Abstract
Thymic stromal lymphopoietin (TSLP) is elevated in asthma and triggers dendritic cell-mediated activation of Th2 inflammatory responses. Although TSLP has been shown to be produced mainly by airway epithelial cells, the regulation of epithelial TSLP expression has not been extensively studied. We investigated the expression of TSLP in cytokine- or TLR ligand-treated normal human bronchial epithelial cells (NHBE). The mRNA for TSLP was significantly up-regulated by stimulation with IL-4 (5.5-fold) and IL-13 (5.3-fold), weakly up-regulated by TNF-α, TGF-β, and IFN-β, and not affected by IFN-γ in NHBE. TSLP mRNA was only significantly up-regulated by the TLR3 ligand (dsRNA) among the TLR ligands tested (66.8-fold). TSLP was also induced by in vitro infection with rhinovirus. TSLP protein was detected after stimulation with dsRNA (120 ± 23 pg/ml). The combination of TNF-α and IL-4 produced detectable levels of TSLP protein (40 ± 13 pg/ml). In addition, TSLP was synergistically enhanced by a combination of IL-4 and dsRNA (mRNA; 207-fold, protein; 325 ± 75 pg/ml). The induction of TSLP by dsRNA was dependent upon NF-κB and IFN regulatory factor 3 (IRF-3) signaling via TLR3 as indicated by a study with small interfering RNA. The potent topical glucocorticoid fluticasone propionate significantly suppressed dsRNA-dependent TSLP production in NHBE. These results suggest that the expression of TSLP is induced in airway epithelial cells by stimulation with the TLR3 ligand and Th2 cytokines and that this response is suppressed by glucocorticoid treatment. This implies that respiratory viral infection and the recruitment of Th2 cytokine producing cells may amplify Th2 inflammation via the induction of TSLP in the asthmatic airway.
The asthmatic airway is characterized by an infiltrate of eosinophils and Th2-type T cells (Th2 cells). Disease exacerbations of asthma are usually triggered by infection with airway-targeting viruses, especially rhinovirus, coronavirus, influenza virus, respiratory syncytial virus, and adenovirus (1– 6). These viruses preferentially infect nasal and airway epithelial cells through their entry receptors. Infection by respiratory viruses can variably destroy the epithelial barrier, depending on the type of virus. The damage of epithelial cells results in both increased epithelial permeability and increased penetration of allergens and injurious materials, events that may contribute to the exacerbation of asthma by respiratory virus infection (1– 6). Increasing evidence suggests that airway epithelial cells do not simply act as a physical barrier but also function in the regulation of immune responses though the production of cytokines and chemokines and via interaction with cells of immune systems (7–12). It is now known that rhinovirus infection of airway epithelial cells induces the production of cytokines such as IL-1β, IL-6, IL-11, and G-CSF, chemokines such as CCL5 (RANTES), CCL11 (eotaxin-1), CCL24 (eotaxin-2), and CXCL8 (IL-8) and the expression of adhesion molecules such as ICAM-1 and VCAM-1, resulting in the recruitment of inflammatory cells and the amplification of tissue inflammation (13–16).
Thymic stromal lymphopoietin (TSLP)3 is an IL-7-like cytokine molecule that was first cloned in humans in 2001 (17–19). TSLP is produced by epithelial cells, skin keratinocytes, stromal cells, smooth muscle cells, lung fibroblasts, and mast cells (20). TSLP expression is not found in most hemopoietic cells including T cells, B cells, NK cells, monocytes, and dendritic cells (DC), with the exception of mast cells (20). The TSLP receptor is a heterodimeric receptor consisting of the IL-7 receptor α-chain and a common γ-like TSLP-specific receptor (TSLP receptor (TSLPR); also known as CRLF2) (18, 21). Human TSLPR is expressed on heart, skeletal muscle, kidney, liver, and DC (18, 2121). TSLP functionally activates different cell types in humans and mice. In the mouse, TSLP promotes an early stage of B cell and T cell development (20, 22, 23). In contrast, in humans TSLP activates myeloid cells such as CD11c+ myeloid DC (mDC) but does not have direct effects on T cells, B cells, NK cells, or neutrophils (18, 20).
DC are essential for the Th differentiation of naive CD4+ T cells in response to aeroallergens. TSLP strongly induces the expression of MHC I and II and that of costimulatory molecules such as CD40, CD80, and CD86 on mDC (20). Recent studies have shown that TSLP-stimulated mDC induce naive CD4+ T cells to differentiate into Th2 cells that produce IL-4, IL-13, and TNF-α but not IL-10 and IFN-γ (24, 25). This contrasts with TLR ligands that usually stimulate mDC to produce Th1-inducing cytokines such as IL-12, IL-27, and IFN-α, cytokines that are not produced by TSLP-stimulated mDC (20, 24). Further contributing to Th2 activation, TSLP-stimulated mDC produce the Th2-attracting chemokine TARC (20). OX40L, a TNF ligand family member, is induced by TSLP on mDC and has been reported to be a key regulator of Th2 differentiation (24).
TSLP has been shown to be highly involved in the pathogenesis of inflammatory diseases. High expression of TSLP has been found in the skin of patients with acute and chronic atopic dermatitis, while TSLP is not detectable in normal skin or nonlesional skin of patients (20). The expression of TSLP is confined mainly to keratinocytes of the apical layers of the epidermis in patients with atopic dermatitis (20). Skin-specific overexpression of TSLP in mice induces an atopic dermatitis-like phenotype including acanthosis, intraepidermal spongiosis, hyperkeratosis, and inflammatory cell infiltrates into skin (26). In addition to atopic dermatitis, TSLP also has been found to be increased in asthmatic airways (27). High amounts of TSLP have been found in bronchoalveolar lavage fluid in a mouse asthma model and lung-specific TSLP transgenic mice show airway inflammation including the massive infiltration of inflammatory cells, goblet cell hyperplasia, and airway hyper responsiveness, whereas mice lacking the TSLPR exhibit strong Th1 responses and fail to develop an inflammatory lung response to Ag (28, 29). Although TSLP has been shown to be produced mainly by airway epithelial cells and is involved in the pathogenesis of inflammatory diseases, the regulation of TSLP expression has not been extensively studied.
