The inhibition of dopamine (DA) uptake and the increase of extracellular DA with consequent activation of DA receptors in specific brain regions such as the nucleus accumbens (NAc) and dorsal striatum are an important, but may be not an exclusive mechanism for behavioral excitation induced by psychostimulants [2, 5, 14, 15, 24, 25]. A typical spectrum of acute cocaine-induced arousal effects in animal models includes locomotor activation and stereotyped behavior consisting of continuous sniffing, rearing, licking and gnawing. At low and moderate doses, cocaine preferentially enhances locomotor activity and this effect correlates with the decrease in DA clearance in the NAc of freely moving rats [24]. At high doses, the effect of cocaine on the stereotyped activity became predominant [1]. It is unknown whether a strong relationship exists between the stereotypy and cocaine-induced DA uptake changes in the NAc.
In this study we have employed fast-scan cyclic voltammetry (FSCV) on freely moving rats to determine whether a correlation exists between the increase in the stereotyped behavior and DA uptake inhibition following cocaine (20 mg/kg, i.p.) administration. The FSCV was chosen since the general characteristics of this technique allow an examination of the DA uptake kinetics without DA release or metabolism contributions [1, 28, 30].
Male Sprague-Dawley rats (300-350 g; Charles River, Raleigh, NC) were housed on a 12:12 light/dark cycle with food and water ad libitum. Rats were group housed before surgery and singly housed after surgery. All protocols were approved by the Institutional Animal Care and Use Committee at Wake Forest University. Rats were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) and placed in a stereotaxic frame. A guide cannula (Bioanalytical Systems, West Lafayette, IN) was positioned above the NAc core (AP + 1.3, L+1.3, V-2.0 mm from bregma). An Ag/AgCl reference electrode was implanted in the contralateral hemisphere. A bipolar stimulating electrode was lowered to the ventral tegmental area ipsilateral to the guide cannula at 5.2 mm posterior and 1.0 mm lateral to bregma. The stimulating electrode depth was optimized to evoke DA release in the NAc (24 rectangular pulses, 60 Hz, 120 μA, 2 ms/phase, biphasic), monitored using a carbon fiber microelectrode inserted through the guide cannula. The rats were individually housed and allowed to recover for 48 hrs, then they were placed in the test chamber and a new carbon fiber electrode was inserted into the NAc core. The reference and carbon fiber electrodes were connected to a head-mounted voltammetric amplifier (UNC Electronics Design Facility, Chapel Hill, NC) attached to a swivel at the top of the test chamber. Voltammetric recordings were made at the carbon fiber electrode every 100 ms by applying a triangular waveform (-0.4 to +1.2 V, 300 V/s). Data were digitized (National Instruments, Austin, TX) and stored on a computer. DA release was evoked every 5 min with electrical stimulations (24 rectangular pulses, 60 Hz, 120 μA, 2 ms/phase, biphasic) and detected by a carbon fiber electrode. At least four stable stimulations of DA were collected, and then a single dose of cocaine (20 mg/kg, i.p.) or saline was injected. Stimulations and recordings were collected at 5 min intervals for 2 h following the cocaine injection. Carbon fiber microelectrodes were calibrated in vitro with known concentrations of DA (2-5 μM). Calibrations were done in triplicate and the average value for the current at the peak oxidation potential was used to normalize in vivo signals to DA concentration. DA uptake was determined from the clearance rate of DA following the termination of the stimulus. DA uptake was assumed to following Michaelis-Menten kinetics, and the change in DA during and after stimulated release was fit using the equation:
where f is the stimulation frequency (Hz), [DA]p is the concentration of DA released per stimulus pulse, and Vmax is the maximal rate of DA uptake. The baseline value of Km was taken to be 0.16 μM, a value determined in rat brain synaptosomes [22]. The derivative form of the above equation was used to simulate the DA response. DA signals for each rat were fit individually at all time points after cocaine injection.
