic gene expression and accumulation [46,47]. In addition, ethylene appears to be involved in controlling the expression of an alcohol dehydrogenase gene from grape [48]. Furthermore, some inhibitors of ethylene biosynthesis can delay berry ripening [49]. Ethylene-related transcripts displayed some very unique and intriguing patterns of expression (Figure 5B) indicating that this signaling pathway is differentially expressed along berry development. One transcript (1617012_at, TC68057) encoding a putative Ethylene Response Factor 1 (ERF1), a putative ethylene output gene, displayed a steady increase in abundance with maximal expression at ripening (Figure 5B) indicating a potential post-véraison role for this signaling pathway. An ethylene-induced transcription factor (1619585_at, TC62897) exhibited transcript accumulation during the lag (E-L stages 32 to 34) and early véraison (E-L stage 35) stages of development. A putative ethylene co-activator (1621552_at, TC66829) protein displayed biphasic peak transcript abundance at E-L stages 32 and 35. The transcript abundance of ACC oxidase (1615952_s_at, TC56709), the enzyme responsible for the last step in ethylene biosynthesis, was highest at E-L stage 32, the start of the lag phase, and then declined throughout the remainder of berry development. Interestingly, the transcript abundance of an ethylene receptor ERS1 (1622402_at, TC62349) and EIN4/ETR5 (1618518_at, TC55908) were at their lowest during E-L stages 32 to 33 until véraison, but then increased at a later stage (E-L stage 38). Ethylene pathway activation in grape berry appears to occur within a three week period of berry development (weeks 6 to 8 after anthesis; E-L stages 30 to 33) when the highest ethylene (ACC) content and transcript abundance of ACC oxidase were detected in Cabernet Sauvignon [46]. This hypothesis is supported by the observation that application of exogenous ethylene 8 weeks after anthesis hastened the ripening of the grape berries and resulted in a decrease in average cell size. In contrast, if the same ethylene treatment was applied during earlier stages of berry development (at 4, 5, 6 or 7 weeks), maturation was delayed [47].
According to the Arabidopsis model of ethylene signaling, reduced expression of transcripts and activity of receptors increases the sensitivity to ethylene, whereas increased receptor expression and activity decreases sensitivity [50]. In tomato, the expression of most genes encoding ethylene receptors increases during fruit development. In parallel, high levels of ethylene are expressed to counterbalance the negative effect of increased receptor expression on the ethylene signaling pathway [51]. In grape berry, the slight decreases observed in ethylene receptor transcript expression occurring between E-L stages 31 and 32 and the peak of ethylene accumulation during this same period, indicate a higher sensitivity to ethylene during the early stages of berry development. This would be expected to lead to a greater activation of the ethylene signaling pathway prior to véraison.
As in grape berry, strawberry is able to produce significant levels of ethylene during fruit development, but not to the same extent as climacteric fruits. Recently, three ethylene receptors have been identified in strawberry [52]. Two of them (FaEtr1 and FaErs1) display the same pattern of expression during fruit development as those observed for ERS1 ethylene receptor during grape berry development. In addition, the highest rates of ethylene production in strawberry were detected in very young green fruits. Following this, the hormone decreases continuously until the White stage of fruits. Following this stage, ethylene showed a slight but steady increase for the remainder of development. When considered together, the similarities of expression of ethylene receptors during fruit development for both grapes and strawberries coupled with the concomitant ethylene production during the early steps of fruit development indicate a conserved mechanism for ethylene perception between these fruits prior to ripening.
Brassinosteroids
Brassinosteroids (BR) have recently been implicated in playing an important role in berry development [53]. Castasterone concentrations are low during the early stages of berry development and then increase at véraison [53]. Brassinosteroids have been shown to increase cell size [54] indicating that berry enlargement may be affected by castasterone levels. BRH1 RING finger protein (1617572_at, TC66046) transcript abundance, which is known to be down-regulated by exogenous application of BR, decreased during E-L stages 31 to 35, but increased in fully mature berries (Figure 5C). The transcript abundance of the Brassinosteroid Receptor 1 gene (BRI1, 1612516_at, TC56501) peaks at the start of the lag phase (E-L stage 32) and then declines thereafter. The transcript abundance of BRU1 (1608945_at, TC54729), which is a BR-responsive transcript encoding a xyloglucan endotransglycosylase (XET), showed a transient increase in abundance at véraison. In the same family, transcripts for another BR-responsive protein (1619068_at, TC60314) declined with berry development. Clearly, there are many significant changes in transcript abundance that are associated with brassinosteroid responses during berry development.
Gibberellins
Very little is known about the role of gibberellin (GA) in grape berry development except a possible role in cell enlargement. Biologically active concentrations of GA are high in flowers and in fruits just after anthesis, but then drop to lower levels over the course of berry development [53,55]. There is a second peak of active GA at the start of the lag phase and it is 77 times higher in the seed compared to the berry mesocarp [56]. The transcript abundance of two putative GA receptors, GIDL1 and GIDL2 (1618181_at, TC67464; 1620071_at, TC56624, respectively), increased during berry development (Figure 5D). Interestingly, the transcript abundance of the GA signaling pathway repressor, GAI1 (1606777_s_at, TC56894), declines transiently at véraison. The transcript abundance of a putative GA β-hydroxylase (1610610_at, TC66284) declines over the course of berry development (Figure 5D) more or less coincident with the known accumulation pattern of GA1 in developing berries.
Auxins
The mechanisms by which the phytohormone indole-3-acetic acid (IAA) regulates berry development are complex and not fully understood. Increased auxin production produced through the action of an ovule-specific auxin-synthesizing transgene enhanced fecundity in grapes [57]. Earlier reports indicated that auxin concentrations were high during early Phase I and declined following véraison [55] consistent with the role of this phytohormone in promoting cell division and expansion during Phase I. Treatment of grape berries with synthetic auxin-like compound, benzothiazole-2-oxyacetic acid (BTOA) delayed ripening [45]. A more recent study showed that auxin concentrations remain relatively constant over the course of berry development [53].
Our data indicate that there are numerous transcript responses to auxin (Figure 5E). The transcript abundance of Aux22 (1614660_at, TC53887), which forms heterodimers with auxin response factors (ARF) in order to repress auxin responses, increased after véraison (Figure 5E). Transcripts for both Auxin Response Factor 2 (ARF2, 1613813_a_at, TC65541) and a Small Auxin Up RNA protein (SAUR) (1609591_at, TC63193) increased after véraison, whereas transcripts for a different SAUR transcript (1606566_at, TC62299) and an Auxin-induced Response Factor, ARF18 (1616225_at, TC52772) both declined in a very similar pattern during berry development. A transcript (1619610_at, TC56575) encoding IAA-amino acid hydrolase, which is involved in IAA homeostasis, was highly expressed during the later stages of berry development (Figure 5E). The synthesis and hydrolysis of IAA conjugates, which function in both permanent inactivation and temporary storage of auxin [58], may play an important role in the control of IAA concentrations as berry development progresses. IAA-amino acid hydrolase may provide for local concentrations of auxins within the berries to promote mesocarp cell enlargement. Several transcripts (1611479_at, CD799903; 1617179_at, CF414958; 1610034_at, TC59892) related to auxin transport and perception also displayed increased abundance at the onset of véraison. Given the importance of auxin-mediated processes in developing berries, more research needs to be conducted to elucidate the mode of action of auxin signaling and response pathways.
Methyl jasmonate and cytokinins
Methyl jasmonate (MeJA) is known to promote the synthesis and accumulation of terpenes and resveratrol in berry cell cultures [59,60], however, its effects in vivo are not well understood. The transcript abundance of 12-oxophytodienoate reductase (12-OPR) (1607601_at, TC61395), which is involved in jasmonate biosynthesis [61], and a constitutive pathogen-response 5 protein (1614324_at, CF213899), both decreased with berry development (Figure 5F). Less is known about the role of cytokinins in berry development. The transcript abundance of cytokinin oxidase (1620306_at, TC69712), which degrades cytokinin [62], decreased over berry development, whereas a known cytokinin-response regulator, a Type-A response regulator (1612955_at, TC52530), showed a steady increase in transcript abundance over berry development (Figure 5F).
New candidates genes associated with calcium signaling, flavonoid transport and flavor
Calcium has many essential roles in plant growth and development [63], however, the role of calcium signaling in grape berry development is largely unexplored. Recently, an ABA-responsive calcium-dependent protein kinase (CDPK) was described that was specifically expressed in the seed and flesh of berries with increased transcript abundance over berry development and ripening [64]. In the current study, a large number of genes with functions related to calcium sequestration, transport and signaling were found to display developmentally regulated expression patterns (Figure 6A; Table 3).
Figure 6.
Expression of potential candidates Unigenes. A) Black solid round (1614028_at, TC67285)-cation-transporting ATPase, red solid triangle (1622073_at, CF404214)-calcium-transporting ATPase, green solid triangle (1617237_s_at, TC66680)-Ca2+/H+ exchanger, blue solid diamond (1618587_at, TC64370)-calmodulin-repressor of gene silencing. B) Black solid round (1619917_s_at, TC69505)-glutathione-S-transferase, red solid triangle (1609870_at, TC58286)-glutathione-S-transferase conjugating ATPase, green solid triangle (1607560_at, TC62162)-multi-drug secondary transporter like protein (MATE), blue solid diamond (1611091_s_at, TC54724)-VvMYBPA1, orange solid square (1618504_at, TC61713)-MYC transcription factor. C) Black solid round (1608603_at, TC56956)-phloroglucinol O-methyltransferase, red solid triangle (1613542_at, TC62584) O-methyltransferase, green solid triangle (1620469_at, CF209780)-O-methyltransferase, blue solid diamond (1616348_at, TC52353)-S-adenosyl-L-methionine:benzoic acid/salicylic acid carboxyl methyltransferase orange solid square (1612552_at, TC57170)-S-adenosyl-L-methionine:salicylic acid carboxyl methyltransferase.
Table 3.
Transcripts (TFR pool) related to calcium categorized by the first hit in the MIPS2 catalog
| Probeset ID | GenBank Annotation | VvGI5 | UniProt ID | Gene Name Description | Function | Profile | Fold Change |
| 1616662_at | CF404703 | TC59643 | Q9LIK7 | Ca2+/ATPase | Ca transport | 3 | 27.96 |
| 1617237_s_at | CF207946 | TC66680 | O64455 | Ca2+/H+ exchanger (VCAX1) | Ca transport | 14 | 2.56 |
| 1614028_at | CB976052 | TC62785 | Q7X8B5 | Ca2+-transporting ATPase 8 | Ca transport | 16 | 2.32 |
| 1619731_at | CB972437 | CB972437 | Q93YX7 | Type IIB calcium ATPase | Ca transport | 21 | 2.2 |
| 1622073_at | CF404214 | CF404214 | Q9LIK7 | Calcium-transporting ATPase 13 | Ca transport | 5 | 2.05 |
| 1615486_at | CF415476 | TC69351 | Q5D6H2 | Cyclic Nucleotide-Gate Channel 2 | Ion channel | 3 | 8.21 |
| 1621591_at | CB981532 | TC66482 | Q94AS9 | Cyclic nucleotide-gated ion channel 4 | Ion channel | 10 | 3.01 |
| 1609527_at | CD802146 | TC64117 | Q6ZHE3 | Cyclic nucleotide-binding transporter 1 | Ion channel | 8 | 2.06 |
| 1613268_at | CB342482 | TC53213 | O65717 | Cyclic nucleotide-gated ion channel 1 | Ion channel | 12 | 2.05 |
| 1614456_at | BQ797488 | BQ797488 | Q8L706 | Ca2+-dependent lipid-binding protein | Lipid binding | 11 | 3.83 |
| 1614582_at | BQ799084 | BQ799084 | Q8LJ85 | Calreticulin | Protein folding and stabilization | 12 | 2.37 |
| 1611917_at | CB972164 | TC58290 | Q39817 | Calnexin | Protein folding and stabilization | 1 | 2.31 |
| 1612291_at | CB347450 | TC67746 | P93508 | Calcium-binding protein | Protein folding and stabilization | 3 | 2.2 |
| 1622324_at | CF568845 | TC63952 | Q39817 | Calnexin | Protein folding and stabilization | 1 | 2.04 |
| 1612443_at | CF211151 | TC68392 | Q7X996 | CBL-interacting protein kinase 20 | Signal transduction | 11 | 12.97 |
| 1610295_at | BQ797947 | TC57947 | Q8W1D5 | CBL-interacting protein kinase 5 | Signal transduction | 4 | 12.35 |
| 1618587_at | CF518131 | TC64370 | Q9AXG2 | Calmodulin | Signal transduction | 21 | 11.43 |
| 1618447_at | CA815141 | TC53225 | Q6ETM9 | CBL-interacting protein kinase 21 | Signal transduction | 3 | 7.75 |
| 1611127_at | CF510878 | TC64442 | Q8L3R2 | Calmodulin | Signal transduction | 12 | 5.33 |
| 1610922_at | CF404315 | TC68116 | Q1SFZ7 | CBL-interacting protein kinase 21 | Signal transduction | 3 | 3.43 |
| 1606980_at | CF211606 | TC69501 | Q008R9 | Calcium sensor homolog | Signal transduction | 2 | 3.23 |
| 1618045_at | CF216119 | TC53057 | Q676U1 | CBL-interacting protein kinase 20 | Signal transduction | 21 | 2.9 |
| 1611172_at | CB003645 | TC52484 | Q8LK24 | SOS2-like protein kinase | Signal transduction | 16 | 2.81 |
| 1612269_at | CB345885 | TC53895 | Q3HRN8 | Calcineurin B | Signal transduction | 13 | 2.74 |
| 1606859_at | CF518881 | CF518881 | Q3HRN8 | Calcineurin B | Signal transduction | 13 | 2.74 |
| 1613576_s_at | CF201676 | TC60874 | P62200 | Calmodulin 1/11/16 | Signal transduction | 11 | 2.7 |
| 1622351_at | CA810859 | TC60874 | P62200 | Calmodulin 1/11/16 | Signal transduction | 11 | 2.26 |
| 1611555_at | CB971903 | TC54154 | Q9SS31 | Calmodulin-related protein 2 | Signal transduction | 13 | 2.22 |
| 1608587_at | CD799705 | TC62151 | Q5D875 | Calcium-dependent protein kinase CDPK1444 | Signal transduction | 10 | 2 |
| 1614600_s_at | CF213754 | TC52150 | Q9ZT86 | Calcium-binding protein | Unclassified protein | 7 | 2.89 |
| 1616580_at | CF206767 | TC55591 | Q84Y18 | CAX-interacting protein 4 | Unclassified protein | 11 | 2.63 |
Calcium homeostasis within the cytosol is tightly controlled by membrane spanning Ca2+-ATPases and H+/Ca2+ exchangers, which typically maintain low concentrations of Ca2+ in the cytosol and restore this concentration following signaling-related transient changes in calcium levels. Transcripts encoding plasma membrane Ca2+-ATPase genes (1614028_at, TC62785; 1622073_at, CF404214), which are closely related to ACA8 and ACA13, respectively, in Arabidopsis thaliana, showed increased transcript abundance during E-L stages 33 and 34 and in later developmental stages. Interestingly, ABA markedly and rapidly stimulates the expression of the ACA8 gene in cell cultures of Arabidopsis thaliana [65]. A tonoplast Ca2+/H+ exchanger (1617237_s_at, TC66680), which is a close homolog of CAX3 from A. thaliana and plays a key role in cytosolic Ca2+ homeostasis [66], showed a transient increase in transcript abundance at E-L stages 34, indicating a possible role for calcium signaling around véraison.
ABA accumulates until two weeks after the beginning of véraison before decreasing later in berry development [67]. Thus, it is likely that ABA is directly or indirectly involved in the control of Ca2+ signaling and homeostasis events, particularly around véraison.
The increased expression of several Unigenes encoding calmodulin or calcium interacting protein kinases (see Table 3) supports this hypothesis [68]. One Unigene encoding a calmodulin-related suppressor of gene silencing (1618587_at, TC64370) displayed a pronounced pattern with two peaks of expression at E-L stage 32 and at E-L stage 35 corresponding to two transitions of berry development (Phases I to II and Phases II to III). This Unigene displayed a 10-fold change in its transcript abundance across berry development and may be involved in the suppression of posttranscriptional gene silencing (PTGS) by interacting with a proteinase known to suppress PTGS in plants [69]. This correlation indicates a possible role for calcium in regulating the activity of the PTGS mechanisms. To date, only one paper reported the possibility of the involvement of PTGS in the regulation of gene expression during plant development [70]. Further investigations are necessary to evaluate the real impact of this Unigene in the triggering of véraison.
Phenolic compounds, derived from flavonoids (anthocyanins, tannins and flavonols), are the major wine constituents responsible for organoleptic properties such as color and astringency. Twenty-one Unigenes encoding biosynthetic enzymes of the general phenylpropanoid and flavonoid pathways were found to exhibit differential mRNA expression patterns across berry development (Table 4). The vast majority of these genes are expressed predominantly in the skin [71].
Table 4.