Functional cytokine receptors and TLRs are known to be expressed on airway epithelial cells. In the present study we investigated whether TSLP is induced by cytokines and TLR ligands in airway epithelial cells. We discovered that TSLP was strongly and significantly induced by the Th2 cytokines IL-4 and IL-13 and the TLR3 ligand dsRNA and that the combination of IL-4 and dsRNA synergistically enhanced TSLP production in airway epithelial cells. These findings imply that respiratory viral infection and the recruitment of Th2 cytokine-producing cells may amplify Th2 inflammation via the induction of TSLP in the asthmatic airway.
Materials and Methods
Reagents
Recombinant human TNF-α, IL-4, IL-13, IFN-β, IFN-γ, IFN-λ1, and TGF-β were purchased from R&D Systems. The synthetic bacterial lipoprotein N-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2-RS)-propyl]-[R]-Cys-[S]-Ser-[S]-Lys4 trihydrochloride (Pam3CSK4), zymosan, peptidoglycan (PGN) from Staphylococcus aureus, lipoteichoic acid (LTA) from Staphylococcus aureus, polyinosinic-polycytidylic acid (dsRNA), LPS from Escherichia coli serotype 0111:B4, recombinant flagellin from Salmonella typhimurium, synthetic diacylated lipoprotein S-(2,3-bispalmitoyloxypropyl) Cys-Gly-Asp-Pro-Lys-His-Pro-Lys-Ser-Phe (FSL-1), R-837, and CpG oligodeoxynucleotide M362 (CpG-C) were purchased from InvivoGen. Fluticasone propionate (FP) and DMSO were purchased from Sigma-Aldrich. The small interfering RNA (siRNA) against TLR3, melanoma differentiation-associated gene 5 (MDA5), retinoic acid-inducible gene I (RIG-I), dsRNA-dependent protein kinase (PKR), IFNαβ receptor 2 (IFNAR2), NF-κB p65 (RELA), NF-κB p50 (NFKB1), IFN regulatory factor-3 (IRF-3), STAT6, and the no effect control were obtained from Qiagen.
Cell culture, treatments, transfection, and rhinovirus infection
Normal human bronchial epithelial cells (NHBE) were obtained from Cambrex. NHBE were maintained in serum-free bronchial epithelial cell growth medium (Cambrex). NHBE were plated in 24-well culture plates coated with collagen (Vitrogen; Cohesion Technologies). Before stimulation, NHBE were cultured in bronchial epithelial cell growth medium without hydrocortisone for at least 2 days. NHBE were stimulated with one of the following at the indicated concentration: 100 ng/ml TNF-α, 100 ng/ml IL-4, 100 ng/ml IL-13, 1000 U/ml IFN-β, 100 ng/ml IFN- γ, 100 ng/ml IFN- λ1, 100 ng/ml TGF-β, 1 µg/ml Pam3CSK4, 10 µg/ml PGN, 10 µg/ml LTA, 25 µg/ml dsRNA, 1 µg/ml LPS, 10 ng/ml flagellin, 1 µg/ml FSL-1, 10 µg/ml R-837, and 4 µg/ml CpG-C for 6 h.
NHBE were seeded at 3 × 104 cells per well in 24-well culture plates and were cultured for 2 days. At 40–60% confluence, cells were transfected with siRNA against TLR3, MDA5, RIG-I, PKR, IFNAR2, RELA, NFKB1, IRF-3, STAT6, or control RNA at 5 nM using HiPerFect transfection reagent (Qiagen) following the manufacturer’s instructions. Viability of the cells was usually >80% in siRNA transfected cells. The viability of transfected cells with siRNA against TLR3 and IRF-3 was rarely <80% (TLR3, once; IRF-3, twice), but we did not use these cells. The transfected cells were further grown for 48 h and then stimulated with 25 µg/ml dsRNA for 6 h.
Rhinovirus serotype 16 (RV16) was a gift from W. Busse and E. Dick University of Wisconsin, Madison). RV16 stocks were amplified and purified based on a previously published protocol (30). NHBE were infected with RV16 at a multiplicity of infection (MOI) of 2 or 10. RV16-infected NHBE were cultured for 6 or 24 h at 33°C in the presence or absence of 100 ng/ml IL-4.
Real-time PCR
Total RNA was extracted using RNeasy (Qiagen) and treated with DNase I (Qiagen) according to the manufacturer’s instructions. Single-strand cDNA was synthesized with SuperScript II reverse transcriptase (Invitrogen) and random primers. Real-time RT-PCR was performed with the Taq-Man method using an Applied Biosystems 7500 sequence detection system (Applied Biosystems) as described previously (31). Primer and probe sets for two genes, TSLP (sense, 5′-CCCAGGCTATTCGGAAACTCA-3′; antisense, 5′-ACGCCACAATCCTTGTAATTGTG-3′; and MGB probe, 5′-AAGGAAAGTCACAACCAATAA-3′) and GAPDH (sense, 5′-GAAGGTGAAGGTCGGAGTC-3′; antisense, 5′-GAAGATGGTGATGGGATTTC-3′; and FAM/TAMRA labeled probe, 5′-CAAGCTTCCCGTTCTCAGCC-3′) were synthesized by Applied Biosystems. Primer and probe sets for TLR3, MDA5, RIG-I, PKR, IFNAR2, RELA, NFKB1, STAT6, retinoid X receptor α (RXRA) and Hes1 were purchased from Applied Biosystems. To determine the exact copy number of the target genes, quantified aliquots of purified PCR fragments of the target genes were serially diluted and used as standards in each experiment. Aliquots of cDNA equivalent to 10 ng of total RNA were used for real-time PCR. The mRNA expression levels were normalized to the median expression of a housekeeping gene (GAPDH).
