Questions about the effects of patents and licensing are becoming critical in the United States, Europe and other developed countries as more genes are discovered and patented, and as genetic testing becomes an integral part of standard medical care. The award of patents for the diagnostic test for haemochromatosis, a progressive iron-overload disease, joins an ever-growing list of such tests that have been, or will very soon be, patented. We have found that US laboratories have refrained from offering clinical-testing services for haemochromatosis because of the patents. A lot of clinical study is needed to validate and extend the early discovery of a disease gene such as that for haemochromatosis, so our results give us reason to fear that limiting clinical testing will inhibit further discovery as well as the understanding that emerges naturally from broad medical adoption1.
As highlighted by the looming patent battle over testing for a breast-cancer mutation between Myriad Genetics and the French Curie and Gustave Roussy institutes (see ref. 2), restrictive licensing and monopolization of clinical-testing services will not be limited to the United States for long. Indeed, four patents relating to haemochromatosis testing are pending in the European Patent Office, suggesting that the situation in the United States described here may soon spread to Europe.
Patent concerns
New human genes are being patented as rapidly as they are discovered3,4. Gene patents generally cover the clinical diagnosis of mutations, as well as using the gene sequence in potential therapies. Setting aside the debate about the ethics of allowing any patenting of human gene sequences, many are concerned about the ramifications of gene patents for biomedical research and clinical medicine.
Unfortunately, there are few empirical data about the effects of patents on the translation of genomic discoveries into medical advances, so it is not clear how justified these concerns might be. Here, we present the results of a survey of US laboratories’ adoption and use of genetic testing for hereditary haemochromatosis.
Hereditary haemochromatosis is a common autosomal recessive disease, affecting 1 in 200 to 1 in 300 people of northern European descent, with a carrier frequency of up to 1 in 10 (ref. 5). As much as 80–85% of haemochromatosis is caused by the two most common mutant alleles of the HFE gene (C282Y and H63D). Haemochromatosis can be treated by periodic therapeutic phlebotomy (such as regular blood donation), so it is a candidate for population screening — and there is a potentially large market for clinical genetic-testing services.
We have discovered that many US laboratories began genetic testing for haemochromatosis before the patents were awarded, but 30% of those in our survey reported discontinuing or not developing genetic testing in the light of the exclusive licence granted on the patents covering clinical-testing services. This result raises obvious concerns about test quality, patient access to testing services, the costs of clinical testing, innovation of testing methods, and the potential for placing limitations on clinical research.
The US patents (numbers 5,712,098; 5,753,438; and 5,705,343) covering the HFE genetic test were first issued to Mercator Genetics in early 1998. The patents grant the right to exclude others from testing for two mutations, C282Y and H63D. Mercator went out of business after spending about US$10 million developing its patented method of positional cloning and discovering the association between HFE mutations and haemochromatosis. Progenitor merged with Mercator and was assigned its pending and issued patents. Progenitor then licensed the patents exclusively for clinical testing to SmithKline Beecham Clinical Laboratories (SBCL) for an up-front payment and guaranteed continuing fees worth around $3 million. SBCL’s exclusivity and payment guarantees continued until a kit became available for use by clinical laboratories. (Exclusive licensing of gene patents is common, particularly for clinical diagnostic uses6.)
Controlling interest
In the summer of 1998, SBCL began enforcing its patent rights. The company wrote to laboratories, stating its willingness to grant sublicences for an up-front fee of $25,000 from academic laboratories, and 5 to 10 times more than this from commercial laboratories, plus royalties of as much as $20 per test. In January 1999, SBCL was sold to Quest Diagnostics, but the sale was not completed until autumn 1999. During and after the sale, SBCL and Quest curtailed active enforcement of the patents, creating uncertainty for laboratories that were —or were interested in — performing HFE testing.
In April 1999, Bio-Rad Laboratories acquired the portfolio of pending and issued patents covering HFE and its mutations from Progenitor, subject to the exclusive clinical-testing licence held by SBCL. After the acquisition of SBCL in late 1999, Quest did not enforce the clinical-testing licence and, in October 2000, transferred it to Bio-Rad for terms that were not made public. In 2001, Bio-Rad began offering a test kit consisting of analyte-specific reagents for the C282Y and H63D alleles. According to several laboratory directors to whom we have spoken recently, Bio-Rad is now offering to license laboratories to perform testing without its kits — but at a cost that makes its kit more economically attractive than the laboratories’ own tests, with up-front payments inversely proportional to the testing volume of the laboratory, plus a per test fee of about $20.
Our data show that patents inhibited development and validation of clinical assays.
US laboratory survey
To understand how gene patents affected laboratories, we ran a pilot survey in November 1998 of laboratory directors and staff attending two national meetings7. Drawing on these results, we developed a comprehensive telephone survey, the results of which we report here, to assess the impact of the HFE patents and the SBCL licensing strategy on clinical-laboratory practices. We identified 117 laboratories in the GeneTests database (see www.genetests.org) and the Association for Molecular Pathology test directory that were or seemed capable of offering the HFE test. With approval of the University of Pennsylvania committee on studies involving human beings, one of us (A.G.K.) carried out interviews in July and August 1999. Snowball sampling (asking respondents for referrals) yielded 11 additional laboratories, for a total sample of 128. We completed 119 interviews (93%) — staff at 9 laboratories declined to participate.