Transcripts (TFR pool) related to flavonoid metabolism categorized by the first hit in the MIPS2 catalog within specific sub-sections of the flavonoid pathway
| Probeset ID | GenBank Annotation | VvGI5 | UniProt ID | Gene Name Description | Function | Profile | Fold Change |
| 1617171_s_at | AF000371 | TC51696 | O22303 | UDP glucose:flavonoid 3-o-glucosyltransferase (UFGT) | Anthocyanin Pathway | 11 | 46.79 |
| 1614441_at | BQ798241 | TC57653 | Q9SWY6 | Anthocyanidin synthase (ANS) | Anthocyanin Pathway | 11 | 12 |
| 1618112_at | CB971725 | TC70789 | Q9LTA3 | Anthocyanidin-3-glucoside rhamnosyltransferase | Anthocyanin Pathway | 3 | 9.39 |
| 1611309_at | CF210457 | TC58629 | Q8H1R1 | Dihydroflavonol 4-reductase (DFR) | Common Pathway | 19 | 7.36 |
| 1611739_at | CF403783 | TC64266 | Q8H224 | Flavonoid 3'-hydroxylase (F3'H) | Common Pathway | 2 | 5.68 |
| 1620675_at | CB969894 | TC51699 | P93799 | Dihydroflavonol 4-reductase (DFR) | Common Pathway | 3 | 5.21 |
| 1617019_at | BQ800456 | TC67173 | O80407 | Chalcone synthase (CS) | Common Pathway | 3 | 5.17 |
| 1607739_at | CF415693 | TC70298 | P41090 | Flavanone 3-hydroxylase (F3H) | Common Pathway | 3 | 2.93 |
| 1608379_at | CF202029 | TC40489 | Q8H8H7 | Flavanone 3-hydroxylase (F3H) | Common Pathway | 21 | 2.55 |
| 1607732_at | AF020709 | TC63806 | O22519 | Chalcone synthase (CS) | Common Pathway | 3 | 2.48 |
| 1608761_at | CB982029 | TC53331 | Q9FLV0 | Flavanone 3-hydroxylase (F3H) | Common Pathway | 18 | 2.02 |
| 1611542_at | CB971080 | TC51691 | P43311 | Polyphenol oxidase (PPO) | Flavonoid Catabolism | 3 | 28.9 |
| 1622651_at | CF215945 | TC58764 | P93622 | Polyphenol oxidase (PPO) | Flavonoid Catabolism | 5 | 3.79 |
| 1608791_at | CB978059 | TC66577 | Q84TM1 | Flavonol synthase (FLS5) | Flavonol Pathway | 3 | 5.12 |
| 1621051_at | CN006197 | - | Q40285 | Flavonol 3-O-glucosyltransferase | Flavonol Pathway | 13 | 3.94 |
| 1615401_at | CB342555 | TC55331 | Q40285 | Flavonol 3-O-glucosyltransferase | Flavonol Pathway | 15 | 2.43 |
| 1618155_at | CD004374 | TC54048 | Q40288 | Flavonol 3-O-glucosyltransferase 6 | Flavonol Pathway | 10 | 2.27 |
| 1612134_at | CF204393 | TC53206 | Q5FB34 | Anthocyanin reductase (ANR) | Proanthocyanidin Pathway | 3 | 34.12 |
| 1615174_s_at | CD011073 | TC68741 | Q4W2K6 | Leucoanthocyanidin reductase 2 (LAR2) | Proanthocyanidin Pathway | 13 | 4.08 |
| 1608212_at | CK138122 | TC54322 | Q84V83 | Leucoanthocyanidin reductase 2 (LAR2) | Proanthocyanidin Pathway | 13 | 3.52 |
The mechanisms by which anthocyanins accumulate in the vacuole of grape berry skin cells during Phase III are not fully understood. These compounds must be transported from the site of synthesis in the cytosol to their final destination, the vacuole. Several models have been proposed for sequestering anthocyanins in the vacuole in Arabidopsis thaliana. One model [72] indicates the action of a glutathione-S-transferase (GST) in facilitating the transfer of anthocyanins into the vacuole. Another model indicates that a transporter of the multidrug-resistance-associated protein family could facilitate the transport of an anthocyanin-GST complex into the vacuole [73]. Here, the Unigene transcript encoding a GST (1619917_s_at, TC69505; Figure 6B) displayed a 63-fold increase in abundance during the stages in berry development in which flavonoids accumulate (Figure 6B). This Unigene is closely related to a GST homolog known to be involved in anthocyanin sequestration [74]. This Unigene also displays a skin-specific expression pattern [71], which is consistent with the tissue localization of anthocyanins. A Unigene homologous to a glutathione-S-conjugate transporting ATPase (1609870_at, TC58286) showed a peak of expression at véraison (E-L stage 34). While not yet characterized in detail, this Unigene belongs to the ABC transporter sub-family, members of which are known to transport anthocyanins [74]. The putative multi-drug transporter (1607560_at, TC62162), which is known to be involved in the sequestration of tannins into vacuoles [75], exhibited peak transcript abundance at E-L stage 32 followed by a decline, and is consistent with the pattern of maximal tannin accumulation that occurs a few weeks before véraison.
Specific members of the MYB transcription factor family play critical roles in the regulation of flavonoid metabolism during grape berry development [76]. We detected four transcripts encoding MYB transcription factors that have been previously characterized in grape berry (see Table 4) [77-80]. VvMYBPA1 (1611091_s_at, TC54724) regulates the proanthocyanidin (condensed tannins) pathway in the grape berry [77]. In the Shiraz cultivar, VvMYBPA1 peak expression appears to occur during E-L stages 34 and 35 in the skin and seeds, whereas, in Cabernet Sauvignon this gene is expressed at an earlier developmental stage (E-L stages 32) (Figure 6B). Such differences are likely to be cultivar-dependent. In the same way, the MYC family of transcription factors also plays a key role in regulation of the anthocyanin pathway. One MYC transcription factor transcript (1618504_at, TC61713), which shares strong amino acid sequence identity with MYC genes known to be involved in the regulation of anthocyanin production [81], displayed a pattern of transcript accumulation that decreased from the beginning of berry development until E-L stage 35 and then increased for the remainder of fruit development (Figure 6B). Furthermore, this Unigene is preferentially expressed in the skin [71]. These expression patterns correlate well with the accumulation of anthocyanins and proanthocyanins.
In grape berries, volatile aroma compounds, such as terpenes, benzenoids, and phenylpropanoids, accumulate in exocarp and mesocarp tissues following the initiation of berry ripening [38,82]. Three transcripts (Figure 6C) encoding O-methyltransferases, which may participate in the biosynthesis of volatile compounds, were also detected [83]. The first Unigene (1608603_at, TC56956), which encodes a putative phloroglucinol O-methyltransferase, is involved in the biosynthesis of volatile 1,3,5-trimethoxybenzene, a compound not previously described in grape [83], displayed a very high transcript abundance at the beginning of berry development (E-L stage 31) before decreasing after véraison until E-L stage 36 and then increasing again in mature berries (Figure 6C). The second Unigene (1613542_at, TC62584) was expressed at E-L stage 31, but then declined. The third Unigene (1620469_at, CF209780) displayed very low transcript abundance with a slight increase following véraison (Figure 6C). Finally, two S-adenosyl-L-methionine (SAM):salicylic acid carboxyl methyltransferases were identified with developmentally-induced expression patterns. The first Unigene (1616348_at, TC52353) showed a broad peak of expression between E-L stages 32 to 35, whereas the second Unigene (1612552_at, TC57170) showed increased transcript abundance after véraison (E-L stage 34) (Figure 6C). Such genes are thought to play important roles in scent production or plant defense [84]. Little correlation between the level of sequence similarity and the structural similarity of their substrates has been observed for most of these protein families, so that gene functions have to be assigned following detailed biochemical testing [85].
Organic acid and proline metabolism
The acid:sugar balance at harvest is an important factor of wine quality as it affects important sensory attributes [15]. Two major organic acids that contribute to titratable acidity, tartrate and malate, are the most abundant organic acids in grapes and reach maximal concentrations around the end of Phase I (E-L stage 32; see Table 5). Tartrate concentrations were found to peak at E-L stage 32 and then declined steadily until harvest, E-L stage 38 (Figure 7A). Tartrate concentrations decreased in parallel with three different transcripts encoding L-idonate dehydrogenase (1622252_at, TC52651; 1613165_s_at, TC52651; 1612918_at, TC52651), a key enzyme in tartrate biosynthesis [86]. The innermost region of the berry pulp surrounding the seed has been shown to contain the highest tartrate concentrations [87]. Consistent with this observation, tartrate synthase transcripts have been shown to be more abundant in seeds than in outer mesocarp and skin tissues [71].
Table 5.
Transcripts (TFR pool) related to organic and phenolic acid metabolism
| Probeset ID | GenBank Annotation | VvGI5 | UniProt ID | Gene Name Description | Function | Profile | Fold Change |
| 1608526_at | CB974198 | TC66314 | Q5NBP4 | AOBP-like protein | Organic Acid | 11 | 6.48 |
| 1618209_at | CF373021 | TC56127 | P82281 | Ascorbate peroxidase | Organic acid | 3 | 3.59 |
| 1617448_at | BQ795936 | TC54982 | Q9M6B3 | Malate dehydrogenase | Organic Acid | 10 | 3.48 |
| 1606935_at | CB969531 | TC66898 | Q9SAK4 | Succinic semialdehyde dehydrogenase 1 | Organic acid | 3 | 3.05 |
| 1620641_at | CF511421 | TC52472 | Q39540 | AOBP-like protein | Organic Acid | 9 | 2.11 |
| 1611871_at | CF415063 | TC54132 | Q84UH4 | Dehydroascorbate reductase | Organic Acid | 19 | 2.1 |
| 1609147_at | CB979150 | TC55437 | Q645N0 | Malate dehydrogenase (cytosolic) | Organic Acid | 11 | 2.01 |
| 1607417_at | CF512464 | TC53733 | Q8L7U8 | Cinnamyl-alcohol dehydrogenase CAD1 | Phenolic Acid | 2 | 80.03 |
| 1614643_at | CF214966 | TC51729 | Q43237 | Caffeoyl-CoA O-Methyltransferase | Phenolic Acid | 2 | 34.34 |
| 1611265_at | CF513719 | TC51900 | Q49LX7 | 4-coumarate:CoA ligase | Phenolic Acid | 11 | 25.11 |
| 1620342_at | CF207053 | TC64352 | Q00763 | Caffeic acid 3-O-methyltransferase 1 | Phenolic Acid | 11 | 18.69 |
| 1610935_at | CF404728 | TC64481 | Q75W19 | Ferulate-5-hydroxylase (FAH1) | Phenolic Acid | 2 | 13.64 |
| 1619682_x_at | CF205002 | TC62835 | Q9M560 | Caffeic acid O-Methyltransferase | Phenolic Acid | 2 | 13.58 |
| 1616434_s_at | AF239740 | TC62835 | Q9M560 | O-methyltransferase | Phenolic Acid | 2 | 11.53 |
| 1609307_at | CD715818 | TC66040 | O24145 | 4-coumarate--CoA ligase (At4CL1) | Phenolic Acid | 2 | 10.6 |
| 1619450_s_at | CF215109 | TC52364 | Q00763 | O-methyltransferase | Phenolic Acid | 2 | 10.28 |
| 1607475_s_at | CD012393 | TC64352 | Q3SCM5 | Caffeic acid O-methyltransferase | Phenolic Acid | 11 | 9.32 |
| 1614423_at | CF517687 | TC66815 | Q6DMZ8 | Cinnamoyl CoA Reductase | Phenolic Acid | 2 | 8.61 |
| 1620650_s_at | CF207485 | TC69704 | Q9ATW1 | Cinnamyl-alcohol dehydrogenase | Phenolic Acid | 1 | 5.95 |
| 1616191_s_at | CB971061 | TC70715 | Q3HM04 | Cinnamate-4-Hydroxylase | Phenolic Acid | 3 | 5.78 |
| 1613542_at | CF209028 | TC62584 | Q7X9J0 | O-methyltransferase | Phenolic Acid | 2 | 5.54 |
| 1622267_at | CF516149 | TC64537 | O65152 | Cinnamyl-alcohol dehydrogenase | Phenolic Acid | 3 | 4.48 |
| 1619320_at | CB974305 | TC66743 | P31687 | 4-coumarate:CoA ligase 3 (4CL3) | Phenolic Acid | 3 | 4.21 |
| 1619808_at | CB972340 | TC54722 | O65152 | Cinnamyl-alcohol dehydrogenase | Phenolic Acid | 3 | 3.96 |
| 1611249_s_at | CF517155 | TC51769 | O65152 | Cinnamyl-alcohol dehydrogenase | Phenolic Acid | 3 | 3.93 |
| 1613831_at | CD801016 | TC58955 | Q5I6D6 | Sinapyl alcohol dehydrogenase | Phenolic Acid | 3 | 3.36 |
| 1613548_at | CB009193 | TC68990 | Q8H8C9 | 4-coumarate:CoA ligase | Phenolic Acid | 11 | 3.19 |
| 1615439_at | CF213244 | TC63112 | P30359 | Cinnamyl alcohol dehydrogenase 2 | Phenolic Acid | 2 | 2.38 |
| 1609327_at | CF208599 | TC68572 | A1YIQ2 | Cinnamyl-alcohol dehydrogenase 1 | Phenolic Acid | 2 | 2.33 |
| 1607163_at | CF415171 | - | Q8LSQ3 | 4-coumarate:CoA ligase | Phenolic Acid | 3 | 2.22 |
| 1613511_at | BQ796246 | TC59682 | Q65CJ7 | Hydroxyphenylpyruvate reductase | Phenolic Acid | 11 | 2.22 |
| 1616445_at | CD716014 | TC57545 | Q9LYJ0 | Cinnamoyl CoA Reductase | Phenolic Acid | 16 | 2.11 |
Figure 7.
Organic acids and amino acids: metabolites and transcripts. A) Black solid round-tartrate, red solid triangle (1622252_at, TC52651)-L-idonate dehydrogenase, green solid triangle (1613165_s_at, TC52651)-L-idonate dehydrogenase, blue solid diamond (1612918_at, TC52651)-L-idonate dehydrogenase. B) Black solid round-malate, red solid triangle (1612546_at, TC68207)-cytosolic MDH, green solid triangle (1609147_at, TC55437)-cytosolic MDH, blue solid diamond (1622059_at, TC69439)-mitochondrial malate dehydrogenase (MDH), orange solid square (1617448_at, TC54982)-mitochondrial MDH, lavender open square (1609345_s_at, TC57092)-malic enzyme. C) Black solid round-proline, red solid triangle (1619565_at, TC52705)-pyrroline-5-carboxylate synthetase, green solid triangle (1617293_s_at, BQ792635)-proline dehydrogenase, blue solid diamond (1610800_at, CK906448)-proline transporter. *: Transcripts that do not pass the two-fold ratio. All compounds amounts were normalized by a ribitol standard (25 mg/L).
Like tartrate, malate concentrations peaked around E-L stage 32, but then declined more rapidly than tartrate during berry ripening (Figure 7B). In contrast to the good correlation between tartrate and L-idonate dehydrogenase transcript abundance, there is a less obvious correlation between malate concentrations and the transcript abundance of Unigenes encoding malate dehydrogenases (Figure 7B). Transcript abundance for two isogenes encoding cytosolic NAD-dependent malate dehydrogenases (1612546_at, TC68207; 1609147_at, TC55437), which catalyze the interconversion of malate to oxaloacetate, increased during ripening. Transcripts for mitochondrial isoforms of the enzyme (1622059_at, TC60439; 1617448_at, TC54982) also increased over this same time period. In contrast, the transcript abundance of a NADP-dependent malic enzyme (1609345_s_at, TC57092), which catalyzes the oxidative decarboxylation of malate to pyruvate, declined slightly from E-L stages 34 to 36, but then increased by stage 38 (Figure 7B). The slight increase in the expression of all of these enzymes together may contribute to the declining concentrations of malate during ripening. Very little is known about the mechanisms of malate transport processes in the phloem/xylem and within developing grape berries. The regulation of malate concentrations in berries appears to be quite complex. More research is needed to elucidate this well known developmental process.
Mature berries contain unusually high concentrations of free proline; proline being the most abundant amino acid in Cabernet Sauvignon [88,89]. Proline concentrations increased significantly at véraison and remained high until berries were fully ripe (Figure 7C). Transcripts encoding pyrroline-5-carboxylate synthetase (1619565_at, TC52705), the key regulatory enzyme in proline biosynthesis, remained relatively constant with a small peak of expression occurring at E-L stage 35 (Figure 7C). Proline dehydrogenase transcripts (1617293_s_at, BQ792635), which encode the first enzymatic step in proline catabolism, increased only during the latter stages of berry development. These mRNA expression patterns are consistent with earlier reports and with protein expression patterns of these enzymes [88]. Proline accumulation correlated poorly with steady-state transcript and protein abundance changes for these two enzymes indicating that proline production is regulated by posttranslational mechanisms [88]. Steady-state transcripts encoding a proline transport protein (1610800_at, CK906448) also increased in conjunction with proline abundance.
Sugar metabolism
Sugar accumulation in grape berries has been well studied because sugar content is a key factor in producing wine. In contrast to organic acids, hexose sugars (i.e., Glc and Fru) begin to accumulate substantially in the lag phase (Phase II) and continue thereafter. In grapevines, carbohydrates produced during photosynthesis are exported from the leaf as sucrose and transported in the phloem to the berry cluster [90,91]. Prior to véraison, most sugars imported into the berries are metabolized with little if any storage of these compounds. Following véraison, however, sugars accumulate in the vacuole to high levels in the form of glucose and fructose following the enzymatic cleavage of sucrose (mainly in the apoplast, but also in the cytoplasm and vacuole). Monosaccharide transporters direct the transport of these sugars through different organelles [92].
In the berries in this study, fructose was more abundant than glucose; in contrast sucrose concentrations remained relatively low and constant throughout berry development (Figure 8A). Transcript abundance for the Unigene encoding sucrose synthase (1609402_at, TC62599), increased gradually over berry development consistent with increased hexose accumulation in the berry. This Unigene has high homology with the sucrose synthase (CiTSUSA) in Citrus unshiu [93]. CiTSUSA also increases with fruit development and catalyzes the reaction in the cleavage direction (sucrose to UDP-glucose and fructose). Komatsu et al. [93] suggest that the action of this gene may be important for sink strength.
Figure 8.
Hexose sugars, transporters, and starch: metabolites and transcripts. A) Black solid round-fructose, red solid triangle-glucose, green solid triangle-sucrose. B) Black solid round (1609402_at, TC62599)-sucrose synthase, red solid triangle (1608257_at, TC68135)-sucrose-6-phosphate phosphatase, green solid triangle (1611613_at, TC60693)-invertase (GIN1), blue solid diamond (1612836_at, TC57719)-invertase (GIN2), orange solid square (1620628_at, TC67908)-neutral invertase, lavender open square (1611027_at, TC56057)-acidic invertase, brown open triangle (1616255_at, TC57339)-fructokinase. C) Black solid round (1616083_at, TC51694)-VvHT1 (hexose transporter 1), red solid triangle (1615257_at, TC65400)-VvHT6 (hexose transporter 6), green solid triangle (1615697_at, TC51724)-VvSUC27 (sucrose transporter), blue solid diamond (1608991_at, TC60060)-plastidial glucose transporter, orange solid square (1613408_at, TC66667)-polyol transporter, lavender open square (1619379_at, TC58801)-plastidial triose phosphate transporter, brown open triangle (1622157_at, TC61733)-plastidial triose phosphate transporter. D) Black solid round (1615571_at, TC53551)-starch synthase, red solid triangle (1613601_at, TC67353)-starch synthase, green solid triangle (1617068_at, TC54621)-plastidial alpha-glucan, water dikinase, blue solid diamond (1617941_at, TC62494)-plastidial alpha-glucan, water dikinase, orange solid square (1622120_at, TC54533)-starch phosphorylase, lavender open square (1613188_at, TC70258)-α-amylase, brown open triangle (1617124_at, TC67979)-β-amylase. All compounds amounts were normalized by a ribitol standard (25 mg/L).