ELISA
Concentrations of the TSLP protein in cell-free supernatants were measured with a specific ELISA kit (R&D Systems). The minimal detection limit for this kit was 31.25 pg/ml.
Sequence analysis
The sequences of the TSLP gene (accession no. NW_922751.1) and TSLP mRNA (accession no. NM_033035.3) were obtained from GenBank. We searched the proximal 5000-bp promoter region of TSLP for putative NF-κB binding sites by using the consensus sequence GGGRNNYYCC (N indicates any nucleotide, R indicates A or G, and Y indicates C or T) and searched for putative STAT6 binding sites using the consensus sequence TTCN3/4GAA (32–34). We searched the proximal 1900 bp 3′ UTR of TSLP for AU-rich elements by using the sequence AUUUA (35, 36).
Statistical analysis
All data are reported as the mean ± SEM unless otherwise noted. Differences between groups were analyzed using the paired Student’s t test and considered to be significant when p < 0.05.
Results
TSLP induction by dsRNA and IL-4
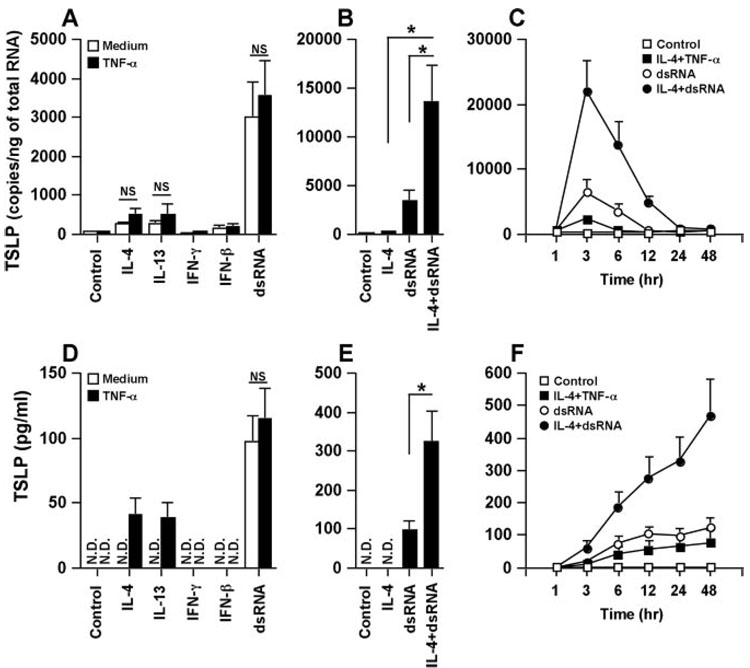
Increasing evidence indicates that airway epithelial cell-derived TSLP is involved in the pathogenesis of allergic diseases, including asthma. To test the possible role of the regulation of TSLP production in airway epithelial cells, NHBE were treated with cytokines including TNF-α, IL-4, IL-13, IFN-γ, IFN-β, IFN-λ1, and TGF-β and TLR ligands including Pam3CSK4 (TLR2 ligand), zymosan (TLR2 ligand), PGN (TLR2 ligand), LTA (TLR2 ligand), dsRNA (TLR3 ligand), LPS (TLR4 ligand), flagellin (TLR5 ligand), FSL-1 (TLR2/6 ligand), R-837 (TLR7 ligand), and CpG-C (TLR9 ligand) for 6 h. Messenger RNA for TSLP was significantly up-regulated by stimulation with IL-4 (5.5-fold, n = 4; p < 0.05) and IL-13 (5.3-fold, n = 4; p < 0.05), weakly up-regulated by TNF-α (1.5-fold), TGF-β (2.3-fold) and IFN-β (3.0-fold), and not affected by IFN-γ and IFN-λ1 in NHBE (Fig. 1A). Messenger RNA for TSLP was only significantly up-regulated by the TLR3 ligand (dsRNA) among the TLR ligands tested (66.8-fold, n = 7; p < 0.05) (Fig. 1A and data not shown). To confirm this phenomenon at the protein level, we measured the production of TSLP using ELISA. Significant levels of TSLP (control; undetectable, dsRNA-treated; 97 ± 20 pg/ml, n = 7) were detected in the supernatant only after stimulation with dsRNA (Fig. 1D). In contrast with dsRNA-treated NHBE, TSLP protein was not detected in the supernatant of TNF-α-, IL-4-, IL-13- or IFN-β-treated NHBE (Fig. 1D). To further examine the details of the induction of mRNA for TSLP by dsRNA, we determined the concentration and the time dependence of the response in NHBE. TSLP mRNA was induced by concentrations of dsRNA as low as 2.5 ng/ml and peak levels of TSLP mRNA were observed at 3 h (Fig. 1, B and C).
FIGURE 1.
