Abstract
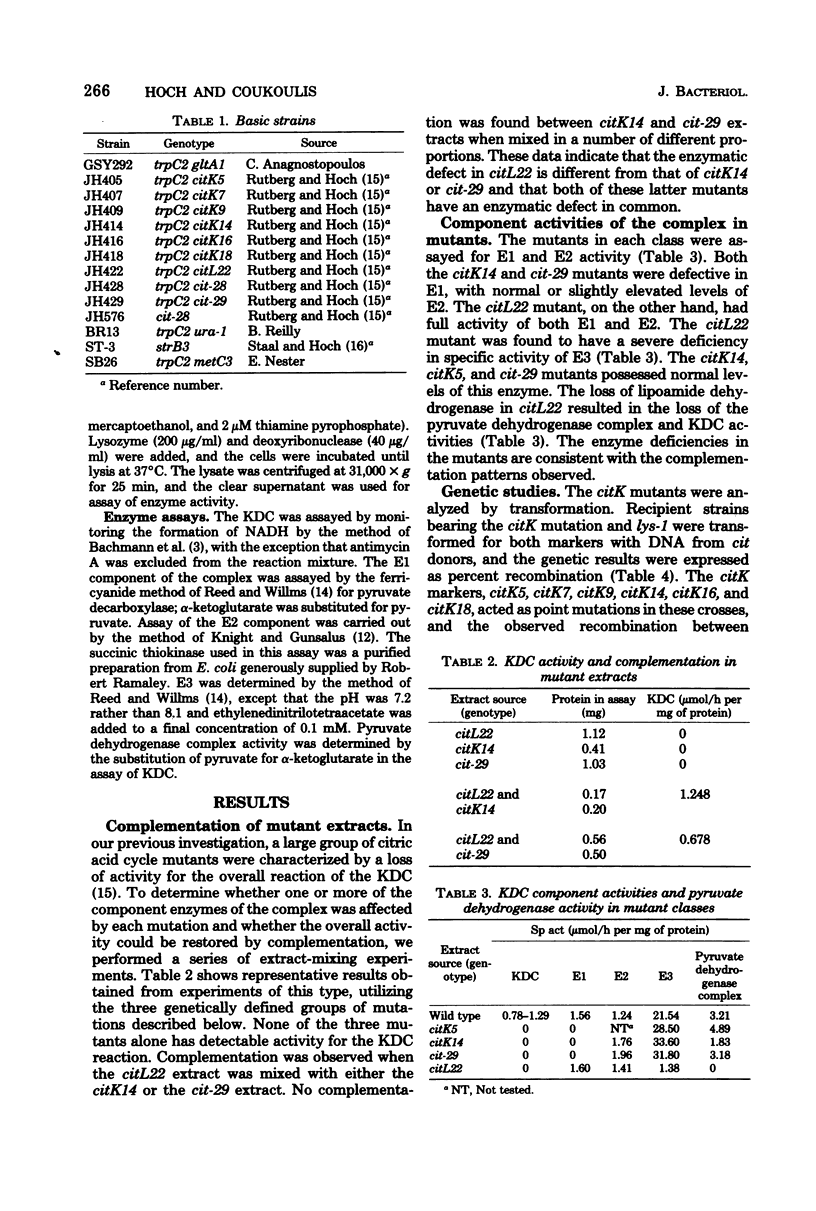
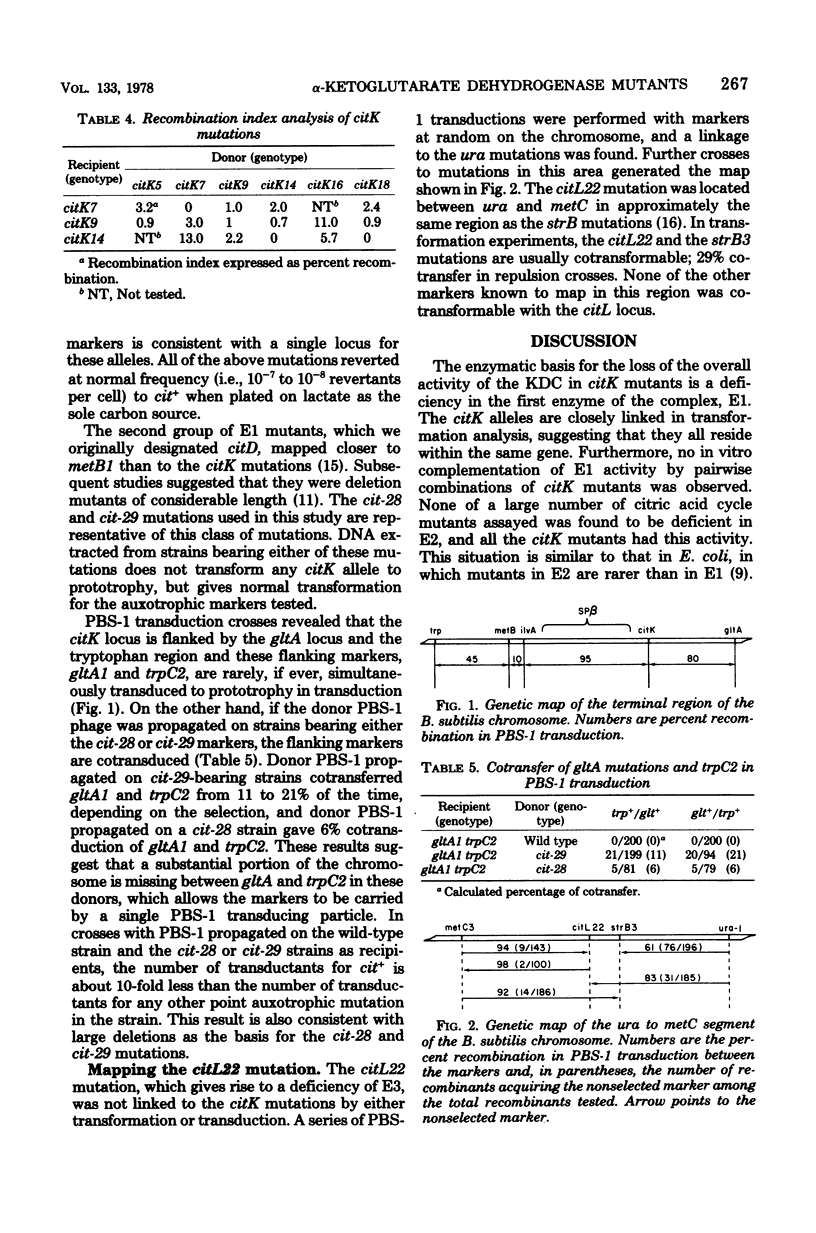
The enzymatic defects in a number of Bacillus subtilis mutants of the alpha-ketoglutarate dehydrogenase complex lacking activity have been investigated. Mutants in the citK locus, as well as a series of deletions of unknown length covering the citK locus, are deficient in E1 of the complex, alpha-ketoglutarate dehydrogenase, but have normal activities of E2, dehydrolipoyl transsuccinylase, and E3, lipoamide dehydrogenase. The citK mutants and the citL22 mutant show in vitro complementation of alpha-ketoglutarate dehydrogenase complex activity. The citL22 mutant is severely deficient in lipoamide dehydrogenase activity, and, as a result, lacks activity for both the alpha-ketoglutarate and the pyruvate dehydrogenase complexes. Thus, the E3 components of both complexes are identical. The citL22 mutation maps between ura and metC on the chromosome.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann E., Allmann D. W., Green D. E. The membrane systems of the mitochondrion. I. The S fraction of the outer membrane of beef heart mitochondria. Arch Biochem Biophys. 1966 Jul;115(1):153–164. doi: 10.1016/s0003-9861(66)81051-2. [DOI] [PubMed] [Google Scholar]
- Goldstein B. J., Zahler S. A. Uptake of branched-chain alpha-keto acids in Bacillus subtilis. J Bacteriol. 1976 Jul;127(1):667–670. doi: 10.1128/jb.127.1.667-670.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest J. R., Creaghan I. T. Further studies with lipoamide dehydrogenase mutants of Escherichia coli K12. J Gen Microbiol. 1974 Mar;81(1):237–245. doi: 10.1099/00221287-81-1-237. [DOI] [PubMed] [Google Scholar]
- Guest J. R., Creaghan I. T. Gene-protein relationships of the alpha-keto acid dehydrogenase complexes of Escherichia coli K12: isolation and characterization of lipoamide dehydrogenase mutants. J Gen Microbiol. 1973 Mar;75(1):197–210. doi: 10.1099/00221287-75-1-197. [DOI] [PubMed] [Google Scholar]