Sucrose-6-phosphate phosphohydrolase (SPP) (1608257_at, TC68135), which catalyzes the last step in sucrose synthesis, showed a slight increase in transcript abundance after E-L stage 32 and then remained relatively constant throughout the remainder of berry development (Figure 8B). In contrast, the transcript abundance of two vacuolar invertases, GIN1 and GIN2 (1611613_at, TC60693; 1612836_at, TC57719), which catalyze the catabolism of sucrose to fructose and glucose, declined over the course of berry development (Figure 8B), consistent with an earlier report [94]. The mRNA expression of these two invertases is consistent with the early increases in sugar accumulation during Phase II (E-L stages 32 to 34). On the other hand, transcript abundance for a neutral invertase (1620628_at, TC67908) and a cell wall acid invertase (1611027_at, TC56057) remained relatively constant during the course of berry development consistent with earlier reports on the amount and activity of these enzymes in developing berries [95]. In grape berries, sucrose cleavage is largely catalyzed by cell wall bound invertases [95]. Sucrose cleavage is usually associated with cell wall invertase activity at the onset of ripening, together with a shift towards apoplastic phloem unloading of sugars in berries during this same period of time [95]. Finally, transcripts encoding fructokinase (1616255_at, TC57339), which catalyzes the formation of fructose-6-phosphate and may regulate starch formation, declined in abundance in a similar manner as GIN1 and GIN2 following a peak of expression at E-L stage 32.
In most sink cells, sucrose is either cleaved by invertase into glucose and fructose or degraded by sucrose synthase into uridine-5'-diphosphate (UDP) glucose and fructose for subsequent metabolism and biosynthesis [96,97]. Cell wall invertases appear to play the main role in the cleavage of sucrose during Phase III of berry development [95]. However, the increase in sucrose synthase during Phase III of berry development indicates that this isogene may participate in the catabolism of sucrose to fructose and glucose. Alternatively, this sucrose synthase isogene may play a critical role in cellulose synthesis associated with Phase III cell expansion similar to its role in cotton fiber elongation [98]. Two cellulose synthase isogenes (1607069_at, TC53461; 1611149_at, TC56091) displayed increased transcript abundance during Phase II and III, consistent with this hypothesis (see Table 1). Additional developmentally regulated transcripts related to carbohydrate metabolism and transport are summarized in Table 6.
Table 6.
Transcripts (TFR pool) related to carbohydrate metabolism and transport categorized by the first hit in the MIPS2 catalog
| Probeset ID | GenBank Annotation | VvGI5 | UniProt ID | Gene Name Description | Function | Profile | Fold Change |
| 1618061_a_at | CF514699 | TC52548 | O78327 | Transketolase | Amino Acids Metabolism | 20 | 3.44 |
| 1614105_at | CB968800 | TC70460 | Q4JIY3 | Pyruvate dehydrogenase | Amino Acids Metabolism | 11 | 2.2 |
| 1616700_at | CB910092 | TC53526 | Q9SLY2 | Sucrose synthase | Carbohydrate metabolism | 3 | 227.67 |
| 1611613_at | BQ796771 | TC60693 | Q9S944 | Invertase | Carbohydrate metabolism | 3 | 61.86 |
| 1622656_at | CF215745 | TC61716 | Q5NA70 | Glucan endo-1,3-b-glucosidase | Carbohydrate metabolism | 2 | 53.85 |
| 1614716_at | CB978853 | TC58640 | Q6Z8F4 | Phosphoribulokinase | Carbohydrate metabolism | 12 | 51.76 |
| 1617719_at | CB975632 | TC55314 | Q6IV07 | UDP-glucose:protein transglucosylase | Carbohydrate metabolism | 2 | 40.93 |
| 1607442_at | CF403717 | - | Q50HW0 | Glucuronosyltransferase | Carbohydrate metabolism | 2 | 38.54 |
| 1622115_at | CD004218 | TC60627 | Q9SRX8 | b-glucosidase | Carbohydrate metabolism | 10 | 26.34 |
| 1611970_at | CF207195 | TC62847 | Q9LKY6 | Glucose acyltransferase | Carbohydrate metabolism | 3 | 25.74 |
| 1620679_at | CB972076 | TC53351 | Q9LV33 | b-glucosidase | Carbohydrate metabolism | 14 | 16.26 |
| 1621352_at | BQ794457 | TC59789 | Q8GT41 | Invertase inhibitor | Carbohydrate metabolism | 11 | 16.06 |
| 1608932_at | CB982469 | TC63201 | Q59J80 | Glucosyltransferase | Carbohydrate metabolism | 11 | 15.38 |
| 1620347_at | CA814065 | TC66065 | Q5QPZ6 | Glycosyltransferase | Carbohydrate metabolism | 10 | 15.36 |
| 1622282_at | CD712313 | TC54393 | Q7XAE2 | Fructokinase | Carbohydrate metabolism | 2 | 13.54 |
| 1616642_at | BQ800221 | TC64250 | Q9FEP9 | Glycerol-3-phosphate acyltransferase | Carbohydrate metabolism | 16 | 11.07 |
| 1616255_at | CF516475 | TC57339 | O82616 | Fructokinase | Carbohydrate metabolism | 12 | 10.13 |
| 1612918_at | CB972844 | TC52651 | Q9MBD7 | NAD-dependent sorbitol dehydrogenase | Carbohydrate metabolism | 2 | 9.14 |
| 1608393_at | CF403620 | TC64860 | O22658 | ADP-glucose pyrophosphorylase | Carbohydrate metabolism | 7 | 8.79 |
| 1621067_at | CF511425 | TC51908 | Q8W3C8 | Glucose acyltransferase | Carbohydrate metabolism | 3 | 8.2 |
| 1612883_at | CB911656 | TC60606 | O22060 | Sucrose-phosphate synthase 1 | Carbohydrate metabolism | 16 | 7.92 |
| 1617035_s_at | CF205538 | TC64995 | Q9XGN4 | Galactinol synthase | Carbohydrate metabolism | 11 | 7.73 |
| 1609652_s_at | CF215703 | TC59328 | Q9FNI7 | Glucosyltransferase | Carbohydrate metabolism | 3 | 7.5 |
| 1617309_at | CB922444 | TC59505 | Q8LFT7 | Aldehyde dehydrogenase | Carbohydrate metabolism | 10 | 7.26 |
| 1619190_at | CD720196 | TC54797 | Q6H5W0 | Alcohol dehydrogenase | Carbohydrate metabolism | 3 | 6.66 |
| 1616107_s_at | CD715446 | TC67979 | Q94EU9 | b-amylase | Carbohydrate metabolism | 9 | 6.34 |
| 1618409_at | CF514784 | TC52918 | Q94G86 | Glucan endo-1,3-b-glucosidase | Carbohydrate metabolism | 3 | 6.26 |
| 1620624_at | CB969436 | TC52478 | Q94IP3 | UDP-Glucose Transferase | Carbohydrate metabolism | 2 | 6.21 |
| 1611680_at | CF415491 | TC58448 | Q50HU7 | Glycosyltransferase | Carbohydrate metabolism | 2 | 5.8 |
| 1612465_at | CF568806 | TC53602 | O65736 | b-galactosidase | Carbohydrate metabolism | 4 | 5.76 |
| 1618071_at | CF518536 | TC54381 | Q9M8Y0 | O-linked GlcNAc transferase | Carbohydrate metabolism | 16 | 5.7 |
| 1611804_at | CF513259 | TC62252 | Q9ZVX4 | Glucose acyltransferase | Carbohydrate metabolism | 12 | 5.52 |
| 1617454_at | BQ798893 | - | Q8VYG2 | Galactokinase | Carbohydrate metabolism | 2 | 5.43 |
| 1612836_at | CF403299 | TC57719 | Q9S943 | Invertase | Carbohydrate metabolism | 3 | 5.28 |
| 1618517_at | CB971627 | TC53602 | Q93X58 | b-galactosidase | Carbohydrate metabolism | 4 | 5.01 |
| 1622074_at | BQ794083 | - | Q84JP7 | Phosphoenolpyruvate carboxylase kinase | Carbohydrate metabolism | 12 | 4.66 |
| 1615571_at | CB983156 | TC53551 | Q9FNF2 | Starch synthase | Carbohydrate metabolism | 9 | 4.58 |
| 1622543_at | CB977855 | TC61696 | Q84V96 | Aldehyde dehydrogenase | Carbohydrate metabolism | 1 | 4.57 |
| 1610724_at | CB916342 | TC63651 | Q652S1 | Fructose/tagatose bisphosphate aldolase | Carbohydrate metabolism | 11 | 4.57 |
| 1620997_at | CD799067 | TC63159 | Q84LI1 | Galactose dehydrogenase | Carbohydrate metabolism | 2 | 4.55 |
| 1619223_s_at | CB005867 | TC52910 | Q9SLS2 | Sucrose synthase | Carbohydrate metabolism | 2 | 4.46 |
| 1615614_at | CF405918 | TC54197 | Q9M3I0 | Glucosyltransferase | Carbohydrate metabolism | 2 | 4.28 |
| 1622065_at | CD801714 | - | Q94FA7 | Fructose-bisphosphatase | Carbohydrate metabolism | 3 | 4.17 |
| 1622503_at | CF203022 | TC69704 | Q9ATW1 | Mannitol dehydrogenase | Carbohydrate metabolism | 1 | 4.16 |
| 1615634_at | CB970085 | TC69016 | Q8L9U9 | Glucose acyltransferase | Carbohydrate metabolism | 12 | 3.86 |
| 1607324_at | CD719348 | TC54773 | P94078 | a-mannosidase | Carbohydrate metabolism | 2 | 3.85 |
| 1611112_at | CB971308 | TC51885 | Q7XPW5 | Phosphomannomutase | Carbohydrate metabolism | 3 | 3.85 |
| 1616325_at | CF211815 | TC53040 | Q6Q2Z9 | Phosphoenolpyruvate carboxylase | Carbohydrate metabolism | 3 | 3.84 |
| 1617068_at | CF519166 | TC54621 | Q9SGX4 | Water dikinase | Carbohydrate metabolism | 18 | 3.8 |
| 1611604_at | CB916873 | TC54851 | Q8LPJ3 | a-mannosidase | Carbohydrate metabolism | 3 | 3.78 |
| 1620724_at | CB915307 | TC66445 | O48628 | Phosphofructo-1-kinase | Carbohydrate metabolism | 11 | 3.73 |
| 1616500_at | AF194175 | TC52882 | Q9FZ00 | Alcohol dehydrogenase | Carbohydrate metabolism | 10 | 3.69 |
| 1612870_s_at | CF201540 | TC66152 | Q0DAH4 | GDP-4-keto-6-deoxy-D-mannose-3,5-epimerase-4-reductase | Carbohydrate metabolism | 3 | 3.66 |
| 1608527_at | CF515950 | TC58983 | Q9FJ95 | Sorbitol dehydrogenase | Carbohydrate metabolism | 10 | 3.6 |
| 1619457_at | CB969731 | TC63406 | P93653 | Trehalose-6-phosphate synthase | Carbohydrate metabolism | 12 | 3.58 |
| 1611154_at | CF204490 | - | Q42954 | Pyruvate kinase | Carbohydrate metabolism | 3 | 3.54 |
| 1614552_at | CB978862 | TC54160 | Q5SMZ1 | Aldose 1-epimerase | Carbohydrate metabolism | 12 | 3.52 |
| 1608263_a_at | BQ794795 | TC51761 | Q9M6B4 | Alcohol dehydrogenase | Carbohydrate metabolism | 11 | 3.5 |
| 1617186_at | CF415580 | TC70119 | O65856 | Glucose-6-phosphate dehydrogenase | Carbohydrate metabolism | 1 | 3.49 |
| 1608907_s_at | CA809004 | TC51713 | Q9XGN4 | Galactinol synthase | Carbohydrate metabolism | 11 | 3.4 |
| 1613182_at | CB982869 | - | Q6PP98 | Pyruvate dehydrogenase kinase | Carbohydrate metabolism | 11 | 3.37 |
| 1612414_at | CD715284 | TC58601 | Q42910 | Pyruvate phosphate dikinase | Carbohydrate metabolism | 10 | 3.34 |
| 1622120_at | CF519014 | TC54533 | P27598 | Starch phosphorylase | Carbohydrate metabolism | 21 | 3.33 |
| 1606536_at | CB971452 | - | Q8S9A7 | Glucosyltransferase | Carbohydrate metabolism | 3 | 3.29 |
| 1622606_at | CB910226 | TC52786 | Q6DW08 | GDP-mannose pyrophosphorylase | Carbohydrate metabolism | 3 | 3.28 |
| 1610766_at | CF212685 | TC53291 | Q7Y152 | Galactokinase | Carbohydrate metabolism | 11 | 3.24 |
| 1615270_at | CF208284 | TC70917 | Q6K963 | Callose synthase | Carbohydrate metabolism | 21 | 3.23 |
| 1615167_at | CF519116 | TC65652 | Q9LFQ0 | Glycosylation enzyme | Carbohydrate metabolism | 12 | 3.2 |
| 1606774_at | CF415165 | TC70261 | Q8L7J4 | Pyruvate kinase | Carbohydrate metabolism | 11 | 3.14 |
| 1609402_at | BQ794844 | TC62599 | Q9SLY2 | Sucrose synthase | Carbohydrate metabolism | 11 | 3.09 |
| 1608100_at | CF404013 | TC51810 | Q8S569 | Phosphoenolpyruvate carboxylase | Carbohydrate metabolism | 2 | 3.07 |
| 1609470_at | CF203556 | - | Q8LFZ9 | Sucrase | Carbohydrate metabolism | 5 | 3.04 |
| 1614023_at | CF414667 | - | P46275 | Fructose-1,6-bisphosphatase | Carbohydrate metabolism | 2 | 3.01 |
| 1618726_at | CF211103 | TC60540 | Q5JNJ1 | Trehalose-6-phosphate synthase/phosphatase | Carbohydrate metabolism | 4 | 3 |
| 1614982_at | CF211066 | TC61602 | Q9C9P3 | GDP-mannose pyrophosphorylase | Carbohydrate metabolism | 3 | 2.99 |
| 1616783_at | CF405837 | TC58450 | P93344 | Aldehyde dehydrogenase | Carbohydrate metabolism | 11 | 2.95 |
| 1616630_at | CF603093 | TC56347 | Q94LX9 | Phosphoenolpyruvate carboxylase | Carbohydrate metabolism | 16 | 2.95 |
| 1620905_at | CF215819 | TC68052 | Q6RK07 | UDP-glucose dehydrogenase | Carbohydrate metabolism | 21 | 2.89 |
| 1621861_at | CF209183 | TC65564 | Q94AS2 | b-amylase | Carbohydrate metabolism | 11 | 2.87 |
| 1613188_at | CA817889 | TC70258 | Q5BLY1 | a-amylase | Carbohydrate metabolism | 11 | 2.85 |
| 1608207_at | CB343787 | TC63660 | Q84V96 | Aldehyde dehydrogenase | Carbohydrate metabolism | 3 | 2.7 |
| 1611808_at | CF205006 | TC67979 | Q94EU9 | b-amylase | Carbohydrate metabolism | 9 | 2.69 |
| 1610410_at | CB342966 | TC61245 | O64733 | Glucosyltransferase | Carbohydrate metabolism | 9 | 2.67 |
| 1611851_at | BQ799617 | TC52022 | Q9FIK0 | Phosphofructo-1-kinase | Carbohydrate metabolism | 10 | 2.67 |
| 1609545_at | CF514819 | TC52560 | Q4R0T9 | ADP-sugar diphosphatase | Carbohydrate metabolism | 11 | 2.64 |
| 1617368_at | CF512540 | - | E1313 | Glucan endo-1,3-b-glucosidase | Carbohydrate metabolism | 3 | 2.63 |
| 1615623_at | CF511813 | TC55899 | O64733 | Glucose acyltransferase | Carbohydrate metabolism | 2 | 2.63 |
| 1620375_at | CA814054 | TC62155 | Q8LK43 | Glycogene synthase kinase-like kinase | Carbohydrate metabolism | 7 | 2.58 |
| 1613601_at | CB978458 | TC67353 | O64927 | Starch synthase | Carbohydrate metabolism | 3 | 2.56 |
| 1618125_at | BQ798742 | - | Q94KE3 | Pyruvate kinase | Carbohydrate metabolism | 16 | 2.52 |
| 1617941_at | CB914224 | TC62494 | O81505 | Water dikinase | Carbohydrate metabolism | 11 | 2.52 |
| 1621073_at | CB914439 | TC55380 | Q7XEL0 | GDP-mannose-3",5"-epimerase | Carbohydrate metabolism | 3 | 2.51 |
| 1620165_at | CA817563 | TC56014 | Q84YG5 | Isoamylase | Carbohydrate metabolism | 11 | 2.51 |
| 1613060_at | CF214238 | TC53819 | Q9M3B6 | Pyruvate kinase | Carbohydrate metabolism | 18 | 2.51 |
| 1620904_at | CF609568 | TC58209 | Q9SAD5 | b-1,4-N-acetylglucosaminyltransferase | Carbohydrate metabolism | 16 | 2.49 |
| 1611027_at | CB978747 | TC56057 | Q3L7K5 | Invertase | Carbohydrate metabolism | 20 | 2.49 |
| 1608995_at | BQ796616 | TC54941 | Q84NI6 | a-galactosidase | Carbohydrate metabolism | 11 | 2.48 |
| 1622806_at | CB009073 | TC63769 | Q6VWJ5 | Fructokinase | Carbohydrate metabolism | 1 | 2.48 |
| 1609510_at | CF513342 | TC69905 | Q0WV85 | O-linked GlcNAc transferase | Carbohydrate metabolism | 16 | 2.47 |
| 1609232_at | CA811215 | TC56883 | Q9ZVJ5 | Phosphoglucomutase | Carbohydrate metabolism | 15 | 2.45 |
| 1613514_s_at | CF202452 | TC54941 | Q9M442 | a-galactosidase II | Carbohydrate metabolism | 11 | 2.44 |