Effect of cytokines and TLR ligands on the up-regulation of TSLP in airway epithelial cells. A, NHBE were incubated for 6 h with 100 ng/ml TNF-α, 100 ng/ml IL-4, 100 ng/ml IL-13, 1000 U/ml IFN-β, 100 ng/ml IFN-γ, 100 ng/ml IFN-λ1, 100 ng/ml TGF-β, 1 µg/ml Pam3CSK4, 10 µg/ml PGN, 10 µg/ml LTA, 25 µg/ml dsRNA, 1 µg/ml LPS, 10 ng/ml flagellin, 1 µg/ml FSL-1, 10 µg/ml R-837, and 4 µg/ml CpG-C as indicated and then mRNA was extracted and analyzed for TSLP using real-time PCR. B, NHBE were incubated for 6 h with 2.5–25,000 ng/ml dsRNA and then the expression of mRNA for TSLP was analyzed by real-time PCR. C, NHBE were incubated with 25 µg/ml dsRNA (●) or vehicle control (■) for 1–48 h and then the expression of mRNA for TSLP was analyzed by real-time PCR. The copy number is expressed as the number of transcripts per nanogram of total RNA. D, Detection of TSLP protein by ELISA in the culture supernatant of NHBE stimulated with cytokines and dsRNA for 24 h. Results shown are mean ± SEM of 4–7 independent experiments. N.D., not detectable. *, p < 0.05.
Effect of combinations of cytokines and dsRNA on the expression of TSLP
TNF-α is usually expressed at inflammatory sites and may contribute to an increase in Th1 and Th2 cytokine-dependent gene expression (32, 37–39). To examine whether or not the combination of Th1 and Th2 cytokines with TNF-α enhanced cytokine-dependent TSLP expression, NHBE were treated with combinations of TNF-α and cytokines including IL-4, IL-13, IFN-γ, and IFN-β. The addition of TNF-α only weakly enhanced TSLP mRNA expression induced by IL-4 (2.0-fold, n = 4) or IL-13 (2.0-fold, n = 4) (Fig. 2A). However, detectable levels of the TSLP protein were only found in the supernatant after stimulation with TNF-α plus IL-4 (40 ± 13 pg/ml, n = 4) and TNF-α plus IL-13 (38 ± = 12 pg/ml, n = 4) but not with the individual cytokines (Fig. 2D). We also examined the effect of combined stimulation with cytokines and the TLR3 ligand dsRNA on the expression of TSLP in NHBE. We found that IL-4 synergistically and significantly enhanced dsRNA-dependent TSLP mRNA expression (4.1-fold, n = 5; p < 0.05) (Fig. 2B) with peak levels at 3 h (Fig. 2C). Elevated levels of TSLP persisted until 24 h after stimulation using the combination of IL-4 and dsRNA (Fig. 2C). In addition, IL-4 synergistically and significantly enhanced dsRNA-dependent TSLP protein production (24 h, 325 ± 75 pg/ml, n = 5; p < 0.05) (Fig. 2E). The combination of IL-4 and dsRNA time-dependently induced the production of TSLP for up to 48 h after stimulation, the duration of the experiment (Fig. 2F). In contrast, the production of TSLP by dsRNA only or in combination with TNF-α and IL-4 had plateaued by 12 h after stimulation (Fig. 2F).
FIGURE 2.
Effect of combination of cytokines and dsRNA on the up-regulation of TSLP. NHBE were incubated for 6 h (A) or 24 h (D) with 100 ng/ml IL-4, 100 ng/ml IL-13, 100 ng/ml IFN-γ, 1000 U/ml IFN-β, and 25 µg/ml dsRNA in the presence or absence of 100 ng/ml TNF-α as indicated. NHBE were incubated for 6 h (B) or 24 h (E) with 100 ng/ml IL-4, 25 µg/ml dsRNA, or a combination of IL-4 and dsRNA. C and F, NHBE were incubated for 1–48 h with medium control (◻), 100 ng/ml IL-4 plus 100 ng/ml TNF-α(◼), 25 µg/ml dsRNA (○), and 100 ng/ml IL-4 plus 25 µg/ml dsRNA (●). The level of TSLP mRNA was determined by real-time PCR (A–C). Concentrations of TSLP protein in the culture supernatant were measured by ELISA (D–F). Results shown are mean ± SEM of 4–7 independent experiments. NS, Not significant; N.D., not detectable. *, p < 0.05.
TSLP induction by rhinovirus
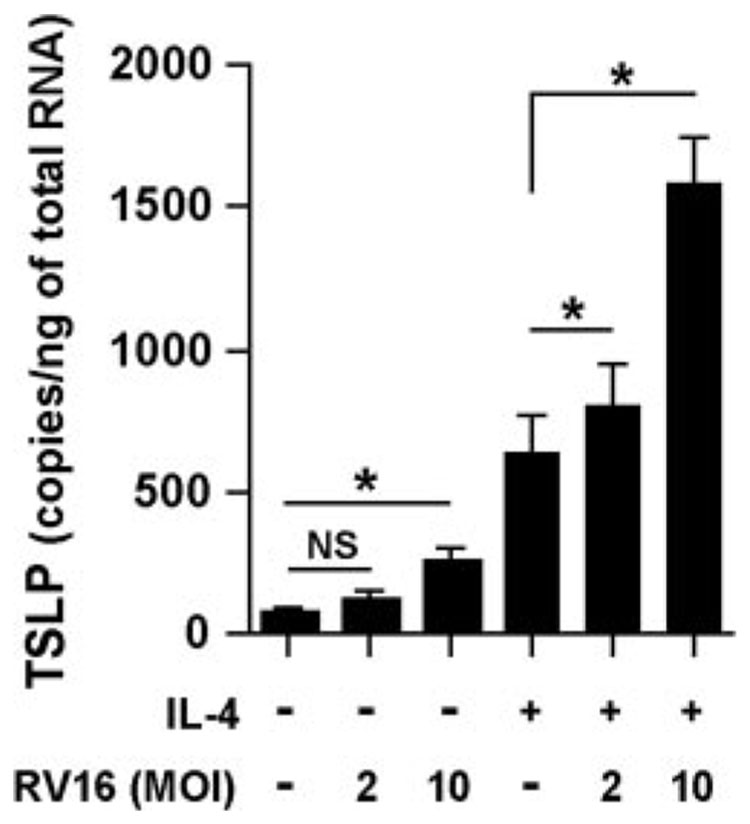
Rhinoviruses are single-stranded RNA viruses belonging to the Picornaviridae family and synthesize dsRNA during their replication. Exacerbations of asthma are commonly triggered by infection with rhinovirus (1–6). To test the effect of rhinovirus infection on the induction of TSLP in airway epithelial cells, NHBE were infected with RV16 in the presence or absence of IL-4. Messenger RNA for TSLP was significantly up-regulated by RV16 at a MOI of 10 (Fig. 3). The combination of IL-4 and RV16 also synergistically and significantly enhanced TSLP expression in NHBE (Fig. 3). To confirm this phenomenon at the protein level, we measured the production of TSLP. TSLP was only detected in supernatants from cells stimulated with IL-4 and challenged with RV16 at a MOI of 10 for 24 h (32 ± 3 pg/ml, n = 3; p < 0.05 vs all other conditions).