Drug-induced behaviors were evaluated simultaneously with the monitoring of DA signals by FSCV. Stereotypy was measured during the 1-min period prior to electrical stimulation using a 0-6-point scale [5, 9, 21]: 0, asleep or inactive; 1, episodes of normal activity; 2, discontinuous activity with bursts of prominent sniffing or rearing; 3, continuous stereotyped activity such as sniffing or rearing along a fixed path; 4, stereotyped sniffing or rearing that is fixated in one location; 5, focused stereotyped behavior with bursts of licking or gnawing; 6, continuous licking or gnawing. Statistical analyses were carried out by a two-way ANOVA with Bonferroni post tests. Spearman correlation analysis was used to evaluate the relationship between cocaine-induced changes in stereotyped behavior and apparent Km in individual rats. Statistical procedures were performed using Graph Pad Prism (Graph Pad Software Inc., San Diego, CA, USA). The data are presented as mean ± S.E.M. The criterion of significance was set at P < 0.05.
The amplitude of DA signal measured in rat NAc markedly increased after cocaine (20 mg/kg, i.p.) injection (Fig. 1). The kinetic analysis revealed significant change in the apparent Km with no change in Vmax, consistent with competitive DA transporter (DAT) inhibition. There were significant main effects for both drug (F=139.0, P<0.0001) and time (F=7.57, P<0.0001). Bonferroni post tests indicated significant effects of cocaine on DA uptake in the 5, 10, 15, 25, 40 and 60 min after injection (P<0.001) (Fig. 2A). The increase in apparent Km was maximal (about 600% of controls) within10-15 min after drug administration. No change in the apparent Km was observed in saline-treated rats. Following cocaine administration there was a marked elevation in stereotypical behavior such as sniffing and rearing (Fig. 2B). Both drug (F=162.4, P<0.0001) and time (F=12.52, P<0.0001) showed significant effects. Bonferroni post tests revealed significant increases in the stereotypic activity in 5, 10, 15, 25, 40 and 60 min after cocaine administration (P<0.001). Throughout the duration of the experiment the changes in stereotypy score were temporal coincident with Km changes. To further evaluate the relationship between Km and stereotyped behavior quantitatively, the two measures were correlated. Statistical analysis revealed a significant positive correlation between cocaine-induced Km change and stereotypy score (r=0.84, P<0.05) (Fig. 3).
Figure 1.

Representative traces of electrically evoked DA signals detected by FSCV in rat NAc core before and 10 min after cocaine (20 mg/kg, i.p.) injection. These signals had an oxidation peak at +0.6 V and a reduction peak at -0.2 V vs. Ag/AgCl reference, identifying the released species as DA.
Figure 2.

Effect of cocaine on the apparent Michaelis-Menten rate constant for DA uptake (Km) (A) and stereotyped behavior of rats (B). Three pretreatment points were collected and then saline or cocaine (20 mg/kg, i.p.) was administrated. The time of drug administration is indicated by the arrow. *P<0.001. Data are mean ± SEM values from 6 rats.
Figure 3.
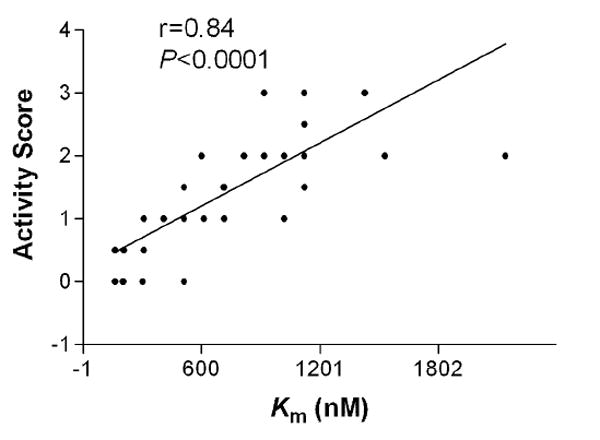
Correlation between cocaine-induced increases in the Km and psychomotor activation. Stereotypy was measured during the 1-min period prior to electrical stimulation using Waddington scale.