| 1613025_at | CF403382 | TC69507 | Q9SNY3 | GDP-mannose 4,6 dehydratase 1 | Carbohydrate metabolism | 21 | 2.43 |
| 1614514_at | CF405361 | TC66847 | Q84V39 | Glucan endo-1,3-b-glucosidase | Carbohydrate metabolism | 2 | 2.42 |
| 1608156_at | CF207998 | TC58210 | Q9XEY7 | Trehalase | Carbohydrate metabolism | 11 | 2.4 |
| 1612056_at | BQ795970 | - | Q5BMC5 | Phosphomannose isomerase | Carbohydrate metabolism | 19 | 2.39 |
| 1612295_at | CF512417 | TC67968 | Q5VMJ5 | Pyrophosphate-dependent phosphofructo-1-kinase | Carbohydrate metabolism | 15 | 2.38 |
| 1609079_at | BQ796278 | TC60979 | Q94KE3 | Pyruvate kinase | Carbohydrate metabolism | 13 | 2.36 |
| 1615874_at | CF403960 | TC54126 | Q93XR7 | Fructose-6-phosphate,2-kinase\/fructose-2,6-bisphosphatase | Carbohydrate metabolism | 2 | 2.35 |
| 1608883_at | CA818676 | TC60515 | Q94AA4 | Pyrophosphate-dependent phosphofructo-1-kinase | Carbohydrate metabolism | 15 | 2.34 |
| 1616002_s_at | CB345569 | TC52261 | Q8LL68 | Aldolase | Carbohydrate metabolism | 3 | 2.29 |
| 1620865_at | CB917214 | TC66899 | Q7XBE4 | Enolase | Carbohydrate metabolism | 11 | 2.29 |
| 1607147_at | CF404016 | - | Q5BLY0 | a-amylase | Carbohydrate metabolism | 10 | 2.28 |
| 1607727_at | CB976321 | TC57680 | Q5IH14 | Sucrose-6-phosphate phosphatase | Carbohydrate metabolism | 11 | 2.26 |
| 1614707_at | BQ799313 | TC53692 | P32811 | a-glucan phosphorylase | Carbohydrate metabolism | 21 | 2.25 |
| 1610277_at | CF208016 | TC70514 | Q50HW6 | b-1,3-glucuronosyltransferase | Carbohydrate metabolism | 2 | 2.25 |
| 1621432_s_at | CD005042 | TC52007 | Q8VXZ7 | a-galactosidase | Carbohydrate metabolism | 10 | 2.18 |
| 1621053_at | CF414284 | TC63955 | Q6VWJ5 | Fructokinase | Carbohydrate metabolism | 3 | 2.16 |
| 1621719_at | CF404994 | TC65554 | Q8LGH6 | Dihydrolipoamide S-acetyltransferase | Carbohydrate metabolism | 3 | 2.14 |
| 1619373_at | CB920390 | TC69024 | P80572 | Alcohol dehydrogenase | Carbohydrate metabolism | 3 | 2.13 |
| 1614612_at | CF513589 | TC63370 | Q9LSG3 | Glucose acyltransferase | Carbohydrate metabolism | 2 | 2.13 |
| 1615252_at | BQ792622 | TC60550 | Q5N8H1 | Hydrolase-like protein | Carbohydrate metabolism | 3 | 2.08 |
| 1612568_at | CF405938 | TC67425 | Q9LIB2 | Glycogen phosphorylase B | Carbohydrate metabolism | 13 | 2.07 |
| 1614153_at | CF207979 | TC54491 | Q7EYK9 | Glucose-6-phosphate 1-dehydrogenase | Carbohydrate metabolism | 4 | 2.03 |
| 1621378_at | BQ794342 | TC61809 | Q42581 | Ribose-phosphate pyrophosphokinase 1 | Nucleotide metabolism | 11 | 4.5 |
| 1607578_at | CF415519 | TC56533 | O22141 | Nucleotide sugar epimerase | Nucleotide metabolism | 2 | 4.09 |
| 1608708_at | CF211873 | TC53982 | Q9SU83 | Nucleotide pyrophosphatase | Nucleotide metabolism | 18 | 3.73 |
| 1616669_at | CF209174 | TC54382 | Q3EAE2 | dTDP-4-dehydrorhamnose reductase | Nucleotide metabolism | 3 | 3.45 |
| 1609246_s_at | CF206363 | TC54199 | Q655Y8 | UDP-glucose 4-epimerase | Nucleotide metabolism | 4 | 3.14 |
| 1607889_a_at | CB976234 | TC58106 | Q6IVK4 | UDP-glucuronate decarboxylase 2 | Nucleotide metabolism | 4 | 2.55 |
| 1622819_at | BQ798887 | TC59368 | O22141 | Nucleotide sugar epimerase | Nucleotide metabolism | 2 | 2.52 |
| 1616344_at | CF209136 | TC68545 | Q6XP48 | UDP-glucose 4-epimerase | Nucleotide metabolism | 21 | 2.52 |
| 1614498_at | CF213286 | TC57825 | O65781 | UDP-galactose 4-epimerase | Nucleotide metabolism | 21 | 2.32 |
| 1620930_s_at | CF212327 | TC51843 | Q6IVK4 | UDP-glucuronate decarboxylase 2 | Nucleotide metabolism | 4 | 2.21 |
| 1614184_at | CF604220 | TC66293 | Q9SA77 | UDP-galactose 4-epimerase | Nucleotide metabolism | 21 | 2.13 |
| 1618478_at | CF515277 | - | O64749 | UDP-galactose-4-epimerase | Nucleotide metabolism | 20 | 2.11 |
| 1616383_at | CF609704 | TC59968 | Q8L9F5 | dTDP-glucose 4-6-dehydratase | Nucleotide metabolism | 21 | 2.06 |
| 1615814_at | CB920915 | TC56030 | Q7FAH2 | Glyceraldehyde-3-phosphate dehydrogenase | Phosphate Metabolism | 10 | 2.87 |
| 1622715_s_at | CA809281 | TC51781 | P12858 | Glyceraldehyde-3-phosphate dehydrogenase | Phosphate Metabolism | 3 | 2.45 |
| 1618277_at | CF568829 | TC56963 | Q8VWN9 | Glyceraldehyde-3-phosphate dehydrogenase | Phosphate Metabolism | 21 | 2.22 |
| 1616083_at | CB009608 | TC51694 | Q9ZR63 | Hexose transporter (VvHT1) | Transport | 2 | 12.37 |
| 1610527_at | CA815926 | TC52979 | Q84QH3 | Sorbitol transporter | Transport | 2 | 5.49 |
| 1615257_at | CB972713 | TC65400 | Q4U339 | Hexose transporter (VvHT6) | Transport | 15 | 4.7 |
| 1619691_at | CF211807 | TC62520 | Q4U339 | Hexose transporter (VvHT6) | Transport | 14 | 3.69 |
| 1613408_at | CB347178 | TC66667 | P93075 | Sucrose transporter (BvST1) | Transport | 11 | 2.92 |
| 1608991_at | CA816013 | TC60060 | Q8GTR0 | Sugar transporter | Transport | 10 | 2.86 |
| 1610298_at | CB972367 | TC53493 | Q8LES0 | Golgi nucleotide sugar transporter (GONST) 4 | Transport | 2 | 2.71 |
| 1615697_at | AF021810 | TC51724 | Q4JLW1 | Sucrose transporter (VvSuc27) | Transport | 3 | 2.44 |
| 1611331_at | CF201541 | TC69532 | Q69M22 | Golgi nucleotide sugar transporter (GONST) 4 | Transport | 7 | 2.2 |
| 1612481_at | CF213270 | - | Q6ID34 | Glycerol 3-phosphate transporter | Transport | 4 | 2.03 |
Hexose and triose phosphate transport
The transcript abundances of numerous hexose and triosephosphate transporters varied considerably over the course of berry development (Figure 8C) indicating that each may fulfill different transport roles. The transcript abundance for a VvHT1 (1616083_at, TC51694), a previously described hexose transporter (VvHT1) located at the sieve cell-companion cell interface in the phloem and thought to play a major role in providing energy (mainly from glucose) for cell division and cell growth during the early stages of berry development [99], was high during Phase I, but then declined rapidly during ripening; this is largely consistent with an earlier report [18]. A second hexose transporter, VvHT6 (1615257_at; TC65400) exhibited a peak in transcript abundance near the start of véraison (E-L stage 34), which correlated well with hexose accumulation in the berries (Figure 8A), indicating that this transporter may play a significant role in hexose accumulation during berry ripening. Another previously described sucrose transporter (VvSUC27; 1615697_at, TC51724) [100], exhibited decreased transcript abundance throughout berry development consistent with earlier observations.
A putative plastidic glucose transporter (1608991_at, TC60060) showed increased transcript abundance up to E-L stage 34 and then remained constant throughout berry ripening (Figure 8C). The transcript abundance of a putative plasma membrane sugar/polyol transporter (1613408_at, TC66667), which resembles the AtPLT5 gene from A. thaliana [101] and is also capable of hexose transport, increased gradually over the course of berry development. In addition, two transcripts encoding a plastidial phosphate translocator-like (PTL) protein (1619379_at, TC58801) and a plastidial triosephosphate/phosphate translocator, TPT (1622157_at, TC61733) [102] displayed similar expression patterns that peaked at E-L stage 34 and then declined with berry ripening. The observed patterns of expression of the plastidial glucose and triosephosphate transporters indicate that both glucose and triosephosphates may be mobilized as export products as a result of active starch metabolism in plastids of developing and ripening berries.
Finally, a sorbitol transporter (Figure 9) that has high homology with a cherry sorbitol transporter (PcSOT2) [103], has high transcript abundance early in fruit development as it does in cherry fruit. This transporter has high specificity for sorbitol as compared to its isomer, mannitol [103]. We were able to detect a sugar alcohol in our polar extracts using GC-MS, but were unable to distinguish whether it was sorbitol or mannitol. Further work will be done to distinguish sorbitol from mannitol. Note, however, that sorbitol has been detected in the sap of grapevines [104].
Figure 9.
Transcriptomic mapping of transcripts related sucrose and starch metabolism along berry development. SPS: sucrose phosphate synthase-(1614674_at, TC60623), SPP: sucrose phosphate phosphorylase – (1608257_at, TC68135) SUSY: sucrose synthase – a) (1616700_at, TC53526) b) (1619223_s_at, TC52910) c) (1609402_at, TC62599) INV: invertase – a) (1620628_at, TC67908) b) (1611027_at, TC56057) c) (1612836_at, TC57719) d) (1611613_at, TC60693) HK: hexokinase-(1611419_at – TC53318) FK: fructokinase – a) (1628006_at, TC63769), b) (1622282_at, TC54393), c) (1621053_at, TC63955), d) (1616255_at, TC57339) SuT: sucrose transporter – a) (1620256_at, AF021808) b) (1622221_at, AF021809) c) (1615697_at, TC51724) d) (1615257_at, TC65400) NPP: nucleotide pyrophosphatase – (1620770_at, TC53085) SDH: sorbitol dehydrogenase – (1608527_at, TC58983) ST: sorbitol transporter – (1610527_at, TC52979) AGPase: ADP-glucose phosphatase – a) (1608393_at, TC64860) b) (1610928_at, TC64860) SBE: starch branching enzyme – (1621790_at, TC65671) SS: starch synthase – a) (1615571_at, TC53551) b) (1613601_at, TC67353) SP: starch phosphorylase – a) (1622120_at, TC54533) b) (1614707_at, TC53692) α AM: α-amylase – (1613188_at, TC70258) β AM: β-amylase – a) (1617124_at, TC67979) b) (1611808_at, CF205006) SEX: water dikinase – (1617941_at, TC62494) TPT: triose phosphate transporter – a) (1608991_at, TC60060) b) (1619379_at, TC58801) c) (1622157_at, TC61733). Each square from left to right corresponds to the expression of the probe sets from stage 31 through stage 38. Nonsignificant: Does not pass the ANOVA filter.
Starch metabolism
Starch metabolism in developing and ripening grape berries is poorly understood. Starch synthase I catalyzes the elongation of glucans by the addition of glucose residues from ADP-glucose through the formation of α-1,4 linkages and is a major determinant for the synthesis of transient starch reserves in plants [105]. Our data indicate that starch metabolism is significant in berries. Starch concentrations declined significantly during Phase III of berry development; E-L stage 35, 36 and 38 were equal to 774 ± 57, 715 ± 54 and 554 ± 28 μg of glucose per g fresh weight of berry, respectively (mean ± SE).
Furthermore, the transcript abundance of numerous transcripts involved in starch metabolism changed during berry development. One plastidial soluble starch synthase Unigene (1615571_at, TC53551) displayed increasing transcript abundance, while a second Unigene (1613601_at, TC67353) displayed decreasing transcript abundance during berry development (Figure 8D). A transcript for the plastidial α-glucan, water dikinase (Gwd) gene (1617941_at, TC62494), which encodes an enzyme that is a regulator of starch mobilization and is essential for starch degradation [106], showed increased accumulation during berry development much like starch synthase I (1615571_at, TC53551). A second Gwd isogene (1617068_at, TC54621), showed peak transcript expression at E-L stage 35, but declined in fully ripe berries. Expression of plastidial α-1,4 glucan phosphorylase (Starch phosphorylase L isozyme, 1622120_at, TC54533), a starch mobilization enzyme that phosphorylates amylopectin to catalyze the release of glucose-1-phosphate, was nearly coordinate with the expression of this latter Gwd isogene. Finally, transcripts encoding the starch degrading enzymes, α-amylase (1613188_at, TC70258) and β-amylase (1617124_at, TC67979), both showed increased abundance during berry development (Figure 8D). Grape berries are likely to contain intact and functional plastids at véraison and at later stages of ripeness as shown by in situ fixation of exocarp and mesocarp cells [107].
Figure 9 summarizes the major pathways of hexose sugars and polysaccharide flux and putative transport processes in the developing berry as defined by the combined transcriptomic and metabolite analyses performed in this study. Abridged gene expression patterns for key regulatory genes involved in both sucrose and starch metabolism are shown. One can easily visualize the coordinate transcript expression patterns for the entire pathway along berry development. It is not apparent from this analysis why fructose concentrations would be higher than glucose in berries. This indicates that the regulation of these hexoses by hexokinase genes, whose transcripts did not significantly change (data not shown), is more complex than what can be discerned from a simple examination of transcript profiles.
Photosynthesis and carbon assimilation
During berry development transcripts encoding proteins associated with photosynthesis-related functions are strongly expressed during the flowering stage and the so-called "herbaceous phase" or Phase I of berry development with expression declining during the later stages of berry maturation [17,18]. In our data, around 100 Unigenes with photosynthesis-related functions were identified with most displaying a steady or transient decline in the transcript abundance across berry development (Additional file 5, Table 7). Similarly, transcripts encoding enzymes with roles in carbon assimilation also exhibited a declining pattern of expression. For instance, Unigenes encoding Calvin cycle enzymes such as glyceraldehyde-3-phosphate dehydrogenase (1615814_at, TC56030; 1622715_s-at, TC51781), phosphoribulokinase (1614716_at, TC58640), transketolase (1618061_a_at, TC52548) as well as ribulose biphosphate carboxylase/oxygenase small subunit (1612848_x_at, TC64044) were highly expressed and then declined during Phase III of berry development consistent with previous reports [18].
Table 7.