Ninety-two respondents (77%) were laboratory directors, 12 were supervisors (10%), and the rest were other types of laboratory staff. Two-thirds (80) of the laboratories in our sample were affiliated with universities, hospitals or other nonprofit institutions. Most (111; 93%) respondents reported knowing about the HFE patents; 61 had first heard about them from colleagues or at meetings, and 35 from SBCL’s letter. Overall, 54 respondents received the letter, and the 58 laboratories performing HFE testing were more likely than those not offering the test to have received the letter (odds ratio = 4.4, P < 0.001).
Significantly, in September 1999, 31 (26%) laboratories reported that they had not developed and were not performing the test, and another 5 (4%) said they had stopped performing the test. There was no difference between commercial and nonprofit laboratories. Of these 36 laboratories, 22 reported that the patents were “the reason” and 10 said the patents were one of several reasons they had not developed or had stopped offering the test.
We believe that testing volume is a dominant factor in a laboratory’s decision to carry out a particular clinical test. For hospital-based laboratories in the United States, the non-reimbursed expenses incurred by sending samples out to other laboratories for testing can be very high, motivating institutions to develop in-house tests if testing volume justifies the costs of development. Although we did not ask for specific reasons why laboratories were not performing HFE testing, it is likely that low test volume was one of the factors.
In sum, the patents on HFE had a measurable effect on the development and availability of HFE testing services in the United States, as many laboratories that had the capability to perform the test reported not doing so because of the patents.
We asked respondents when they started offering the HFE test, and present a timeline (Fig. 1) that covers the filing of patent applications, publication of the HFE discovery8, and issuance of the patents. Superimposed on this timeline is the accumulation of respondent laboratories as each began HFE testing. The mean time from publication to adoption (of the truncated distribution) was 14 months. Significantly, 35 laboratories (60% of the 58 performing HFE testing at the time of our survey) reported introducing the clinical test before the first patent was issued.
Figure 1.
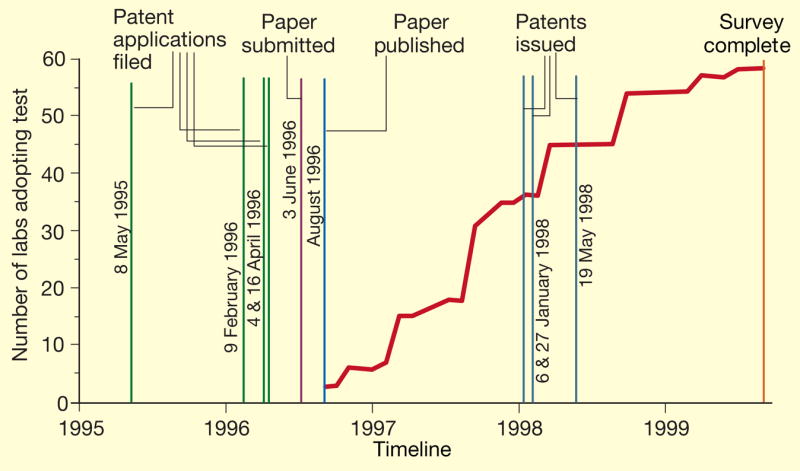
Timeline showing HFE patent applications, publication, patent grants and the cumulative number of laboratories at the times they began offering the clinical test following publication of the gene discovery in ref. 8.
We cannot say, on the basis of our data, whether the decrease in the rate of laboratory adoption after the date the patents were issued was because laboratory staff perceived that there was inadequate demand to justify their development of the test or because of concerns about patent enforcement. Although our respondents overall reported that the patents weighed heavily in their decisions not to perform HFE testing, we do not know when those decisions were made, and that responses could have been biased by hindsight or by the nature of our questioning.
Our data show that there was very rapid adoption of HFE testing by laboratories soon after the cloning of HFE was published (ref. 8), roughly 17 months before the first patent was issued and almost 2 years before SBCL began enforcement. It is clear, as is typical with genetic tests, that the patents were unnecessary for rapid translation of the HFE discovery into clinical-testing services1. On the contrary, our data show that the patents inhibited adoption, perhaps by creating a financial risk for laboratories, and a disincentive to develop and validate a clinical assay that could be stopped by patent enforcement.
Of course, without the potential value of the patented discovery, the investment of venture capital in Mercator Genetics might not have been made and the gene discovery delayed. Yet the question remains whether the exclusive licensing strategy embarked on by Progenitor and SBCL was the best method for capturing financial patent rewards in lieu of, for example, broad non-exclusive licensing of all laboratories that wished to perform the testing with payment of a reasonable per-test royalty, or development and sale of a test kit, the latter being the current strategy of Bio-Rad. But this last strategy may compromise test quality by restricting laboratories to using a single kit, thereby limiting innovation and development of alternative potentially higher-quality or lower-cost methods.