FIGURE 3.
Effect of rhinovirus infection on the up-regulation of TSLP in airway epithelial cells. NHBE were infected with RV16 at a MOI of 2 or 10 and then cultured for 6 h at 33°C in the presence or absence of 100 ng/ml IL-4. The level of TSLP mRNA was determined by real-time PCR. The results are shown as the mean ± SEM of four independent experiments. NS, not significant. * p < 0.05.
Mechanisms of TSLP expression by dsRNA
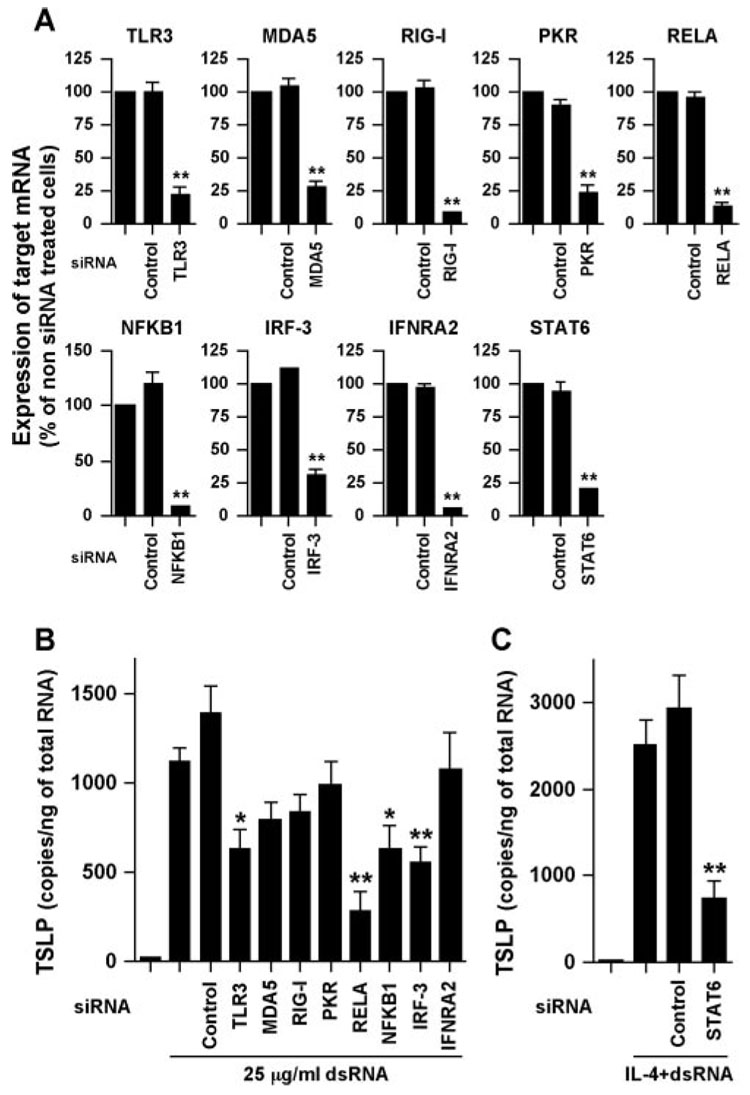
dsRNA is a surrogate for viral RNA and viral replication and is sensed by TLR3 and the recently identified cytosolic RNA helicases RIG-I and MDA5 (40, 41). dsRNA is also recognized by the classical dsRNA recognition protein PKR. Activation of TLR3, RIG-I, or MDA5 by dsRNA transduces its signals to NF-κB and IRF-3 and induces proinflammatory genes and type I IFNs including IFN-β. Induction of IFN-βsecondarily induces numerous IFN-inducible genes. To clarify the mechanism of TSLP induction by dsRNA, we knocked down molecules involved in dsRNA signaling. NHBE were transfected with siRNA against control RNA, TLR3, MDA5, RIG-1, PKR, RELA, NFKB1, IRF-3, or IFNAR2 and then stimulated with dsRNA for 6 h. Control experiments demonstrated that target molecules were significantly suppressed by siRNA against each target molecule but not by control siRNA, and siRNA did not inhibit expression of the housekeeping gene GAPDH (Fig. 4A and data not shown). Induction of TSLP by dsRNA was significantly inhibited by siRNA against TLR3, RELA, NFKB1, and IRF-3 but not by siRNA targeting MDA5, RIG-I, PKR, IFNAR2, or control RNA (Fig. 4B). These results suggest that TSLP is directly induced by dsRNA in airway epithelial cells and that the response is mediated via a TLR3-, NF-κB-, and IRF-3-dependent but IFN-independent pathway. Enhancement of dsRNA-dependent TSLP expression by IL-4 was significantly inhibited by siRNA against STAT6 (Fig. 4C).
FIGURE 4.