Transcripts (TFR pool) related to Energy metabolism within specific sub-sections
| Probeset ID | GenBank Annotation | VvGI5 | Uniprot ID | Gene Name Description | Function | Profile | Fold Change |
| 1612882_at | CD720949 | TC64621 | A5BS41 | ATP-dependent transmembrane transporter | ATP binding | 2 | 5.55 |
| 1612645_at | CB344170 | TC55708 | P32980 | ATP synthase | ATP binding | 3 | 3.59 |
| 1616533_at | CB339497 | TC62259 | P31853 | ATP synthase B' chain | ATP binding | 12 | 2.88 |
| 1618182_at | CF604629 | TC63054 | P19023 | ATP synthase beta chain | ATP binding | 21 | 2.23 |
| 1607759_at | CD798264 | TC67523 | Q43433 | Vacuolar ATP synthase subunit B isoform 2 | ATP binding | 11 | 2.16 |
| 1620500_at | CB349662 | CB349662 | Q67IU5 | Ribulose 1,5-bisphosphate carboxylase small subunit | Carbon dioxide fixation | 3 | 19.73 |
| 1613936_x_at | CF568996 | CF568996 | O22077 | Ribulose bisphosphate carboxylase small chain | Carbon dioxide fixation | 3 | 2.36 |
| 1616918_s_at | CB345541 | TC56391 | Q40281 | Rubisco activase | Carbon dioxide fixation | 3 | 2.22 |
| 1619681_at | CD799678 | TC70996 | Q9C5C7 | Rubisco expression protein | Carbon dioxide fixation | 11 | 2.18 |
| 1612848_x_at | CF202280 | CF202280 | P10795 | Ribulose bisphosphate carboxylase | Carbon dioxide fixation | 3 | 2.13 |
| 1620551_s_at | CB339855 | TC56836 | O98997 | Rubisco activase | Carbon dioxide fixation | 3 | 2.13 |
| 1610491_at | CD010750 | TC57419 | Q8LF17 | Ribulose-1,5-bisphosphate carboxylase/oxygenase | Carbon dioxide fixation | 12 | 2.13 |
| 1616847_s_at | CA816751 | TC68454 | O22077 | Ribulose bisphosphate carboxylase small chain | Carbon dioxide fixation | 3 | 2.12 |
| 1616435_at | CB974220 | TC68219 | P08927 | RuBisCO subunit binding-protein beta subunit | Carbon dioxide fixation | 3 | 2.1 |
| 1622299_s_at | CK136935 | CK136935 | Q9LKH8 | NADPH-protochlorophyllide oxidoreductase | Chlorophyll biosynthesis | 3 | 5.3 |
| 1619717_at | CF210684 | TC59048 | Q9SDT1 | NADPH:protochlorophyllide oxidoreductase | Chlorophyll biosynthesis | 3 | 4.79 |
| 1606624_at | CF606923 | TC64589 | Q43082 | Porphobilinogen deaminase | Chlorophyll biosynthetic process | 1 | 2.5 |
| 1617935_at | CB974545 | TC68056 | Q7YJS8 | NADH dehydrogenase 49kDa subunit | Complex 1 | 16 | 5.55 |
| 1611418_at | CB342953 | TC56161 | Q7YJ08 | NAD(P)H-quinone oxidoreductase | Complex 1 | 14 | 5.09 |
| 1609373_at | CD800734 | TC60468 | Q6KGY1 | NADH dehydrogenase | Complex 1 | 14 | 3.05 |
| 1614095_at | CF606244 | TC62268 | P06261 | NAD(P)H-quinone oxidoreductase | Complex 1 | 21 | 2.83 |
| 1609421_at | BQ795266 | TC62811 | O65414 | NADH dehydrogenase | Complex 1 | 11 | 2.75 |
| 1617757_at | CA818465 | TC58524 | Q6YSN0 | NADH dehydrogenase | Complex 1 | 21 | 2.42 |
| 1612005_s_at | CB004075 | TC64671 | Q68S01 | NADH dehydrogenase | Complex 1 | 3 | 2.27 |
| 1610869_at | CF515388 | TC56269 | Q8H2T7 | NADH dehydrogenase subunit | Complex 1 | 16 | 2.23 |
| 1610347_s_at | CF202826 | CF202826 | Q0ZIW2 | NAD(P)H-quinone oxidoreductase | Complex 1 | 10 | 2.1 |
| 1609391_s_at | CF404650 | TC53103 | Q41001 | Copper Binding Protein | Copper ion binding | 2 | 26.11 |
| 1620588_at | CD801157 | CD801157 | Q8LED5 | Mavicyanin | Copper ion binding | 6 | 25.27 |
| 1621220_at | CB919187 | TC59624 | Q9M510 | Dicyanin | Copper ion binding | 11 | 17.36 |
| 1610220_at | CB973621 | TC68272 | O81500 | Copper Binding Protein | Copper ion binding | 3 | 14.36 |
| 1617350_at | CB975555 | TC58747 | Q39131 | Copper Binding Protein | Copper ion binding | 3 | 10.21 |
| 1611332_at | CF371813 | TC59560 | Q653S5 | Blue copper binding protein (bcb) | Copper ion binding | 1 | 7.64 |
| 1607270_at | CB923224 | TC60083 | Q9ZRV5 | Copper Binding Protein | Copper ion binding | 2 | 5.87 |
| 1620744_at | CF403966 | TC65998 | P17340 | Plastocyanin, chloroplast precursor | Copper ion binding | 3 | 4.41 |
| 1617046_at | CF512505 | TC54856 | O23230 | Trichohyalin | Copper ion binding | 3 | 2.23 |
| 1609233_at | CF512410 | TC54170 | Q84RM1 | Copper Binding Protein | Copper ion binding | 2 | 2.21 |
| 1618207_at | CB347324 | TC52865 | Q9C540 | Cytochrome 561 | Electron carrier activity | 3 | 7.45 |
| 1612624_at | CB974055 | TC58854 | P06449 | Apocytochrome f | Electron carrier activity | 21 | 5.32 |
| 1611598_at | CB970208 | TC60594 | Q9ZSR3 | Cytochrome b-561 | Electron carrier activity | 2 | 4.54 |
| 1606617_at | CF608010 | TC65350 | O23344 | Electron transport | electron carrier activity | 3 | 2.49 |
| 1606704_s_at | CF200937 | CF200937 | P59702 | Cytochrome b559 alpha subunit | Electron carrier activity | 21 | 2.33 |
| 1615927_s_at | CB972155 | TC55109 | Q6Q8B8 | Chloroplast ferredoxin I | Electron carrier activity | 3 | 2.23 |
| 1607800_at | CB972521 | TC52149 | Q84WN3 | Cytochrome c oxidoreductase | Electron transport | 2 | 17.79 |
| 1620504_at | CB342755 | TC52829 | Q84WN3 | cytochrome c oxidoreductase | Electron transport | 7 | 14.78 |
| 1618535_at | CA818656 | CA818656 | Q6V5G1 | Cu2+ plastocyanin | Electron transport | 13 | 6.25 |
| 1615046_at | CF210436 | TC59116 | P41346 | Ferredoxin--NADP reductase | Electron transport | 3 | 5.61 |
| 1614266_at | BQ792322 | TC57184 | Q49KU9 | Cytochrome c heme attachment protein | Electron transport | 16 | 4.17 |
| 1613158_at | CB349843 | CB349843 | O47437 | Cytochrome c oxidase | Electron transport | 16 | 3.56 |
| 1612766_s_at | CF569219 | CF569219 | Q5PY86 | NADH-cytochrome b5 reductase | Electron Transport | 3 | 3.32 |
| 1619756_at | CB003378 | TC59085 | Q9LYC6 | Glutaredoxin | Electron transport | 14 | 2.88 |
| 1620991_at | CB344999 | TC58191 | O24068 | Cytochrome oxidase subunit 3 | Electron transport | 16 | 2.26 |
| 1606445_a_at | CF512668 | TC62694 | P26291 | Cytochrome B6-F complex iron-sulfur subuni | Electron transport | 3 | 2.16 |
| 1608372_at | CF208491 | TC51964 | Q6K7S7 | Cytochrome c biogenesis | Electron transport | 9 | 2.1 |
| 1621402_a_at | CF213496 | TC53161 | P00051 | Cytochrome c | Electron transport | 11 | 2.01 |
| 1607356_at | CB911288 | TC67262 | Q8LCF6 | Hypothetical Protein | ENERGY | 11 | 12.28 |
| 1611972_s_at | CF519112 | TC53292 | A4X6H5 | Cytochrome b | ENERGY | 3 | 11.63 |
| 1615762_at | CD798079 | TC66865 | O80763 | Hypothetical Protein | ENERGY | 10 | 4.82 |
| 1616241_at | CD797326 | CD797326 | Q8VYC5 | Hypothetical Protein | ENERGY | 16 | 4.08 |
| 1609285_at | CF414528 | TC57440 | Q9FFT2 | Hypothetical Protein | ENERGY | 2 | 3.44 |
| 1611820_at | CB914713 | TC69253 | O80763 | Hypothetical Protein | ENERGY | 3 | 3.09 |
| 1606562_at | CF404246 | TC59415 | A3J369 | Nitrilase 1 | ENERGY | 11 | 3.04 |
| 1621817_at | CB978007 | TC64650 | O80763 | Hypothetical Protein | ENERGY | 3 | 3 |
| 1614875_at | CF518552 | TC69033 | A5AU55 | Hypothetical Protein | ENERGY | 11 | 2.73 |
| 1622517_at | CB970523 | TC54809 | Q8W4Z5 | Hypothetical Protein | ENERGY | 3 | 2.39 |
| 1621903_at | CF404558 | TC62294 | Q9FE29 | Hypothetical Protein | ENERGY | 13 | 2.23 |
| 1612648_at | CD798203 | CD798203 | O80763 | Hypothetical Protein | ENERGY | 21 | 2.15 |
| 1622345_at | CB970837 | TC55633 | Q7XTZ0 | Mandelonitrile lyase | Flavoprotein | 2 | 4.87 |
| 1606948_at | CF404230 | CF404230 | Q01JW7 | Mandelonitrile lyase | Flavoprotein | 2 | 3.68 |
| 1622745_at | BQ796736 | TC58626 | Q8L5Q7 | Quinone oxidoreductase | FMN binding | 15 | 19.63 |
| 1615481_at | CB973026 | TC62178 | Q9ZSP7 | Cytochrome b5 DIF-F | Iron ion binding | 3 | 2.57 |
| 1606727_at | BQ799998 | TC62672 | Q58IV4 | Phytochrome C | Light Signaling | 10 | 2.38 |
| 1617604_at | CF609932 | TC59809 | Q94BM7 | Phytochrome A supressor spa1 protein | Light Signaling | 11 | 2.25 |
| 1611135_at | CB983077 | TC51911 | Q9SG92 | Alpha-hydroxynitrile lyase | Lyase activity | 11 | 2.92 |
| 1622108_at | CF405863 | TC56579 | Q9SU40 | Monocopper oxidase | Multicopper oxidase family | 3 | 23.07 |
| 1621115_at | CF609165 | TC64136 | Q9SU40 | Monocopper oxidase | Multicopper oxidase family | 1 | 20.1 |
| 1617992_a_at | CF213671 | TC60094 | P51132 | Ubiquinol--cytochrome-c reductase-like protein | Oxidative Phosphorespiration | 11 | 2.18 |
| 1611597_at | CB918250 | CB918250 | Q8LDU4 | Red chlorophyll catabolite reductase | Oxidoreductase activity | 12 | 2.03 |
| 1613786_at | CD714955 | TC57282 | Q6QY10 | P700 chlorophyll a apoprotein A1 | Photosystem I | 21 | 8.65 |
| 1611364_at | CF211293 | TC52528 | Q9XQB4 | Reaction center subunit III | Photosystem I | 3 | 7.67 |
| 1611464_at | CF215949 | TC59235 | Q9XF85 | Lhca5 protein | Photosystem I | 3 | 7.21 |
| 1621532_at | CB973721 | TC64270 | Q84QE6 | Reaction center subunit X psaK | Photosystem I | 3 | 7.15 |
| 1619903_at | CD720479 | TC65556 | Q40512 | Light-harvesting chlorophyll a/b-binding protein | Photosystem I | 3 | 6.96 |
| 1619629_at | CB340944 | TC66352 | Q5DNZ6 | Chlorophyll a-b binding protein | Photosystem I | 3 | 6.59 |
| 1622534_at | BQ799942 | TC53444 | Q84U30 | Photosystem I-N subunit | Photosystem I | 3 | 6.45 |
| 1611733_s_at | BQ797982 | TC52546 | Q70PN9 | Reaction centre PSI-D subunit precursor | Photosystem I | 3 | 6.44 |
| 1616560_at | CA817733 | TC62550 | Q84WT1 | Light-harvesting chlorophyll a/b binding protein | Photosystem I | 3 | 5.9 |
| 1611515_s_at | CB343423 | TC57304 | O65101 | Reaction center subunit VI | Photosystem I | 3 | 5.33 |
| 1618370_at | CF510718 | TC57721 | Q9SUI4 | Reaction center subunit XI | Photosystem I | 3 | 5.31 |
| 1617771_at | CF414158 | TC58342 | Q8RVJ8 | Reaction centre subunit IV | Photosystem I | 3 | 4.87 |
| 1618127_at | CB968637 | TC62932 | Q9SY97 | Chlorophyll a/b-binding protein | Photosystem I | 3 | 4.74 |
| 1614409_at | CA817387 | TC55189 | P13869 | Chlorophyll a-b binding protein | Photosystem I | 3 | 4.65 |
| 1614593_at | CF511805 | TC52379 | Q00321 | CP29 polypeptide | Photosystem I | 3 | 4.43 |
| 1611924_at | CA817406 | TC63702 | Q646H3 | Reaction center V | Photosystem I | 3 | 4.06 |
| 1611161_at | CF210442 | TC54044 | Q9ZU86 | Expressed protein | Photosystem I | 3 | 3.09 |
| 1622302_s_at | CF207602 | TC54765 | Q40459 | Oxygen-evolving enhancer protein 1 | Photosystem I | 3 | 2.8 |
| 1610245_at | CF209798 | TC53968 | Q41424 | Chlorophyll a/b binding protein | Photosystem II | 3 | 15.82 |
| 1615822_at | CF208321 | TC52049 | Q9XQB1 | LHCII type III chlorophyll a/b binding protein | Photosystem II | 3 | 10.43 |
| 1608311_at | CF202519 | CF202519 | Q7M1K9 | Chlorophyll a/b-binding protein | Photosystem II | 3 | 10.24 |
| 1616940_s_at | CB348709 | TC52113 | Q7M1K9 | Chlorophyll a/b-binding protein | Photosystem II | 3 | 9.95 |
| 1618116_s_at | BQ798823 | TC55659 | Q32291 | Chlorophyll A/B binding protein precursor | Photosystem II | 3 | 7.28 |
| 1611860_at | CF209952 | TC57521 | Q9XQB6 | Chlorophyll a/b-binding protein CP24 | Photosystem II | 3 | 6.6 |
| 1612085_at | CF413799 | TC54542 | Q41387 | Reaction center W protein | Photosystem II | 3 | 5.79 |
| 1621038_at | CF372077 | TC57214 | O64448 | Light harvesting chlorophyll a/b-binding protein precursor | Photosystem II | 3 | 5.77 |
| 1618679_s_at | CB343106 | TC52042 | Q9BBT1 | 44 kDa reaction center protein | Photosystem II | 16 | 5.41 |
| 1610203_at | CD009386 | TC56267 | Q7YJY8 | Photosystem Q(B) protein | Photosystem II | 21 | 5.11 |
| 1613991_at | CF510955 | TC53743 | P80470 | Core complex proteins psbY | Photosystem II | 3 | 4.77 |
| 1607516_at | CB972913 | TC53930 | Q9LRC4 | Oxygen evolving enhancer protein 1 precursor | Photosystem II | 3 | 4.66 |
| 1613428_at | CF207158 | TC52084 | Q5PYQ5 | Chloroplast oxygen-evolving enhancer protein | Photosystem II | 3 | 4.47 |
| 1607961_at | CF415716 | TC57429 | P31336 | 5 kDa protein | Photosystem II | 3 | 4.16 |
| 1614598_at | CF373065 | TC61762 | Q9XQB2 | Chlorophyll a/b binding protein CP29 | Photosystem II | 3 | 4.06 |
| 1613691_s_at | CF511746 | TC54828 | P27518 | Chlorophyll a-b binding protein 151 | Photosystem II | 3 | 3.84 |
| 1613773_s_at | BQ799145 | TC63656 | Q41387 | Reaction center W protein | Photosystem II | 3 | 3.34 |
| 1618031_s_at | CF404451 | TC53833 | Q8GV53 | 10 kDa protein | Photosystem II | 3 | 3.28 |
| 1621351_s_at | CB340283 | TC53732 | Q40961 | Light-harvesting chlorophyll a/b-binding protein precursor | Photosystem II | 3 | 3.2 |
| 1613494_s_at | CA813944 | TC55522 | Q9SLQ8 | Oxygen-evolving enhancer protein 2 | Photosystem II | 3 | 3.05 |
| 1617605_at | CF513977 | TC55526 | Q8HS34 | CP47 protein | Photosystem II | 18 | 2.99 |
| 1618274_at | CB972471 | TC55538 | Q4FFQ9 | Phosphoprotein | Photosystem II | 21 | 2.92 |
| 1610144_at | CB342508 | TC53591 | Q9MTN0 | Uncharacterized 6.9 kDa protein in psbD-trnT intergenic region | Photosystem II | 15 | 2.88 |
| 1611582_s_at | CB970190 | TC70959 | Q02060 | 22 kDa protein | Photosystem II | 3 | 2.69 |
| 1607926_at | CF202256 | CF202256 | Q9AR57 | Putative membrane protein | Photosystem II | 2 | 2.56 |
| 1621978_at | CB837910 | TC56626 | Q9M3M7 | Uncharacterized protein | Photosystem II | 16 | 2.31 |
| 1607803_at | CB975690 | TC52112 | Q06364 | 26S proteasome non-ATPase regulatory subunit 3 | Photosystem II | 21 | 2.31 |
| 1619523_at | CB969438 | TC67627 | Q952R1 | Succinate dehydrogenase | Succinate dehydrogenase activity | 15 | 2.24 |
Circadian cycles
Circadian clocks are signaling networks that enhance an organism's growth, survival, and competitive advantage in rhythmic day/night environments [108]. The plant circadian clock modulates a wide range of physiological and biochemical events, such as stomatal and organ movements, photosynthesis and induction of flowering. A model of circadian rhythm based upon activities of several enzymes has been created involving transcription factors such as CIRCADIAN CLOCK-ASSOCIATED 1 (CCA1) or pseudo-response regulators such as PRR7 [108]. Transcripts for Unigene (1616834_at, TC54726) encoding CCA1 were repressed during the early stages of berry development, but increased in abundance at E-L stage 36. In contrast, one Unigene (1608006_at, TC51808) related to the two-component response regulator APRR7 had a transient peak of expression in the early stages of berry development. This result is consistent with the position and function of these proteins in the circadian clock. Indeed, APRR7 represses CCA1 activity in Arabidopsis thaliana. In grape, these correlations in the transcript abundance indicate the operation of the circadian clock machinery throughout berry development. In addition, those genes are thought to enhance starch mobilization, consistent with previous observations made during Phase III of berry development [109].
Pathogen and disease resistance related proteins
Pathogen-related (PR) proteins are the most abundant class of proteins present in wine and can negatively affect the clarity and stability of wine [110]. During berry development, PR genes are expressed highly throughout various stages of berry growth. Around 30 Unigenes encoding different classes of PR genes were identified with a two-fold ratio or greater expression change (Additional file 5, Table 8). Interestingly, four Unigenes encoding PR1 protein were highly expressed during early berry development, but then declined for the remainder of berry development. PR1 protein is regarded as one of the main down-stream responses of the salicylic acid signaling that plays an important role in Systemic Acquired Resistance. Salycylic acid is thought to accumulate just before véraison, which correlates well with the PR1 mRNA and protein expression [111]. The two main PR proteins that have a significant role in the defense against invading fungal pathogens are β-1,3-glucanase (PR2) and chitinase (PR3) [112]. Five Unigenes encoding β-1,3-glucanase were transiently expressed at different periods of berry development. Unigenes encoding various chitinases were also identified that displayed similar mRNA expression patterns. Some chitinase genes exhibit strong homologies with a chitinase previously observed in grape berry [111]. Another PR protein, which may play a role in grape berry defense, is thaumatin protein (PR5) [113]. Eight Unigenes encoding PR5 proteins were identified and their respective expression patterns span all stages of berry development. Taken together, the expression patterns revealed that these defense-related gene products and enzymes are expressed across all stages of berry development. Such a Systemic Acquired Resistance strategy probably minimizes pathogen invasion as previously suggested [114].
Table 8.