Counting costs
Our data highlight several concerns that have been expressed about patents on biotechnology discoveries. First, at least one study9 has shown that publication of new biotechnology discoveries is delayed because of patenting activities. The paper reporting the cloning of HFE (ref. 8) was submitted more than a year after the first US patent application was filed, and several months after the last of the four applications. Because laboratories can rapidly develop, validate and offer clinical tests, delay in publishing scientifically validated findings of clinical importance can adversely affect patients by delaying access to diagnostic testing.
Second, the two most common mutant alleles, covered by the US patents, account for upto 85% of haemochromatosis in the northern European population; there are many other rare polymorphisms that have clinical relevance10. Laboratories forced to use Bio-Rad’s kit for financial reasons may decide to develop alternative tests for other mutations11, which can increase the cost of testing, the likelihood of laboratory errors because of increased handling of samples, and, if any of the new mutations are also being patented, further increase the cost and licensing complexity for haemochromatosis testing.
Testing may be compromised by limiting labs to a single kit, as developing better or cheaper tests is not encouraged.
Third, gene patents affect the cost and availability of clinical-diagnostic testing. Royalties charged for this and other non-exclusive licences include SBCL’s and Bio-Rad’s charge of up to $20 per test (in addition to substantial up-front payments), and $12.50 per test for Canavan’s disease, $5 per test for Gaucher’s disease, and $2 per test for volume greater than 750 tests a year for the most common allele (ΔF508) of the CFTR gene that causes cystic fibrosis, all with no up-front fees. Although these amounts seem modest, they can present various problems. For example, ‘stacking of royalties’12 occurs for laboratories offering a panel of tests for the Ashkenazi Jewish population, including Tay-Sachs disease, several cystic fibrosis mutations, Gaucher’s disease, Niemann-Pick disease and Canavan’s disease.
One respondent indicated that his cost for this panel of tests was about $100. The royalties for the tests make up about 20% of the cost; this percentage will increase as new tests are added to the panel and technology drives down the marginal cost per test. We believe that royalties must be reasonable and, given the rapid advances being made in testing technology, that they should not be fixed amounts but should be a percentage of the marginal reimbursement, cost or price that can be allocated to the patented test.
Medieval medicine. Haemochromatosis, thought of as a disease of the twenty-first century, can be treated by regular phlebotomy, or bleeding, a medical technique used back in the Dark Ages.

How much is clinical-diagnostic testing being limited by gene patents?
Acknowledgments
Supported in part by the US NIH. The opinions expressed are those solely of the authors. We thank interview respondents; Elizabeth Miller for research assistance; and Michelle Henry and Meredith Weaver for comments. Preliminary results of this survey were presented in testimony to the US Congress in June 2000, and at the 10th Anniversary of ELSI Research Conference, Bethesda, Maryland, 17–19 Jan 2001.
References
- 1.Merz JF. Clin Chem. 1999;45:324–330. [PubMed] [Google Scholar]
- 2.Wadman M. Nature. 2001;413:443. doi: 10.1038/35097228. [DOI] [PubMed] [Google Scholar]
- 3.Thomas SM, Davies ARW, Birtwistle NJ, Crowther SM, Burke JF. Nature. 1996;380:387–388. doi: 10.1038/380387a0. [DOI] [PubMed] [Google Scholar]
- 4.Thomas SM, Birtwistle NJ, Brady M, Burke JF. Nature. 1997;388:709. doi: 10.1038/41853. [DOI] [PubMed] [Google Scholar]
- 5.Gochee PA, Powell LW. Curr Opin Hematol. 2001;8:98–104. doi: 10.1097/00062752-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Schissel A, Merz JF, Cho MK. Nature. 1999;402:118. doi: 10.1038/45907. [DOI] [PubMed] [Google Scholar]
- 7.Cho MK. Preparing for the Millennium: Laboratory Medicine in the 21st Century. Am. Assoc. Clin. Chemistry Press; Orlando, Florida: 1998. pp. 47–53. [Google Scholar]
- 8.Feder JN, et al. Nature Genet. 1996;13:399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 9.Blumenthal D, Campbell EG, Anderson MS, Causino N, Seashore LK. J Am Med Assoc. 1997;277:1224–1228. [PubMed] [Google Scholar]
- 10.Pointon JJ, Wallace D, Merryweather-Clarke AT, Robson KJ. Genet Test. 2000;4:151–161. doi: 10.1089/10906570050114867. [DOI] [PubMed] [Google Scholar]
- 11.Thorstensen K, et al. Genet Test. 2000;4:371–376. doi: 10.1089/109065700750065117. [DOI] [PubMed] [Google Scholar]
- 12.Heller M, Eisenberg R. Science. 1998;280:698–701. doi: 10.1126/science.280.5364.698. [DOI] [PubMed] [Google Scholar]