Effect of the dsRNA-related molecules on the expression of TSLP by dsRNA. NHBE were transfected with siRNA against control RNA, TLR3, MDA5, RIG-1, PKR, RELA, NFKB1, IRF-3, IFNAR2, or STAT6 at 5 nM for 48 h (A) and then stimulated with 25 µg/ml dsRNA (B) or 100 ng/ml IL-4 plus 25 µg/ml dsRNA (C) for 6 h. The levels of mRNAs were determined by real-time PCR. Efficiency of siRNA against target molecules was expressed as a percentage of non-siRNA transfected cells (A). The results are shown as the mean ± SEM of four independent experiments. *, p < 0.05; **, p < 0.005.
Effect of glucocorticoids on the induction of TSLP
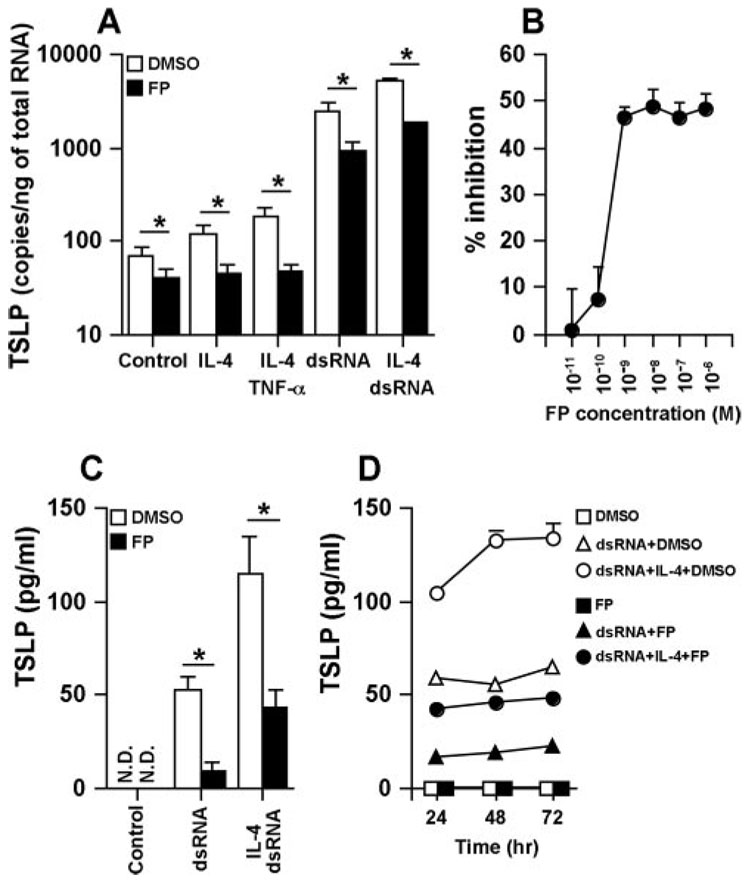
Glucocorticoids are a mainstay in the treatment of diseases characterized by airway inflammation such as asthma and chronic rhinosinusitis (42). We investigated whether glucocorticoids were able to suppress the IL-4- or dsRNA-dependent production of TSLP in airway epithelial cells. NHBE were treated with DMSO (vehicle control) or the potent topical glucocorticoid FP for 2 h and then stimulated with IL-4, TNF-α, dsRNA, or their combination for 6 h (for assessment of mRNA) or 24–72 h (for assessment of protein). Induction of mRNA for TSLP was significantly and dose-dependently inhibited by FP (Fig. 5, A and B). To confirm this phenomenon at the protein level, we measured the production of TSLP. We found significant FP inhibition of the production of TSLP by dsRNA (84% inhibition at 24 h, n = 4; p < 0.05) or the combination of dsRNA and IL-4 (62% inhibition at 24 h, n = 4; p < 0.05) in NHBE (Fig. 5, C and D).
FIGURE 5.
Effect of glucocorticoids on the up-regulation of TSLP by dsRNA and IL-4 in airway epithelial cells. NHBE were preincubated with 0.01% DMSO, 100 nM FP (A, C, and D), or 10−11 to 10−6 M FP (B) for 2 h and then stimulated with 100 ng/ml IL-14, 100 ng/ml TNF-α, and 25 µg/ml dsRNA for 6 h (A and B), 24 h (C), or 24–72 h (D). The level of TSLP mRNA was determined by real-time PCR (A and B). Concentrations of TSLP protein in the culture supernatant were measured by ELISA (C and D). The results are shown as the mean ± SEM of 4–7 independent experiments. N.D., Not detectable. *, p < 0.05.
Discussion
TSLP plays an important role in the DC-mediated activation of Th2 inflammatory responses. Despite the expression in vivo and potential importance of epithelial cell-derived TSLP in allergic diseases including asthma, no studies have tested the effect of cytokines and TLR ligands on the production of TSLP in human airway epithelial cells. In the present study we determined the TSLP expression profiles of normal human bronchial epithelial cells after stimulation with cytokines and TLR ligands. This study provides the first demonstration that the Th2 cytokines IL-4 and IL-13 and rhinovirus infection induce TSLP expression in airway epithelial cells. We have also shown that IL-4 synergistically enhanced dsRNA- and rhinovirus-dependent TSLP production in airway epithelial cells.