Transcripts (TFR pool) related to Pathogenesis-Related proteins within specific sub-sections
| Probeset ID | GenBank Annotation | VvGI5 | Uniprot ID | Gene Name Description | Category | Profile | Fold Change |
| 1613471_at | CF215857 | TC59306 | Q9SW05 | Pathogenesis-related protein | PR1 | 3 | 7.83 |
| 1611058_at | CA814153 | TC67060 | Q7XAJ6 | Pathogenesis related protein 1 | PR1 | 3 | 5.76 |
| 1613816_x_at | CF074673 | TC56938 | Q7XAJ6 | Pathogenesis related protein 1 | PR1 | 21 | 4.74 |
| 1618533_at | CB970020 | TC55782 | Q40374 | Pathogenesis related protein 1 | PR1 | 2 | 2.31 |
| 1615595_at | AF239617 | AF239617 | Q9M563 | β-1,3-glucanase | PR2 | 11 | 10.99 |
| 1620496_at | CF214365 | TC66187 | Q8VY12 | β-1,3-glucanase | PR2 | 15 | 2.3 |
| 1608203_at | CF511734 | TC64974 | Q94EN5 | β-1,3-glucanase | PR2 | 2 | 2.17 |
| 1616183_at | CF405742 | TC62849 | Q94G86 | β-1,3-glucanase | PR2 | 14 | 2.17 |
| 1610324_a_at | CB346041 | TC67051 | Q8L868 | β-1,3-glucanase | PR2 | 12 | 2.05 |
| 1621319_s_at | CB981122 | TC70080 | Q7XAU6 | Chitinase IV | PR3 | 10 | 299.34 |
| 1613461_s_at | AF532966 | AF532966 | Q7XAU6 | Chitinase IV | PR3 | 10 | 162.58 |
| 1607557_at | CF202548 | CF202548 | Q7XAU6 | Chitinase IV | PR3 | 10 | 149.38 |
| 1614551_at | CB343715 | TC51734 | Q6JX04 | Chitinase | PR3 | 2 | 49.79 |
| 1616064_at | CF205270 | CF205270 | O24531 | Chitinase IV | PR3 | 11 | 20.19 |
| 1621583_at | CF404733 | TC62834 | Q6JX04 | Chitinase | PR3 | 1 | 4.93 |
| 1620111_at | CF568854 | CF568854 | Q6JX04 | Chitinase | PR3 | 2 | 3.36 |
| 1606625_at | CF603972 | TC64563 | Q7XB39 | Chitinase IV | PR3 | 19 | 3.21 |
| 1620518_at | CF201341 | CF201341 | O81228 | Pathogenesis related protein 4 | PR4 | 15 | 43.11 |
| 1618835_s_at | BQ797163 | TC58333 | O81228 | Pathogenesis related protein 5 | PR4 | 15 | 25.9 |
| 1612160_at | CF415249 | TC64611 | P50699 | Thaumatin | PR5 | 3 | 34.49 |
| 1618871_at | CF510551 | TC55284 | Q82L96 | Thaumatin | PR5 | 3 | 15.65 |
| 1616617_at | AF195654 | AF195654 | Q9SNY0 | Thaumatin | PR5 | 11 | 11.86 |
| 1607225_at | CB914105 | TC65548 | O65638 | Thaumatin | PR5 | 11 | 8.51 |
| 1614746_at | CF214284 | TC53053 | Q7XST4 | Thaumatin | PR5 | 2 | 5.27 |
| 1607708_at | CF413841 | TC63177 | Q9LZL8 | Thaumatin | PR5 | 2 | 4.22 |
| 1606517_at | CB347191 | TC62530 | Q8LBL4 | Thaumatin-like protein | PR5 | 14 | 3.51 |
| 1622374_at | CB920589 | TC56535 | Q41350 | Thaumatin | PR5 | 2 | 3.34 |
| 1613999_x_at | CF202364 | CF202364 | Q84S31 | Chitinase III | PR8 | 2 | 4.57 |
Quantitative real-time RT-PCR
To validate expression profiles obtained using the Affymetrix GeneChip® Vitis genome array, quantitative real-time RT-PCR was performed on 11 genes using gene-specific primers [Additional file 5, Table 3]. Transcript abundance patterns were calculated along the entire course of berry development. Linear regression ([microarray value] = a[RT-PCR value]+b) analysis showed an overall correlation coefficient of 0.94 indicating a good correlation between transcript abundance assessed by real-time RT-PCR and the expression profiles obtained with the GeneChip® genome arrays (Figure 10).
Figure 10.
Quantitative real-time RT-PCR of eleven transcripts. Comparison between the gene expression ratios reported by the Affymetrix GeneChip® genome array and by real-time RT-PCR. Data were from 11 probe sets across seven developmental stages. The difference in the number of PCR cycles required to produce the same amount of product is plotted against the log2 expression ratio averaged over the first time point. The linear regression line was constrained to pass through the origin. Grey solid square (1615402_at, TC56083)-ferulate-5-hydroxylase, Apricot solid triangle (1606794_at, TC63891)-osmotin precursor, red solid triangle (1616700_at, TC53526)-sucrose synthase, orange solid diamond (1607760_at, TC51695) flavonoid-3'5'-hydroxylase, light green solid round (1611650_at, TC57228)-WRKY7, dark green open square (1616880_at, TC54034)-cinnamoyl alcohol dehydrogenase, dark blue open triangle (1613896_at, TC62182)-nitrate/chloride transporter), blue open triangle (1615722_s_at, TC51776)-aquaporin PIP1.1, lavender open diamond (1611342_at, TC55943)-serine/threonine kinase, pink open circle (1612132_s_at, TC68311)-protein phosphatase 2C, brown cross (1614931_at, TC61058)-MYB transcription factor.
Conclusion
Our large-scale transcriptomic analysis demonstrated that nearly a third (28%) of genes expressed in berries exhibited at least two-fold or greater change in steady-state transcript abundance over the course of seven stages of grape berry development. Approximately two-thirds (64%) of these Unigenes could be assigned a functional annotation with the remaining one-third having obscure or unknown functions. Twenty distinct patterns of expression were resolved in order to illustrate the complex transcriptional regulatory hierarchies that exist to orchestrate the dynamic metabolic, transport, and control processes occurring in developing berries. We provided evidence that phytohormone biosynthesis and responses, particularly for ABA, ethylene, brassinosteroids, and auxins, as well as calcium homeostasis, transport, and signaling processes play critical roles in this developmental process. We also demonstrate that the expression and regulation of genes involved in cell wall biosynthesis and expansion, as well as genes involved in the biosynthesis, transport, and regulation of the phenylpropanoid and flavonoid pathways undergo dynamic changes throughout the course of berry development. Our analysis has revealed candidate genes that may participate in the production of different classes of aroma producing compounds. We have also demonstrated coordinate regulation of transcripts and the accumulation of key metabolites including tartrate, malate, and proline during berry development. A close examination of the behavior of gene expression patterns of genes involved in sugar and starch metabolism indicate that plastidial starch reserves are mobilized to fuel the production of hexose sugars during the ripening and maturation phase (Phase III) of berry development. Finally, our findings provide the first functional genomic information for hundreds of genes with obscure functions that can be exploited for hypothesis testing by traditional functional assays to improve our understanding of the complex developmental processes present in grape berries and to ultimately utilize this information to improve quality traits of wine grapes.
Methods
Plant Materials
Six twenty-year-old Cabernet Sauvignon (Vitis vinifera L.) vines grown on St. George rootstock were used during 2004 for this study. The vines were located at the Shenandoah Vineyard in Plymouth, CA, on a hillside row located in the middle of the vineyard. All plants were equipped with a drip irrigation system and watered daily to keep their water status high. Mid-day stem water potentials were measured weekly with a pressure chamber on two mature leaves per plant for a total of 6 vines [115]. For each measurement, a single leaf per plant was tightly zipped in a plastic bag to eliminate transpiration and covered with aluminum foil to deflect light and heat. After two hours of equilibration time, the petiole was cleanly cut and carefully threaded through a rubber gasket in the lid of a pressure chamber (3005 Plant Water Status Console, Soilmoisture Equipment Corp., Santa Barbara, CA, USA). The foil was removed before sealing the bagged leaf in the chamber. The balancing pressure required to visibly push stem xylem sap to the cut surface was recorded.
Two grape clusters were sampled weekly from each plant (n = 6), one from the south (sunny) and one from the north (shady) side of the plant. The clusters were pooled together for each plant in order to avoid light and temperature effects. Berry development was characterized by monitoring berry diameter, total soluble solids and titratable acidity. The berry diameter was measured with a micrometer for fifteen randomly selected berries per each of two clusters and an average berry size was computed per vine (n = 6). Total soluble solids (°Brix) were assayed (two technical replicates) with a refractometer (BRIX30, USA) from juice crushed from harvested berries from two clusters per vine (n = 6) to estimate total sugar content. Titratable acidity (g/L) of the grape juice was measured by titration to an endpoint of pH 8.4 with a strong base. The same number of repetitions as in °Brix measurements was used.
RNA extraction and microarray hybridization
Total RNA was extracted from berries finely ground in liquid nitrogen using Qiagen RNeasy Plant MidiKit columns (Qiagen Inc., CA) as previously described [116]. The total RNA was further purified using a Qiagen RNeasy Plant Mini Kit (Qiagen, Valencia, CA) according to the manufacturers' instructions. RNA integrity was confirmed by electrophoresis on 1.5% agarose gels containing formaldehyde and quality was confirmed by analysis on an Agilent 2100 Bioanalyzer using RNA LabChip® assays according to the manufacturer's instructions. mRNAs were converted to cDNAs using oligo dT primer containing a T7 RNA polymerase promoter sequence and reverse transcriptase. Biotinylated complementary RNAs (cRNAs) were synthesized in vitro using T7 RNA polymerase in the presence of biotin-labeled UTP/CTP, purified, fragmented and hybridized in the GeneChip® Vitis vinifera Genome Array cartridge (Affymetrix®, Santa Clara, CA). The hybridized arrays were washed and stained with streptavidin phycoerythrin and biotinylated anti-streptavidin antibody using an Affymetrix Fluidics Station 400. Microarrays were scanned using a Hewlett-Packard GeneArray® Scanner and image data was collected and processed on a GeneChip® workstation using Affymetrix® GCOS software.
Microarray data processing
Three biological replicates per experiment were processed to evaluate intra-specific variability. Expression data were processed by RMA (Robust Multi-Array Average) [117] using the R package affy [118]. Specifically, the RMA model of probe-specific background correction was first applied to the PM (perfect match) probes. These corrected probe values were normalized via quantile normalization and a median polish method was applied to compute one expression measure from all probe values. Data quality was verified by digestion curves describing trends in RNA degradation between the 5' end and the 3' end in each probe set. Differentially expressed genes throughout berry development were determined by ANOVA on the RMA expression values [118]. A multiple testing correction [22] was applied to the p-values of the F-statistics to adjust the false discovery rate. Genes with adjusted p-values < 0.05 were extracted for further analysis. Genes having a two-fold ratio (TFR) or greater between at least two time points along berry development were selected for further analyses. The RMA expression data (experiment Vv5) have been deposited in PLEXdb [119].
Microarray data analysis
Clustering of co-regulated genes was performed using the MultiExperimentViewer software part of the TM4 software package (MEV3.1) developed by TIGR [120]. TFR Unigenes were clustered via the Pavlidis Template Matching (PTM) algorithm [24]. The twenty template profiles were selected (by us) as representatives of biological processes occurring during berry development (Additional File 4). The Pearson correlation coefficient between each Unigene and each template profile was used to determine cluster membership: correlation measures greater than 0.75 corresponded to a good match. If genes were well correlated with more than one template profile, the gene was assigned to the cluster with which it had greatest correlation. The p-values associated to the hypothesis test of each correlation coefficient (null hypothesis is that the correlation is zero) were calculated and a multiple testing correction (Benjamini and Hochberg) was applied. Only genes with adjusted p-values ≤ 0.05 and correlations greater than 0.75 were placed into clusters
Unigene Annotation and Functional Analysis
Unigene annotation was updated by nucleotide sequence query of the probe consensus sequence against the UniProt/TrEMBL, NCBI-nr and TAIR protein databases using BLASTX (e-value < 1e-05). Functional categories were assigned automatically by amino acid homology to Arabidopsis thaliana proteins categorized according to the Munich Information Center for Protein Sequences (MIPS) Funcat 2 classification scheme [25]. Bibliographic searches were performed to assign functions to Unigenes exhibiting no homology with Arabidopsis thaliana proteins. Some annotation presented here will be subject to error due to the relatively correlative nature of these associations. It is expected that the annotated data presented here will be used for future hypothesis-driven research that can establish stronger functional analyses and annotations.
Attribution of the 20 clusters to the key developmental phases (I, II or III) (See Figure 6) was decided according to two criteria. The first one was to fit these phases with the time points used in this study. Stage 31 (Modified E-L System) was the only one belonging to the herbaceous phase (Phase I). The lag phase (Phase II) corresponded to stages 32 to 34. The maturation phase (Phase III) included stages 35 to 38. The second criterion was based on the time point at which the maximum average gene expression value was observed across the genes within each cluster. For instance, cluster 1 was included in the Phase I group, because the maximum average expression level was observed at stage 31. The same assignments were made in the other phases (II and III) (See Additional File 5: Table 1). To test for significant differences in the representation of Unigenes within each functional category per developmental phase (Phases I, II and III; see Figure 5), a Pearson's chi-squared test was used [121]. Three comparisons (Phase I against II; I against III and II against III) were performed and results are listed in Additional File 5: Table 2. Differences in frequency for each category between two stages were considered significant for a p-value < 0.05.
Real Time PCR experiments
RNA was extracted and its integrity verified by standard procedures. cDNA was synthesized using an iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's instructions with a uniform 1 μg RNA per reaction volume reverse-transcribed. Primers for genes (Additional File 5: Table 3) assayed by real-time PCR were selected using Primer3 software [122]. Quantitative real-time PCR reactions were prepared using an iTaq SYBR Green Supermix with ROX (Bio-Rad) and performed using the ABI PRISM® 7000 Sequence Detection System (Applied Biosystems, Foster City, CA). Expression was determined for triplicate biological replicates by use of serial dilution cDNA standard curves per gene. In order to assess the performance of the array in a biological context, we examined the transcript abundance of some candidate genes from Cabernet Sauvignon exhibiting changing expression patterns across the 7 time points of berry development. Real-time RT-PCR was performed with the ABI PRISM® 7000 Sequence Detection System (Applied Biosystems, Forster City, CA) under annealing conditions of 50°C for 1 minute and analyzed with ABI PRISM® 7000 SDS software. Analysis of relative gene expression was performed using the method [123]. The data were analyzed using the equation ΔΔCT = (CT,Target - CT,HG) Time X - (CT,Target - CT,HG) Time 0 where Time X is the value at any time point and Time 0 represents the 1X expression of the target gene normalized to ankyrin. Data were calculated from the calibration curve and normalized using the expression curve of an ankyrin gene (1612584_s_at; TC53110), whose mRNA presented an extremely low coefficient of variation (0.056, M Value = 0.1297) through microarray analysis [124].
Metabolite extraction and derivatization
Polar metabolites were extracted and derivatized with a water/chloroform protocol according to previously established procedures [125]. Freeze-dried berry tissue (6 mg) was placed in a standard screw-cap-threaded, glass vial. The tube was then returned to the -80°C freezer until use. Frozen tubes were wrapped in parafilm and freeze-dried overnight. All tissue samples were kept frozen throughout the lyophilization procedure. Upon lyophilization, tubes were capped and returned to the freezer until extraction. The vials were allowed to cool back to room temperature before being handled. The extraction vials were not washed with a methanol/hexane rinse, but all caps and septa were. The vial was incubated in HPLC grade chloroform for 1 hour at 50°C in an oven. A volume of Millipore water was added (m/V) containing 25 mg/L of ribitol as an internal standard and the sample was re-incubated for an additional hour at 50°C. Finally, vials were allowed to cool to room temperature and then spun down at 2,900 × g for 30 minutes. One mL of the polar phase was dried down in a vacuum concentrator. Polar samples were derivatized by adding 120 μL of 15 mg mL-1 of methoxyamine HCl in pyridine, incubated at 50°C for 30 minutes and sonicated until all crystals disappeared. After that, 120 μL of MSTFA + 1% TMCS were added, incubated at 50°C for 30 minutes and immediately submitted for analysis with a Thermo Finnigan Polaris Q230 GC-MS (Thermo Electron Corporation, San Jose, CA, USA). The inlet and transfer lines were held at 240°C and 320°C, respectively. Separation was achieved with a temperature program of 80°C for 3 min, then ramped at 5°C min-1 to 315°C and held for 17 min, using a 60 m DB-5MS column (J&W Scientific, 0.25 mm ID, 0.25 μm film thickness) and a constant flow of 1.0 ml min-1. Derivatized samples (120 μL) were transferred to a 200 μL silanized vial insert and run at an injection split of 200:1 to bring the large peaks to a concentration within the range of the detector. Identity of all organic acids, sugars and amino acids were verified by comparison with standards purchased from Sigma-Aldrich (St. Louis, MO, USA).
Metabolite data processing
Metabolites were identified from the chromatograms using two different software packages: AMDIS (2.64, United States Department of Defense, USA) and Xcalibur (1.3; Thermo Electron Corporation). The software matched the mass spectrum in each peak against three different metabolite libraries: NIST ver. 2.0 library [126], T_MSRI_ID library of the Golm Metabolome Database [127] and our own custom-created UNR library (V1) made from more than 50 standards bought from Sigma-Aldrich. Quantification of the area of the chromatogram peaks was determined using Xcalibur and normalized as a ratio of the area of the peak of the ribitol internal standard.
Starch determination
Starch assays were performed according to Dubois et al. [128]; 100 mg of berry powder from E-L stages (35 to 38) were finely ground and incubated in 5 mL of methanol (80/20; v/v) at 80°C for 40 min. This step eliminates soluble sugars. The methanol extract was removed and the pellet was washed twice with distilled water. The remaining pellet was incubated overnight in 1.2 mL of acetate buffer (40 mM sodium acetate, 60 mM acetic acid) and 0.2 mL of enzymes solution (3 units of amyloglucosidase and 0.25 units of α-amylase); 0.5 mL of the supernatant was mixed with 0.5 mL of water and 1 mL of phenol (5/95; v/v). Thereafter, 5 mL of concentrated sulfuric acid was added and the solution was left to cool for 15 min. Glucose was measured by its absorbance at 483 nm and expressed in terms of μg of glucose per g fresh weight of berry sample. Calibration of the concentration of glucose was performed by determining the absorbance of several concentrations of glucose standards at 483 nm (0, 20, 40, 80, 120, 160, 200 μg ml-1).
Authors' contributions
LGD conceived the experimental design, set up mRNA extraction, performed microarray experiments, RT-PCR, GC-MS, and starch analyses, prepared figures and tables and wrote the initial manuscript draft. JG performed database analysis. MDW and GRC acquired physiological data. RLT performed the identification of housekeeping genes. DRQ supervised the GC-MS analysis. CO performed microarray analyses. DAS contributed to metabolic profiling studies and edited the manuscript. KAS performed all statistical data analysis and edited the manuscript. JCC and GRC contributed equally to the preparation and finalization of the manuscript and conceived the study. All authors have read and approved the final version of the manuscript.
Supplementary Material
Quality control of Vitis GeneChip® genome arrays. The data provided represent the quality controls and commercial specifications of the 21 arrays used in this study. Slide 1. A) Box plot of raw PM (perfect match) probe intensities before and after RMA normalization. Each color indicates a set of three biological replicates. B) RNA degradation plot for all 21 arrays. All lines have similar shapes and similar variation between highest and lowest points. C) Commercial specifications of the Affymetrix Vitis GeneChip® version 1.0.