The temporally and spatially resolved voltammetry measurements of endogenous DA provide the unique opportunity to assess cocaine-induced changes in the DA uptake in the brain regions, which are associated with its rewarding and stimulating properties [10, 18, 29, 30]. Using FSCV coupled with kinetic analysis, cocaine has been found to act as a competitive inhibitor of DA uptake by altering Km [10, 11, 18-20, 29, 30]. A close temporal association was previously observed between the increase of extracellular DA measured by voltammetry in dorsal striatum and stereotyped activity of rats following systemic administration of DA uptake inhibitors [5, 9]. On the other hand, significant correlations were revealed between the raise in accumbal DA and cocaine-induced locomotor activation [24]. These earlier findings are consistent with hypothesis that the stereotypic effects are mainly caused by changes in DA in the dorsal striatum, whereas the locomotor manifestation are mediated through DA changes in the NAc [7, 12, 13]. The present work links for the first time the effect of cocaine on DA uptake in the NAc to the drug-induced stereotypy. Indeed, the time course of the DA uptake changes was closely paralleled the time course of the increase in stereotypy. The magnitudes of cocaine-stimulated stereotypy were positively and significantly correlated with the Km increases in rat NAc. Therefore, while the role of dorsal striatum in the initiation of stereotyped activity may be critical [7, 12, 13], present data indicate that the DAT inhibition, leading to the increase in extracellular DA in the NAc, is also important contributor for the cocaine-induced stereotypy.
The DA uptake inhibition leads to several different pharmacological consequences which could contribute to the behavioral effects of cocaine. It is well known that the inhibition of soma firing rate diminishes striatal release of DA due to the activation of DA autoreceptors. On the other hand, cocaine enhances DA release by way of mobilizing a synapsin-dependent reserve pool of DA-containing synaptic vesicles [27]. There are some speculations that DAT-independent actions of cocaine could be involved in the behavioral activation induced by cocaine [14, 15]. For example, the contribution of DAT-independent, Na+ channel-mediated actions of cocaine was implicated in the locomotor stimulatory effect of cocaine [15]. In light of these findings, the revelation of an extraordinary connection between a single mechanism, which is the DAT blockade in the NAc and cocaine-induced locomotor activation [24, 25], is certainly important. The present study closely links the DAT inhibition with cocaine-induced stereotypy. This is in good agreement with the fact that the psychostimulant-induced arousal effects were not observed in mice with a genetic deletion of the DAT [8]. In fact, in the absence of the DAT, neither a serotonin nor a norepinephrine transporter, which are also targets for cocaine effects, can provide an alternative uptake site for DA clearance in the NAc [4, 16]. However, a psychostimulant-induced increase in accumbal DA release was revealed in these mutants [3, 6, 17]. This unexpected effect can explain the fact that, despite the absence of the DAT, amphetamine and cocaine display rewarding properties [3, 17, 23, 26], acting through newly developed mechanisms in this case [3, 17]. Importantly, no mechanisms which support behavioral excitation are developed in the DAT mutants.
In conclusion, despite the multiple actions exerted by cocaine on brain neurotransmission, the increase in extracellular DA levels due to the DAT inhibition is an essential mechanism for its arousal effects.