IL-4 and IL-13 are mainly produced by Th2 cells and basophils and each have heterodimeric receptors (43). The IL-4Rα-chain is shared by receptors for IL-4 and IL-13 and is essential for the activation of the central transcription factor STAT6 (43). This suggests that STAT6 may be one transcription factor that can induce TSLP expression. As shown in Fig. 1, IL-4 and IL-13 alone were not able to induce detectable TSLP protein production in NHBE, although the mRNA for TSLP was significantly up-regulated by each. However, a combination of IL-4 or IL-13 with the inflammatory cytokine TNF-α led to detectable expression of the TSLP protein (Fig. 2D). In a recent report, Bogiatzi et al. have shown that TSLP is induced by the combination of TNF-α and Th2 cytokines but not by the individual cytokines in skin keratinocytes (44). These results suggest that the activation of NF-κB may support the STAT6-dependent production of TSLP. Previous studies have demonstrated a synergy between NF-κB and STAT6 in the induction of CCL11 (eotaxin-1) in response to TNF-α and IL-4/IL-13 in airway epithelial cells mediated by a composite response element in the eotaxin promoter (32). In the TSLP promoter we found three putative NF-κB binding sites at −734 bp, −929 bp, and −4552 bp and nine putative STAT6 binding sites at −514 bp, −541 bp, −2249 bp, −2356 bp, −3703 bp, −3774 bp, −3829 bp, and −4299 bp within −5000 bp upstream (32–34). We also found one modified consensus site for STAT6 (position is −4557 bp) that overlapped with the 5′-end of a putative NF-κB binding region −4552 bp). Future studies will be required to identify the functional sites among these.
Airway epithelial cells have been shown to express functional TLR, notably TLR2-6 (12, 45, 46). Although TLRs share signal transduction pathways for activation of the transcription factors NF-κB and IRF-3 (47), only dsRNA, a TLR3 ligand and mimic of viral RNA and viral replication, induced TSLP expression in normal human bronchial epithelial cells (Fig. 1). The failure of other TLR ligands tested to induce the expression of TSLP may reflect low levels of receptor expression or adaptor proteins involved in the respective responses.
While this manuscript was under revision, Lee and Ziegler reported that TSLP is not induced by dsRNA in NHBE (48). Our data clearly show that TSLP mRNA was expressed only transiently after activation because it was not detected at 1 h, was highly elevated at 3 h, and then returned to baseline by 12 h after stimulation with dsRNA (Fig. 1 and Fig. 2). The fact that Lee and Ziegler selected 2 and 20 h after stimulation may explain why elevations of TSLP mRNA by dsRNA were not detected in their studies (48). Two separate groups have also recently shown that TSLP is induced by dsRNA in small airway epithelial cells and oral epithelial cells (49, 50). These results suggest that dsRNA-dependent TSLP production is reproducible in human epithelial cells.
It has been reported that dsRNA and IL-4 synergistically enhanced the production of the eosinophil-recruiting chemokines CCL11 and CCL26 (eotaxin-3) in airway smooth muscle cells and airway epithelial cells, respectively (51, 52). Enhancement of CCL26 by the combination of IL-4 and dsRNA was found to be a secondary effect of the induction of the IL-4 receptor by dsRNA in airway epithelial cells (52). In the present study of TSLP expression, dsRNA and IL-4 stimulated NHBE rapidly (Fig. 2, C and F). In addition, enhancement of dsRNA-dependent TSLP expression by IL-4 was significantly inhibited by siRNA against STAT6 in NHBE (Fig. 4C). These data suggest that dsRNA and IL-4 activate different transcription factors that are capable of binding the promoter of TSLP and activating its expression in airway epithelial cells, probably NF-κB, IRF-3 (dsRNA), and STAT6 (IL-4). Future studies will be required to identify the complement of transcription factors that activate the promoter of TSLP.
dsRNA and RNA viruses are recognized by the endosomal receptor TLR3 and also by the cytoplasmic RNA helicases RIG-I and MDA5 and the cytoplasmic serine-threonine kinase PKR (40, 41). Our data suggest that the induction of TSLP by dsRNA was mainly mediated by TLR3 (Fig. 4). As shown in Fig. 4, the induction of TSLP by dsRNA was weakly but not significantly suppressed by siRNA against MDA5 (27% inhibition) and RIG-I (23% inhibition). Future studies will be required to determine whether dsRNA-dependent TSLP production is partially regulated by MDA5 or RIG-I in airway epithelial cells.
The signal transduction pathway of TLR3 has been well studied (47, 53, 54). Ligand-activated TLR3 recruits a Toll/IL-1 receptor (TIR) domain-containing adapter inducing IFN-β (TRIF) via its intracellular TIR domain. TRIF recruits a TGF-β-activated kinase-1 and a TANK-binding kinase 1 that phosphorylate IκB and IRF-3 respectively (47, 53, 54). The phosphorylation of IκB represents a signal for polyubiquitination followed by degradation by the 26 S proteosome, and this enables the translocation of NF-κB into the nucleus and then induces proinflammatory genes such as TNF-α, IL-6, and IL-12 (47, 53, 54). In contrast, phosphorylated IRF-3 dimerizes and translocates into the nucleus and induces IFN-β production. Our data clearly showed that the induction of TSLP by dsRNA was dependent on both NF-κB and IRF-3 (Fig. 4). Usually, IRF-3 critically regulates IFN-β production and then secondarily induces multiple IFN-regulated genes. In recent studies we found that induction of B cell-activating factor of TNF family (BAFF) by dsRNA occurred via an autocrine loop involving IFN-β(31). In the case of TSLP, the induction by dsRNA was not suppressed by siRNA against the IFN receptor IFNAR2 and IFN-β did not strongly induce TSLP production in NHBE (Fig. 1, Fig. 2, and Fig. 4), suggesting that dsRNA-induced TSLP production is independent of autocrine stimulation by IFN-β.
IRF-3 promotes transcription of the IFN-β gene together with other transcription factors such as NF-κB. Although the mechanisms of type I IFN induction by IRF-3 are well established, IFN independent IRF-3 activation is not fully understood. Recent studies suggest that IRF-3 directly induces the transcriptional suppresser Hes1 independently of IFN-β, which in turn inhibits the expression of the nuclear receptor RXRA (55). Inhibition of RXRA by the activation of IRF-3 can reduce the expression of RXRA target genes including the metabolic enzymes CYP3A4 and UGT1A6 (55). The studies of Li et al. (56) indicate that keratinocyte-selective ablation of RXRA and retinoid×receptor βinduces TSLP expression in epidermal keratinocytes, which results in an atopic dermatitis-like syndrome. We therefore investigated whether IRF-3-dependent TSLP expression by dsRNA resulted via the induction of Hes1 and the inhibition of RXRA. Hes1 was not induced by dsRNA in NHBE and RXRA was only weakly suppressed (data not shown). Together, these results suggest that TSLP is induced by dsRNA in airway epithelial cells and that this response occurs via a pathway dependent on NF-κB and IRF-3 but independent of IFN-β and RXRA.