Extensive list of transcripts differentially expressed along berry development. The data provided represent the lists of transcripts that fulfilled the ANOVA filter. Table 1: List of probe sets that passed the ANOVA filter. Table 2: List of Unigenes that passed the ANOVA filter. Table 3: List of probe sets that passed the two-fold ratio (TFR) or greater filter for transcript abundance changes between two stages over berry development. Table 4: List of Unigenes according to Profile number that passed the two-fold ratio (TFR) or greater filter for transcript abundance changes between two stages over berry development.
Principal component analysis of transcriptomic behavior during grape berry development. Hybridization data from each biological replicate were projected as two graphs according to the A) first and second and B) second and third principal components arranged in descending order of variance. These first three principal components allowed clear distinction of the seven developmental stages with spots representing data from each biological replicate: E-L stage 31 (light green), 32 (dark green), 33 (brown), 34 (burgundy), 35 (yellow), 36 (light purple), and 38 (orange). Analysis was performed using GeneANOVA software [118].
Template profiles used for PTM analysis. The data provided represent the schematic trends of transcript profiles across berry development used for defining the template profiles. Phases are indicated as I, II, or III. Numbers indicated E-L stages 31 to 38. Pink shading indicates véraison (E-L stages 34 to 35).
Supplemental data related to the functional analyses of the Unigenes and to real-time RT-PCR. The data provided represent supplemental data related to Figures 3 and 10. Table 1. Attribution of the 20 profiles to Phase I, II or III according to criteria cited in Methods. Table 2. p-values of the Chi-squared tests of distribution of Unigenes within the three main phases of berry development (I, II and III) for each functional category. Differences in distribution considered as significant are indicated by orange shading. Only Unigenes clustered into the 20 PTM profiles were used for this analysis. Table 3. A list of primers used for quantitative real-time RT-PCR experiments.
Acknowledgments
Acknowledgements
This work was supported by grants from the National Science Foundation (NSF) Plant Genome program (DBI-0217653) to G.R.C., J.C.C., and D.A.S. and the Bioinformatics program (DBI-0136561) to K.A.S. The Nevada Genomics and Proteomics Centers are supported by grants from the NIH Biomedical Research Infrastructure Network (NIH-NCRR, P20 RR16464) and NIH IDeA Network of Biomedical Research Excellence (INBRE, RR-03-008). This research was supported, in part, by the Nevada Agricultural Experiment Station, publication # 03077039. The authors are indebted to Rebecca Albion and Kitty Spreeman for their invaluable technical support. The authors would like to especially thank Leon Sobon of Sobon Estate and Shenandoah Vineyards, Amador County, California for allowing us to collect the berry samples used in this study.
Contributor Information
Laurent G Deluc, Email: delucl@unr.edu.
Jérôme Grimplet, Email: jerome.grimplet@sdstate.edu.
Matthew D Wheatley, Email: wheatle8@unr.nevada.edu.
Richard L Tillett, Email: tillett@unr.nevada.edu.
David R Quilici, Email: quilici@unr.edu.
Craig Osborne, Email: Vandal98@aol.com.
David A Schooley, Email: schooley@unr.edu.
Karen A Schlauch, Email: schlauch@unr.edu.
John C Cushman, Email: jcushman@unr.edu.
Grant R Cramer, Email: cramer@unr.edu.
References
- FAOSTAT database (http://faostat.fao.org) Food and Agriculture Organization of the United Nations; 2007. [Google Scholar]
- Wine Institute http://www.wineinstitute.org/
- http://www.wineinstitute.org/industry/statistics/2006/ca_wine_economic_impact.php
- German JB, Walzem RL. The health benefits of wine. Annu Rev Nutr. 2000;20:561–593. doi: 10.1146/annurev.nutr.20.1.561. [DOI] [PubMed] [Google Scholar]
- Wollin SD, Jones PJ. Alcohol, red wine and cardiovascular disease. J Nutr. 2001;131:1401–1404. doi: 10.1093/jn/131.5.1401. [DOI] [PubMed] [Google Scholar]
- Bradamante S, Barenghi L, Villa A. Cardiovascular protective effects of resveratrol. Cardiovasc Drug Rev. 2004;22:169–188. doi: 10.1111/j.1527-3466.2004.tb00139.x. [DOI] [PubMed] [Google Scholar]
- Bruno R, Ghisolfi L, Priulla M, Nicolin A, Bertelli A. Wine and tumors: study of resveratrol. Drugs Exp Clin Res. 2003;29:257–261. [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Dore S. Unique properties of polyphenol stilbenes in the brain: more than direct antioxidant actions; gene/protein regulatory activity. Neurosignals. 2005;14:61–70. doi: 10.1159/000085386. [DOI] [PubMed] [Google Scholar]
- Jones SB, DePrimo SE, Whitfield ML, Brooks JD. Resveratrol-induced gene expression profiles in human prostate cancer cells. Cancer Epidemiol Biomarkers Prev. 2005;14:596–604. doi: 10.1158/1055-9965.EPI-04-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni JJ. Genetic regulation of fruit development and ripening. Plant Cell. 2004;16 Suppl:S170–80. doi: 10.1105/tpc.019158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Given NK, Venis MA, Gierson D. Hormonal regulation of ripening in the strawberry, a non-climacteric fruit. Planta. 1988;174:402–406. doi: 10.1007/BF00959527. [DOI] [PubMed] [Google Scholar]
- Coombe BG. Research on development and ripening of the grape berry. Am J Enol Vitic. 1992;43:101–110. [Google Scholar]
- Coombe BG. Adoption of a system for identifying grapevine growth stages. Aust J Grape Wine Res. 1995;1:100–110. [Google Scholar]
- Noordeloos S, Nagel CW. Effect of sugar on acid perception in wine. Am J Enol Vitic. 1972;23:139–143. [Google Scholar]
- Davies C, Robinson SP. Differential screening indicates a dramatic change in mRNA profiles during grape berry ripening. Cloning and characterization of cDNAs encoding putative cell wall and stress response proteins. Plant Physiol. 2000;122:803–812. doi: 10.1104/pp.122.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters DL, Holton TA, Ablett EM, Lee LS, Henry RJ. cDNA microarray analysis of developing grape (Vitis vinifera cv. Shiraz) berry skin. Funct Integr Genomics. 2005;5:40–58. doi: 10.1007/s10142-004-0124-z. [DOI] [PubMed] [Google Scholar]
- Terrier N, Glissant D, Grimplet J, Barrieu F, Abbal P, Couture C, Ageorges A, Atanassova R, Leon C, Renaudin JP, Dedaldechamp F, Romieu C, Delrot S, Hamdi S. Isogene specific oligo arrays reveal multifaceted changes in gene expression during grape berry (Vitis vinifera L.) development. Planta. 2005;222:832–847. doi: 10.1007/s00425-005-0017-y. [DOI] [PubMed] [Google Scholar]
- Fernandez L, Torregrosa L, Terrier N, Sreekantan L, Grimplet J, Davies C, Thomas MR, Romieu C, Ageorges A. Identification of genes associated with flesh morphogenesis during grapevine fruit development. Plant Mol Biol. 2007;63:307–323. doi: 10.1007/s11103-006-9090-2. [DOI] [PubMed] [Google Scholar]
- Waters DLE, Holton TA, Ablett EM, Slade Lee L, Henry RJ. The ripening wine grape berry skin transcriptome. Plant Sci. 2006;171:132–138. doi: 10.1016/j.plantsci.2006.03.002. [DOI] [Google Scholar]
- Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc Ser B (Stat Method) 1995;57:289–300. [Google Scholar]
- DFCI Grape Gene Index http://biocomp.dfci.harvard.edu/tgi/cgi-bin/tgi/gimain.pl?gudb=grape
- Pavlidis P, Noble WS. Analysis of strain and regional variation in gene expression in mouse brain. Genome Biol. 2001;2:research0042.1–research0042.15. doi: 10.1186/gb-2001-2-10-research0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoof H, Ernst R, Nazarov V, Pfeifer L, Mewes HW, Mayer KF. MIPS Arabidopsis thaliana Database (MAtDB): an integrated biological knowledge resource for plant genomics. Nucleic Acids Res. 2004;32:D373–6. doi: 10.1093/nar/gkh068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman A, Kaur-Sawhney R, Galston AW. Stabilization of oat leaf protoplasts through polyamine-mediated inhibition of senescence. Plant Physiol. 1977;60:570–574. doi: 10.1104/pp.60.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egea-Cortines M, Mizrahi Y. Polyamines in cell division, fruit set and development and seed germination. In: Slocum RD, Flores HE, editor. Biochemistry and physiology of polyamines in plants. Boca Raton , CRC Press; 1991. pp. 143–159. [Google Scholar]
- Geny L, Broquedis M, Martin TJ, Bouard J. Free, conjugated, and wall-bound polyamines in various organs of fruiting cuttings of Vitis vinifera L. cv. Cabernet Sauvignon. Am J Enol Vitic. 1997;48:80–84. [Google Scholar]
- Burtin D, Martin-Tanguy J, Paynot M, Rossin N. Effects of the suicide inhibitors of arginine and ornithine decarboxylase activities on organogenesis, growth, free polyamine and hydroxycinnamoyl putrescine levels in leaf explants of Nicotiana xanthi n.c. cultivated in vitro in a medium producing callus formation. Plant Physiol. 1989;89:104–110. doi: 10.1104/pp.89.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieciechowicz KA, Joy KW, Ireland RJ. The metabolism of asparagine in plants. Phytochemistry. 1988;27:663–671. doi: 10.1016/0031-9422(88)84071-8. [DOI] [Google Scholar]
- Miflin BJ, Habash DZ. The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. J Exp Bot. 2002;53:979–987. doi: 10.1093/jexbot/53.370.979. [DOI] [PubMed] [Google Scholar]
- Nunan KJ, Sims IM, Bacic A, Robinson SP, Fincher GB. Changes in cell wall composition during ripening of grape berries. Plant Physiol. 1998;118:783–792. doi: 10.1104/pp.118.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas TR, Matthews MA, Shackel KA. Direct in situ measurement of cell turgor in grape (Vitis vinifera L.) berries during development and in response to plant water deficits. Plant Cell Environ. 2006;29:993–1001. doi: 10.1111/j.1365-3040.2006.01496.x. [DOI] [PubMed] [Google Scholar]
- Brummell DA, Harpster MH. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol Biol. 2001;47:311–340. doi: 10.1023/A:1010656104304. [DOI] [PubMed] [Google Scholar]
- Saladie M, Rose JK, Cosgrove DJ, Catala C. Characterization of a new xyloglucan endotransglucosylase/hydrolase (XTH) from ripening tomato fruit and implications for the diverse modes of enzymic action. Plant J. 2006;47:282–295. doi: 10.1111/j.1365-313X.2006.02784.x. [DOI] [PubMed] [Google Scholar]
- Sampedro J, Cosgrove DJ. The expansin superfamily. Genome Biol. 2005;6:242. doi: 10.1186/gb-2005-6-12-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotto MC, Martinez GA, Civello PM. Expression of expansin genes in strawberry varieties with contrasting fruit firmness. Plant Physiol Biochem. 2006;44:301–307. doi: 10.1016/j.plaphy.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Lund ST, Bohlmann J. The molecular basis for wine grape quality--a volatile subject. Science. 2006;311:804–805. doi: 10.1126/science.1118962. [DOI] [PubMed] [Google Scholar]
- Lucker J, Bowen P, Bohlman J. Vitis vinifera terpenoid cyclases: functional identification of two sesquiterpene synthase cDNAs encoding (+)-valencene synthase and (-)-germacrene D synthase and expression of mono- and sesquiterpene synthases in grapevine flowers and berries. Phytochemistry. 2004;65:2649–2659. doi: 10.1016/j.phytochem.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Martin DM, Bohlman J. Identification of Vitis vinifera (-)-alpha-terpineol synthase by in silico screening of full-length cDNA ESTs and functional characterization of recombinant terpene synthase. Phytochemistry. 2004;65:1223–1229. doi: 10.1016/j.phytochem.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Mahmoud SS, Croteau RB. Strategies for transgenic manipulation of monoterpene biosynthesis in plants. Trends Plant Sci. 2002;7:366–373. doi: 10.1016/S1360-1385(02)02303-8. [DOI] [PubMed] [Google Scholar]
- Jackson RS. Wine Science: Principles, Practice, Perception. In: Taylor SL, editor. Food Science and Technology International Series. 2nd. San Diego , Academic Press; 2000. [Google Scholar]
- Farina L, Boido E, Carrau F, Versini G, Dellacassa E. Terpene compounds as possible precursors of 1,8-cineole in red grapes and wines. J Agric Food Chem. 2005;53:1633–1636. doi: 10.1021/jf040332d. [DOI] [PubMed] [Google Scholar]
- Ringer KL, Davis EM, Croteau R. Monoterpene metabolism. Cloning, expression, and characterization of (-)-isopiperitenol/(-)-carveol dehydrogenase of peppermint and spearmint. Plant Physiol. 2005;137:863–872. doi: 10.1104/pp.104.053298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C, Boss PK, Robinson SP. Treatment of grape berries, a nonclimacteric fruit with a synthetic auxin, retards ripening and alters the expression of developmentally regulated genes. Plant Physiol. 1997;115:1155–1161. doi: 10.1104/pp.115.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervin C, Tira-Umphon A, Roustan JP, Lamon J, El-Kereamy A, Kanellis A. Ethylene is required for the ripening of grape. Acta Hort. 2005;689:251–256. [Google Scholar]
- El-Kereamy A, Chervin C, Roustan JP, Cheynier V, Souquet JM, Moutounet M, Raynal J, Ford C, Latche A, Pech JC, Bouzayen M. Exogenous ethylene stimulates the long-term expression of genes related to anthocyanin biosynthesis in grape berries. Physiol Plant. 2003;119:175–182. doi: 10.1034/j.1399-3054.2003.00165.x. [DOI] [Google Scholar]
- Tesniere C, Pradal M, El-Kereamy A, Torregrosa L, Chatelet P, Roustan JP, Chervin C. Involvement of ethylene signalling in a non-climacteric fruit: new elements regarding the regulation of ADH expression in grapevine. J Exp Bot. 2004;55:2235–2240. doi: 10.1093/jxb/erh244. [DOI] [PubMed] [Google Scholar]
- Hale CR, Coombe BG, Hawker JS. Effects of ethylene and 2-chloroethylphosphonic acid on the ripening of grapes. Plant Physiol. 1970;45:620–623. doi: 10.1104/pp.45.5.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YF, Etheridge N, Schaller GE. Ethylene signal transduction. Ann Bot (Lond) 2005;95:901–915. doi: 10.1093/aob/mci100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee H, Tieman D. The tomato ethylene receptor gene family: Form and function. Physiol Plant. 2002;115:336–341. doi: 10.1034/j.1399-3054.2002.1150302.x. [DOI] [PubMed] [Google Scholar]
- Trainotti L, Pavanello A, Casadoro G. Different ethylene receptors show an increased expression during the ripening of strawberries: does such an increment imply a role for ethylene in the ripening of these non-climacteric fruits? J Exp Bot. 2005;56:2037–2046. doi: 10.1093/jxb/eri202. [DOI] [PubMed] [Google Scholar]
- Symons GM, Davies C, Shavrukov Y, Dry IB, Reid JB, Thomas MR. Grapes on steroids. Brassinosteroids are involved in grape berry ripening. Plant Physiol. 2006;140:150–158. doi: 10.1104/pp.105.070706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkhadir Y, Chory J. Brassinosteroid signaling: a paradigm for steroid hormone signaling from the cell surface. Science. 2006;314:1410–1411. doi: 10.1126/science.1134040. [DOI] [PubMed] [Google Scholar]
- Cawthon D, Morris J. Relationship of seed number and maturity to berry development, fruit maturation, hormonal changes and uneven ripening of "Concord" (Vitis labrusca L.) grapes. J Am Soc Hort Sci. 1982;107:1097–1104. [Google Scholar]
- Perez FJ, Viani C, Retamales J. Bioactive gibberellins in seeded and seedless grapes: Identification and changes in content during berry development. Am J Enol Vitic. 2000;51:315–318. [Google Scholar]
- Costantini E, Landi L, Silvestroni O, Pandolfini T, Spena A, Mezzetti B. Auxin synthesis-encoding transgene enhances grape fecundity. Plant Physiol. 2007;143:1689–1694. doi: 10.1104/pp.106.095232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel B, Fink GR. ILR1, an amidohydrolase that releases active indole-3-acetic acid from conjugates. Science. 1995;268:1745–1748. doi: 10.1126/science.7792599. [DOI] [PubMed] [Google Scholar]
- Hampel D, Mosandl A, Wust M. Induction of de novo volatile terpene biosynthesis via cytosolic and plastidial pathways by methyl jasmonate in foliage of Vitis vinifera L. J Agric Food Chem. 2005;53:2652–2657. doi: 10.1021/jf040421q. [DOI] [PubMed] [Google Scholar]
- Tassoni A, Fornale S, Franceschetti M, Musiani F, Michael AJ, Perry B, Bagni N. Jasmonates and Na-orthovanadate promote resveratrol production in Vitis vinifera cv. Barbera cell cultures. New Phytol. 2005;166:895–905. doi: 10.1111/j.1469-8137.2005.01383.x. [DOI] [PubMed] [Google Scholar]
- Schaller F, Biesgen C, Mussig C, Altmann T, Weiler EW. 12-Oxophytodienoate reductase 3 (OPR3) is the isoenzyme involved in jasmonate biosynthesis. Planta. 2000;210:979–984. doi: 10.1007/s004250050706. [DOI] [PubMed] [Google Scholar]
- Werner T, Kollmer I, Bartrina I, Holst K, Schmulling T. New insights into the biology of cytokinin degradation. Plant Biol (Stuttg) 2006;8:371–381. doi: 10.1055/s-2006-923928. [DOI] [PubMed] [Google Scholar]
- Hepler PK. Calcium: a central regulator of plant growth and development. Plant Cell. 2005;17:2142–2155. doi: 10.1105/tpc.105.032508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XC, Li MJ, Gao GF, Feng HZ, Geng XQ, Peng CC, Zhu SY, Wang XJ, Shen YY, Zhang DP. Abscisic acid stimulates a calcium-dependent protein kinase in grape berry. Plant Physiol. 2006;140:558–579. doi: 10.1104/pp.105.074971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerana M, Bonza MC, Harris R, Sanders D, De Michelis MI. Abscisic acid stimulates the expression of two isoforms of plasma membrane Ca2+-ATPase in Arabidopsis thaliana seedlings. Plant Biol (Stuttg) 2006;8:572–578. doi: 10.1055/s-2006-924111. [DOI] [PubMed] [Google Scholar]