Acknowledgments
We thank Dr. Jack W. Strandhoy for helpful comments and John Peterson for technical support. This work was funded by NIDA grant DA021634.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blanchard RJ, Hebert MA, Dulloog L, Kaawaloa N, Nishimura O, Blanchard DC. Acute cocaine effects on stereotype and defense: an ethoexperimental approach. Neurosci Biobehav Rev. 1998;23:179–88. doi: 10.1016/s0149-7634(98)00019-0. [DOI] [PubMed] [Google Scholar]
- 2.Brown PL, Kiyatkin EA. Brain temperature change and movement activation induced by intravenous cocaine delivered at various injection speeds in rats. Psychopharmacology (Berl) 2005;181:299–308. doi: 10.1007/s00213-005-2244-0. [DOI] [PubMed] [Google Scholar]
- 3.Budygin EA, Brodie MS, Sotnikova TD, Mateo Y, John CE, Cyr M, Gainetdinov RR, Jones SR. Dissociation of rewarding and dopamine transporter-mediated properties of amphetamine. Proc Natl Acad Sci U S A. 2004;101:7781–6. doi: 10.1073/pnas.0401418101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Budygin EA, John CE, Mateo Y, Jones SR. Lack of cocaine effect on dopamine clearance in the core and shell of the nucleus accumbens of dopamine transporter knock-out mice. J Neurosci. 2002;22:RC222. doi: 10.1523/JNEUROSCI.22-10-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budygin EA, Kilpatrick MR, Gainetdinov RR, Wightman RM. Correlation between behavior and extracellular dopamine levels in rat striatum: comparison of microdialysis and fast-scan cyclic voltammetry. Neurosci Lett. 2000;281:9–12. doi: 10.1016/s0304-3940(00)00813-2. [DOI] [PubMed] [Google Scholar]
- 6.Carboni E, Spielewoy C, Vacca C, Nosten-Bertrand M, Giros B, Di Chiara G. Cocaine and amphetamine increase extracellular dopamine in the nucleus accumbens of mice lacking the dopamine transporter gene. J Neurosci. 2001;21:RC141, 1–4. doi: 10.1523/JNEUROSCI.21-09-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delfs JM, Schreiber L, Kelley AE. Microinjection of cocaine into the nucleus accumbens elicits locomotor activation in the rat. J Neurosci. 1990;10:303–10. doi: 10.1523/JNEUROSCI.10-01-00303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283:397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- 9.Garris PA, Budygin EA, Phillips PE, Venton BJ, Robinson DL, Bergstrom BP, Rebec GV, Wightman RM. A role for presynaptic mechanisms in the actions of nomifensine and haloperidol. Neuroscience. 2003;118:819–29. doi: 10.1016/s0306-4522(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 10.Greco PG, Garris PA. In vivo interaction of cocaine with the dopamine transporter as measured by voltammetry. Eur J Pharmacol. 2003;479:117–25. doi: 10.1016/j.ejphar.2003.08.062. [DOI] [PubMed] [Google Scholar]
- 11.Jones SR, Garris PA, Wightman RM. Different effects of cocaine and nomifensine on dopamine uptake in the caudate-putamen and nucleus accumbens. J Pharmacol Exp Ther. 1995;274:396–403. [PubMed] [Google Scholar]
- 12.Joyce EM, Iversen SD. Dissociable effects of 6-OHDA-induced lesions of neostriatum on anorexia, locomotor activity and stereotypy: the role of behavioural competition. Psychopharmacology (Berl) 1984;83:363–6. doi: 10.1007/BF00428546. [DOI] [PubMed] [Google Scholar]
- 13.Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–22. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- 14.Kiyatkin EA, Brown PL. Dopamine-dependent and dopamine-independent actions of cocaine as revealed by brain thermorecording in freely moving rats. Eur J Neurosci. 2005;22:930–8. doi: 10.1111/j.1460-9568.2005.04269.x. [DOI] [PubMed] [Google Scholar]