- Guest J. R. Gene-protein relationships of the alpha-keto acid dehydrogenase complexes of Escherichia coli K12: Chromosomal location of the lipoamide dehydrogenase gene. J Gen Microbiol. 1974 Feb;80(2):523–532. doi: 10.1099/00221287-80-2-523. [DOI] [PubMed] [Google Scholar]
- Herbert A. A., Guest J. R. Biochemical and genetic studies with lysine+methionine mutants of Escherichia coli: lipoic acid and alpha-ketoglutarate dehydrogenase-less mutants. J Gen Microbiol. 1968 Oct;53(3):363–381. doi: 10.1099/00221287-53-3-363. [DOI] [PubMed] [Google Scholar]
- Herbert A. A., Guest J. R. Studies with alpha-ketoglutarate dehydrogenase mutants of Escherichia coli. Mol Gen Genet. 1969 Oct 13;105(2):182–190. doi: 10.1007/BF00445687. [DOI] [PubMed] [Google Scholar]
- Hoch J. A., Barat M., Anagnostopoulos C. Transformation and transduction in recombination-defective mutants of Bacillus subtilis. J Bacteriol. 1967 Jun;93(6):1925–1937. doi: 10.1128/jb.93.6.1925-1937.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutberg B., Hoch J. A. Citric acid cycle: gene-enzyme relationships in Bacillus subtilis. J Bacteriol. 1970 Nov;104(2):826–833. doi: 10.1128/jb.104.2.826-833.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal S. P., Hoch J. A. Conditional dihydrostreptomycin resistance in Bacillus subtilis. J Bacteriol. 1972 Apr;110(1):202–207. doi: 10.1128/jb.110.1.202-207.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C. L., Kaneda T. Activities of alpha-ketoisovalerate, pyruvate, and alpha-ketoglutarate dehydrogenases in a mutant of Bacillus subtilis. Can J Microbiol. 1976 Apr;22(4):592–597. doi: 10.1139/m76-088. [DOI] [PubMed] [Google Scholar]
- Zahler S. A., Korman R. Z., Rosenthal R., Hemphill H. E. Bacillus subtilis bacteriophage SPbeta: localization of the prophage attachment site, and specialized transduction. J Bacteriol. 1977 Jan;129(1):556–558. doi: 10.1128/jb.129.1.556-558.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