- Cheng NH, Pittman JK, Shigaki T, Lachmansingh J, LeClere S, Lahner B, Salt DE, Hirschi KD. Functional association of Arabidopsis CAX1 and CAX3 is required for normal growth and ion homeostasis. Plant Physiol. 2005;138:2048–2060. doi: 10.1104/pp.105.061218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombe BG, Hale CR. The hormone content of ripening grape berries and the effects of growth substance treatments. Plant Physiol. 1973;51:629–634. doi: 10.1104/pp.51.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack E, Tsai YC, Braam J. Handling calcium signaling: Arabidopsis CaMs and CMLs. Trends Plant Sci. 2005;10:383–389. doi: 10.1016/j.tplants.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Anandalakshmi R, Marathe R, Ge X, Herr JM, Jr., Mau C, Mallory A, Pruss G, Bowman L, Vance VB. A calmodulin-related protein that suppresses posttranscriptional gene silencing in plants. Science. 2000;290:142–144. doi: 10.1126/science.290.5489.142. [DOI] [PubMed] [Google Scholar]
- Fagard M, Boutet S, Morel JB, Bellini C, Vaucheret H. AGO1, QDE-2, and RDE-1 are related proteins required for post-transcriptional gene silencing in plants, quelling in fungi, and RNA interference in animals. Proc Natl Acad Sci U S A. 2000;97:11650–11654. doi: 10.1073/pnas.200217597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimplet J, Deluc LG, Tillett RL, Wheatley MD, Schlauch KA, Cramer GR, Cushman JC. Tissue-specific mRNA expression profiling in grape berry tissues. BMC Genomics. 2007;8:187. doi: 10.1186/1471-2164-8-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura S, Shikazono N, Tanaka A. TRANSPARENT TESTA 19 is involved in the accumulation of both anthocyanins and proanthocyanidins in Arabidopsis. Plant J. 2004;37:104–114. doi: 10.1046/j.1365-313X.2003.01943.x. [DOI] [PubMed] [Google Scholar]
- Goodman CD, Casati P, Walbot V. A multidrug resistance-associated protein involved in anthocyanin transport in Zea mays. Plant Cell. 2004;16:1812–1826. doi: 10.1105/tpc.022574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfenito MR, Souer E, Goodman CD, Buell R, Mol J, Koes R, Walbot V. Functional complementation of anthocyanin sequestration in the vacuole by widely divergent glutathione S-transferases. Plant Cell. 1998;10:1135–1149. doi: 10.1105/tpc.10.7.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debeaujon I, Peeters AJ, Leon-Kloosterziel KM, Koornneef M. The transparent testa12 gene of arabidopsis encodes a multidrug secondary transporter-like protein required for flavonoid sequestration in vacuoles of the seed coat endothelium. Plant Cell. 2001;13:853–872. doi: 10.1105/tpc.13.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AR, Lee E, Bogs J, McDavid DA, Thomas MR, Robinson SP. White grapes arose through the mutation of two similar and adjacent regulatory genes. Plant J. 2007;49:772–785. doi: 10.1111/j.1365-313X.2006.02997.x. [DOI] [PubMed] [Google Scholar]
- Bogs J, Jaffe FW, Takos AM, Walker AR, Robinson SP. The grapevine transcription factor VvMYBPA1 regulates proanthocyanidin synthesis during fruit development. Plant Physiol. 2007;143:1347–1361. doi: 10.1104/pp.106.093203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluc L, Barrieu F, Marchive C, Lauvergeat V, Decendit A, Richard T, Carde JP, Merillon JM, Hamdi S. Characterization of a grapevine R2R3-MYB transcription factor that regulates the phenylpropanoid pathway. Plant Physiol. 2006;140:499–511. doi: 10.1104/pp.105.067231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Ishimaru M, Hiraoka K, Honda C. Myb-related genes of the Kyoho grape (Vitis labruscana) regulate anthocyanin biosynthesis. Planta. 2002;215:924–933. doi: 10.1007/s00425-002-0830-5. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Goto-Yamamoto N, Hirochika H. Retrotransposon-induced mutations in grape skin color. Science. 2004;304:982. doi: 10.1126/science.1095011. [DOI] [PubMed] [Google Scholar]
- Goodrich J, Carpenter R, Coen ES. A common gene regulates pigmentation pattern in diverse plant species. Cell. 1992;68:955–964. doi: 10.1016/0092-8674(92)90038-E. [DOI] [PubMed] [Google Scholar]
- Luan F, Wüst M. Differential incorporation of 1-deoxy-D-xylulose into (3S)-linalool and geraniol in grape berry exocarp and mesocarp. Phytochemistry. 2002;60:451–459. doi: 10.1016/S0031-9422(02)00147-4. [DOI] [PubMed] [Google Scholar]
- Wu S, Watanabe N, Mita S, Dohra H, Ueda Y, Shibuya M, Ebizuka Y. The key role of phloroglucinol O-methyltransferase in the biosynthesis of Rosa chinensis volatile 1,3,5-trimethoxybenzene. Plant Physiol. 2004;135:95–102. doi: 10.1104/pp.103.037051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JR, Nam KH, D'Auria JC, Pichersky E. S-Adenosyl-L-methionine:salicylic acid carboxyl methyltransferase, an enzyme involved in floral scent production and plant defense, represents a new class of plant methyltransferases. Arch Biochem Biophys. 1999;367:9–16. doi: 10.1006/abbi.1999.1255. [DOI] [PubMed] [Google Scholar]
- Pichersky E, Noel JP, Dudareva N. Biosynthesis of plant volatiles: nature's diversity and ingenuity. Science. 2006;311:808–811. doi: 10.1126/science.1118510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBolt S, Cook DR, Ford CM. L-tartaric acid synthesis from vitamin C in higher plants. Proc Natl Acad Sci U S A. 2006;103:5608–5613. doi: 10.1073/pnas.0510864103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possner DRE, Kliewer WM. The localization of acids, sugars, potassium and calcium in developing grape berries. Vitis. 1985;24:229–240. [Google Scholar]
- Stines AP, Naylor DJ, Hoj PB, Van Heeswijck R. Proline accumulation in developing grapevine fruit occurs independently of changes in the levels of Delta1-pyrroline-5-carboxylate synthetase mRNA or protein. Plant Physiol. 1999;120:923–931. doi: 10.1104/pp.120.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stines AP, Grubb J, Gockowiak H, Henschke PA, Hoj PB, Heeswijck RV. Proline and arginine accumulation in developing berries of V. vinifera in Australian vineyards: Influence of vine cultivar, berry maturity and tissue type. Aust J Grape Wine Res. 2000;6:150–158. [Google Scholar]
- Conde C, Silva P, Fontes N, Dias A, Tavares R, Sousa M, Agasse A, Delrot S, Geros H. Biochemical changes throughout grape berry development and fruit and wine quality. Food. 2007;1:1–22. [Google Scholar]
- Swanson CA, El-Shishiny ED. Translocation of sugars in the Concord grape. Plant Physiol. 1958;33:33–37. doi: 10.1104/pp.33.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde C, Agasse A, Glissant D, Tavares R, Geros H, Delrot S. Pathways of glucose regulation of monosaccharide transport in grape cells. Plant Physiol. 2006;141:1563–1577. doi: 10.1104/pp.106.080804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu A, Moriguchi T, Koyama K, Omura M, Akihama T. Analysis of sucrose synthase genes in citrus suggests different roles and phylogenetic relationships. J Exp Bot. 2002;53:61–71. doi: 10.1093/jexbot/53.366.61. [DOI] [PubMed] [Google Scholar]
- Davies C, Robinson SP. Sugar accumulation in grape berries: cloning for two putative vacuolar invertase cDNAs and their expression in grapevine tissue. Plant Physiol. 1996;111:275–283. doi: 10.1104/pp.111.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Wang XL, Wang XF, Xia GH, Pan QH, Fan RC, Wu FQ, Yu XC, Zhang DP. A shift of phloem unloading from symplasmic to apoplasmic pathway is involved in developmental onset of ripening in grape berry. Plant Physiol. 2006;142:220–232. doi: 10.1104/pp.106.081430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger P, Stitt M. Sucrose synthase catalyses a readily reversible reaction in vivo developing potato tubers and other plant tissues. Planta. 1993;189:329–393. doi: 10.1007/BF00194429. [DOI] [PubMed] [Google Scholar]
- Koch KE. Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:509–540. doi: 10.1146/annurev.arplant.47.1.509. [DOI] [PubMed] [Google Scholar]
- Ruan Y. Rapid cell expansion and cellulose synthesis regulated by plasmodesmata and sugar: insights from the single-celled cotton fibre. Funct Plant Biol. 2007;34:1–10. doi: 10.1071/FP06234. [DOI] [PubMed] [Google Scholar]
- Vignault C, Vachaud M, Cakir B, Glissant D, Dedaldechamp F, Buttner M, Atanassova R, Fleurat-Lessard P, Lemoine R, Delrot S. VvHT1 encodes a monosaccharide transporter expressed in the conducting complex of the grape berry phloem. J Exp Bot. 2005;56:1409–1418. doi: 10.1093/jxb/eri142. [DOI] [PubMed] [Google Scholar]
- Davies C, Wolf T, Robinson SP. Three putative sucrose transporters are differentially expressed in grapevine tissues. Plant Sci. 1999;147:93–100. doi: 10.1016/S0168-9452(99)00059-X. [DOI] [Google Scholar]
- Klepek YS, Geiger D, Stadler R, Klebl F, Landouar-Arsivaud L, Lemoine R, Hedrich R, Sauer N. Arabidopsis POLYOL TRANSPORTER5, a new member of the monosaccharide transporter-like superfamily, mediates H+-symport of numerous substrates, including myo-inositol, glycerol, and ribose. Plant Cell. 2005;17:204–218. doi: 10.1105/tpc.104.026641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kore-eda S, Noake C, Ohishi M, Ohnishi J, Cushman JC. Transcriptional profiles of organellar metabolite transporters during induction of crassulacean acid metabolism in Mesembryanthemum crystallinum. Funct Plant Biol. 2005; 32:451–466. doi: 10.1071/FP04188. [DOI] [PubMed] [Google Scholar]
- Gao Z, Maurousset L, Lemoine R, Yoo SD, van Nocker S, Loescher W. Cloning, expression, and characterization of sorbitol transporters from developing sour cherry fruit and leaf sink tissues. Plant Physiol. 2003;131:1566–1575. doi: 10.1104/pp.102.016725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubelakis-Angelakis KA, Kliewer WM. The composition of bleeding sap from Thompson Seedless grapevines as affected by nitrogen fertilization. Am J Enol Vitic. 1979;30:14–18. [Google Scholar]
- Delvalle D, Dumez S, Wattebled F, Roldan I, Planchot V, Berbezy P, Colonna P, Vyas D, Chatterjee M, Ball S, Merida A, D'Hulst C. Soluble starch synthase I: a major determinant for the synthesis of amylopectin in Arabidopsis thaliana leaves. Plant J. 2005;43:398–412. doi: 10.1111/j.1365-313X.2005.02462.x. [DOI] [PubMed] [Google Scholar]
- Li CY, Weiss D, Goldschmidt EE. Effects of carbohydrate starvation on gene expression in citrus root. Planta. 2003;217:11–20. doi: 10.1007/s00425-002-0963-6. [DOI] [PubMed] [Google Scholar]
- Diakou P, Carde JP. In situ fixation of grape berries. Protoplasma. 2001;218:225–235. doi: 10.1007/BF01306611. [DOI] [PubMed] [Google Scholar]
- Hotta CT, Gardner MJ, Hubbard KE, Baek SJ, Dalchau N, Suhita D, Dodd AN, Webb AA. Modulation of environmental responses of plants by circadian clocks. Plant Cell Environ. 2007;30:333–349. doi: 10.1111/j.1365-3040.2006.01627.x. [DOI] [PubMed] [Google Scholar]
- Lu Y, Gehan JP, Sharkey TD. Daylength and circadian effects on starch degradation and maltose metabolism. Plant Physiol. 2005;138:2280–2291. doi: 10.1104/pp.105.061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock KF, Sefton MA, Williams PJ. Taste thresholds of phenolic extracts of French and American oakwood: the influence of oak phenols on wine flavor. Am J Enol Vitic. 1994;45:429–434. [Google Scholar]
- Robinson SP, Jacobs AK, Dry IB. A class IV chitinase is highly expressed in grape berries during ripening. Plant Physiol. 1997;114:771–778. doi: 10.1104/pp.114.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jach G, Gornhardt B, Mundy J, Logemann J, Pinsdorf E, Leah R, Schell J, Maas C. Enhanced quantitative resistance against fungal disease by combinatorial expression of different barley antifungal proteins in transgenic tobacco. Plant J. 1995;8:97–109. doi: 10.1046/j.1365-313X.1995.08010097.x. [DOI] [PubMed] [Google Scholar]
- Tattersall DB, Van Heeswijck R, Hoj PB. Identification and characterization of a fruit-specific, thaumatin-like protein that accumulates at very high levels in conjunction with the onset of sugar accumulation and berry softening in grapes. Plant Physiol. 1997;114:759–769. doi: 10.1104/pp.114.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busam G, Junghanns KT, Kneusel RE, Kassemeyer HH, Matern U. Characterization and expression of caffeoyl-coenzyme A 3-O-methyltransferase proposed for the induced resistance response of Vitis vinifera L. Plant Physiol. 1997;115:1039–1048. doi: 10.1104/pp.115.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan J, Shackel KA. Stem-water potential as a sensitive indicator of water stress in prune trees (Prunus domestica L. cv. French) J Amer Soc Hort Sci. 1992;117:607–611. [Google Scholar]
- Tattersall EAR, Ergul A, AlKayal F, DeLuc L, Cushman JC, Cramer GR. Comparison of methods for isolating high-quality RNA from leaves of grapevine. Am J Enol Vitic. 2005;56:400–406. [Google Scholar]
- Gautier L, Cope L, Bolstad BM, Irizarry RA. affy--analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004;20:307–315. doi: 10.1093/bioinformatics/btg405. [DOI] [PubMed] [Google Scholar]
- Didier G, Brezellec P, Remy E, Henaut A. GeneANOVA--gene expression analysis of variance. Bioinformatics. 2002;18:490–491. doi: 10.1093/bioinformatics/18.3.490. [DOI] [PubMed] [Google Scholar]
- Shen L, Gong J, Caldo RA, Nettleton D, Cook D, Wise RP, Dickerson JA. BarleyBase--an expression profiling database for plant genomics. Nucleic Acids Res. 2005;33:D614–D618. doi: 10.1093/nar/gki123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Liu Z, Vinsavich A, Trush V, Quackenbush J. TM4: a free, open-source system for microarray data management and analysis. BioTechniques. 2003;34:374–378. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- Chernoff H, Lehman EL. The use of maximum likelihood estimates in chi2 tests for goodness-of-fit. Ann Math Stat. 1954;25:579–586. doi: 10.1214/aoms/1177728726. [DOI] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Luu-The V, Paquet N, Calvo E, Cumps J. Improved real-time RT-PCR method for high-throughput measurements using second derivative calculation and double correction. BioTechniques. 2005;38:287–293. doi: 10.2144/05382RR05. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeckling CD, Huhman DV, Farag MA, Smith JT, May GD, Mendes P, Dixon RA, Sumner LW. Metabolic profiling of Medicago truncatula cell cultures reveals the effects of biotic and abiotic elicitors on metabolism. J Exp Bot. 2005;56:323–336. doi: 10.1093/jxb/eri058. [DOI] [PubMed] [Google Scholar]
- NIST Standard Reference Database. National Institute of Standards and Technology; http://www.nist.gov/srd/ [Google Scholar]
- Kopka J, Schauer N, Krueger S, Birkemeyer C, Usadel B, Bergmuller E, Dormann P, Weckwerth W, Gibon Y, Stitt M, Willmitzer L, Fernie AR, Steinhauser D. GMD@CSB.DB: the Golm Metabolome Database. Bioinformatics. 2005;21:1635–1638. doi: 10.1093/bioinformatics/bti236. [DOI] [PubMed] [Google Scholar]
- Dubois M, Gilles K, Hamilton J, Rebers P, Smith F. Colorimetric method for the determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quality control of Vitis GeneChip® genome arrays. The data provided represent the quality controls and commercial specifications of the 21 arrays used in this study. Slide 1. A) Box plot of raw PM (perfect match) probe intensities before and after RMA normalization. Each color indicates a set of three biological replicates. B) RNA degradation plot for all 21 arrays. All lines have similar shapes and similar variation between highest and lowest points. C) Commercial specifications of the Affymetrix Vitis GeneChip® version 1.0.
Extensive list of transcripts differentially expressed along berry development. The data provided represent the lists of transcripts that fulfilled the ANOVA filter. Table 1: List of probe sets that passed the ANOVA filter. Table 2: List of Unigenes that passed the ANOVA filter. Table 3: List of probe sets that passed the two-fold ratio (TFR) or greater filter for transcript abundance changes between two stages over berry development. Table 4: List of Unigenes according to Profile number that passed the two-fold ratio (TFR) or greater filter for transcript abundance changes between two stages over berry development.
Principal component analysis of transcriptomic behavior during grape berry development. Hybridization data from each biological replicate were projected as two graphs according to the A) first and second and B) second and third principal components arranged in descending order of variance. These first three principal components allowed clear distinction of the seven developmental stages with spots representing data from each biological replicate: E-L stage 31 (light green), 32 (dark green), 33 (brown), 34 (burgundy), 35 (yellow), 36 (light purple), and 38 (orange). Analysis was performed using GeneANOVA software [118].
Template profiles used for PTM analysis. The data provided represent the schematic trends of transcript profiles across berry development used for defining the template profiles. Phases are indicated as I, II, or III. Numbers indicated E-L stages 31 to 38. Pink shading indicates véraison (E-L stages 34 to 35).
Supplemental data related to the functional analyses of the Unigenes and to real-time RT-PCR. The data provided represent supplemental data related to Figures 3 and 10. Table 1. Attribution of the 20 profiles to Phase I, II or III according to criteria cited in Methods. Table 2. p-values of the Chi-squared tests of distribution of Unigenes within the three main phases of berry development (I, II and III) for each functional category. Differences in distribution considered as significant are indicated by orange shading. Only Unigenes clustered into the 20 PTM profiles were used for this analysis. Table 3. A list of primers used for quantitative real-time RT-PCR experiments.