- 15.Kiyatkin EA, Leon Brown P. The role of peripheral and central sodium channels in mediating brain temperature fluctuations induced by intravenous cocaine. Brain Res. 2006;1117:38–53. doi: 10.1016/j.brainres.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mateo Y, Budygin EA, John CE, Banks ML, Jones SR. Voltammetric assessment of dopamine clearance in the absence of the dopamine transporter: no contribution of other transporters in core or shell of nucleus accumbens. J Neurosci Methods. 2004;140:183–7. doi: 10.1016/j.jneumeth.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 17.Mateo Y, Budygin EA, John CE, Jones SR. Role of serotonin in cocaine effects in mice with reduced dopamine transporter function. Proc Natl Acad Sci U S A. 2004;101:372–7. doi: 10.1073/pnas.0207805101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mateo Y, Budygin EA, Morgan D, Roberts DC, Jones SR. Fast onset of dopamine uptake inhibition by intravenous cocaine. Eur J Neurosci. 2004;20:2838–42. doi: 10.1111/j.1460-9568.2004.03736.x. [DOI] [PubMed] [Google Scholar]
- 19.Mateo Y, Lack CM, Morgan D, Roberts DC, Jones SR. Reduced dopamine terminal function and insensitivity to cocaine following cocaine binge self-administration and deprivation. Neuropsychopharmacology. 2005;30:1455–63. doi: 10.1038/sj.npp.1300687. [DOI] [PubMed] [Google Scholar]
- 20.Mathon DS, Vanderschuren LJ, Ramakers GM. Reduced psychostimulant effects on dopamine dynamics in the nucleus accumbens of muopioid receptor knockout mice. Neuroscience. 2006;141:1679–84. doi: 10.1016/j.neuroscience.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Murray AM, Waddington JL. The interaction of clozapine with dopamine D1 versus dopamine D2 receptor-mediated function: behavioural indices. Eur J Pharmacol. 1990;186:79–86. doi: 10.1016/0014-2999(90)94062-3. [DOI] [PubMed] [Google Scholar]
- 22.Near JA, Bigelow JC, Wightman RM. Comparison of uptake of dopamine in rat striatal chopped tissue and synaptosomes. J Pharmacol Exp Ther. 1988;245:921–7. [PubMed] [Google Scholar]
- 23.Rocha BA, Fumagalli F, Gainetdinov RR, Jones SR, Ator R, Giros B, Miller GW, Caron MG. Cocaine self-administration in dopamine-transporter knockout mice. Nat Neurosci. 1998;1:132–7. doi: 10.1038/381. [DOI] [PubMed] [Google Scholar]
- 24.Sabeti J, Gerhardt GA, Zahniser NR. Acute cocaine differentially alters accumbens and striatal dopamine clearance in low and high cocaine locomotor responders: behavioral and electrochemical recordings in freely moving rats. J Pharmacol Exp Ther. 2002;302:1201–11. doi: 10.1124/jpet.102.035816. [DOI] [PubMed] [Google Scholar]
- 25.Sellings LH, McQuade LE, Clarke PB. Evidence for multiple sites within rat ventral striatum mediating cocaine-conditioned place preference and locomotor activation. J Pharmacol Exp Ther. 2006;317:1178–87. doi: 10.1124/jpet.105.100339. [DOI] [PubMed] [Google Scholar]
- 26.Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, Wichems C, Lesch KP, Murphy DL, Uhl GR. Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci U S A. 2001;98:5300–5. doi: 10.1073/pnas.091039298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venton BJ, Seipel AT, Phillips PE, Wetsel WC, Gitler D, Greengard P, Augustine GJ, Wightman RM. Cocaine increases dopamine release by mobilization of a synapsin-dependent reserve pool. J Neurosci. 2006;26:3206–9. doi: 10.1523/JNEUROSCI.4901-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wightman RM, Zimmerman JB. Control of dopamine extracellular concentration in rat striatum by impulse flow and uptake. Brain Res Brain Res Rev. 1990;15:135–44. doi: 10.1016/0165-0173(90)90015-g. [DOI] [PubMed] [Google Scholar]
- 29.Wu Q, Reith ME, Kuhar MJ, Carroll FI, Garris PA. Preferential increases in nucleus accumbens dopamine after systemic cocaine administration are caused by unique characteristics of dopamine neurotransmission. J Neurosci. 2001;21:6338–47. doi: 10.1523/JNEUROSCI.21-16-06338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Q, Reith ME, Wightman RM, Kawagoe KT, Garris PA. Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry. J Neurosci Methods. 2001;112:119–33. doi: 10.1016/s0165-0270(01)00459-9. [DOI] [PubMed] [Google Scholar]