We examined whether glucocorticoids can inhibit IL-4- and dsRNA-dependent TSLP production, because glucocorticoids are widely used in the therapeutic management of inflammatory airway diseases. In vivo studies in human subjects have demonstrated that glucocorticoids inhibit the production of IL-4 and IL-5 in cells from bronchoalveolar lavage in allergic asthmatics and reduce the number of IL-4- and IL-13-expressing cells in nasal tissue from patients with allergic rhinitis (57–59). As shown in Fig. 5, the potent topical glucocorticoid FP partially but significantly inhibited the induction of both TSLP mRNA and TSLP protein by dsRNA and IL-4 in NHBE. This suggests that glucocorticoids may inhibit both the production of Th2 cytokines from cells in the airways and the TSLP response of airway epithelial cells to these cytokines. By such effects, glucocorticoids could blunt the differentiation of Th2 cells in allergic airway disease. Future clinical studies will be required to investigate whether glucocorticoids suppress TSLP production in vivo.
The mechanisms of the anti-inflammatory effects of glucocorticoids have been extensively studied (42). The activated glucocorticoid receptor dimerizes and interacts with transcription factors such as NF-κB and AP-1 and represses the genes activated by these transcription factors. Glucocorticoids also exert posttranscriptional control on gene expression by decreasing the stability of mRNA for inflammatory genes. Our previous studies have suggested that NF-κB is not a major target of glucocorticoids in human airway epithelial cells (36, 60). Possible mechanisms of the effect of glucocorticoids on the expression of TSLP may include the influence of destabilizing sequences known as AU-rich elements in the 3′ untranslated region of the transcripts. In fact, the 3′ untranslated of TSLP contains seven AUUUA motifs. Future studies will be required to determine the mechanism of inhibition of TSLP by glucocorticoids in airway epithelial cells.
Although asthma is characterized by Th2-type inflammation, the normal CD4+ T cell response to viral infection is thought to be predominantly of the Th1 type. It has been suggested that in the lower airways of asthmatics with a pre-existing Th2-type allergic microenvironment the responses to viral infection may be skewed toward inappropriate and potentially harmful Th2 responses (5, 6). Indeed, respiratory syncytial virus infection exacerbated the Th2 cytokine response and lung pathologic lesions in a mouse model of allergic asthma, whereas respiratory syncytial virus infection of nonallergic mice did not induce Th2 cytokine response significantly (61). Respiratory syncytial virus infection enhanced the pulmonary Th2 cytokine response only when mice were inoculated after sensitization to OVA (62). Th2 cytokines such as IL-4, IL-5, IL-10, and IL-13 enhanced the expression of ICAM-1, which serves as the rhinovirus entry receptor and has been proposed to be involved in respiratory syncytial virus entry (63, 64). Basal levels of ICAM-1 expression were increased in the nasal epithelial cells from atopic subjects and the airway epithelial cells from asthmatic subjects (65, 66). These data suggest that susceptibility to virus infection is increased in a Th2 environment. Our studies suggest that virus infection may further enhance the development of Th2 responses, creating a vicious cycle. We found that the Th2 cytokine IL-4 and the virus product dsRNA synergistically enhanced TSLP production from epithelial cells (Fig. 2). Importantly, we also found that the rhinovirus, which is the most common known trigger of asthma exacerbations, induced TSLP expression and production in the presence of IL-4 in airway epithelial cells (Fig. 3). It is possible that the TSLP production triggered by the rhinovirus could be higher in the asthmatic airway compared with NHBE due to the elevation of ICAM-1 (the receptor for rhinovirus) and the reduction of the antiviral response in epithelial cells from patients with asthma (65–67). TSLP-activated DC express OX40L and promote the differentiation of TNF-α-producing inflammatory Th2 cells from naive T cells (24). In addition, TLR3 activation strongly induced eosinophil-recruiting chemokines in the presence of Th2 cytokines (51, 52). Together, these findings suggest that viral infection in a Th2 environment may amplify allergic reactions locally. The effect of in vivo infection with respiratory viruses such as rhinovirus on the production of TSLP in airway epithelial cells from patients with asthma is worthy of investigation.
In summary, we report in this study that the expression of TSLP was induced in airway epithelial cells by stimulation with TLR3 ligand and Th2 cytokines and was synergistically induced by the combination of both stimuli; this response was suppressed by glucocorticoid treatment. Our findings indicate that respiratory viral infection and the recruitment of Th2 cytokine-producing cells may amplify Th2 inflammation via the production of TSLP in the asthmatic airway.
Footnotes
This work was supported in part by National Institutes of Health Grants R01 HL068546 and R01 HL078860 and by a grant from the Ernest S. Bazley Trust. A.K. is the recipient of a 2007 Strategic Training in Allergy Research (ST*AR) Program Award from the American Academy of Allergy, Asthma, and Immunology.
Abbreviations used in this paper: TSLP, thymic stromal lymphopoietin; DC, dendritic cells; FP, fluticasone propionate; FSL-1, Cys-Gly-Asp-Pro-Lys-His-Pro-Lys-Ser-Phe; IFNAR, IFN α or β receptor; IRF-3, IFN regulatory factor 3; LTA, lipoteichoic acid; MDA5, melanoma differentiation-associated gene 5; mDC, myeloid DC; MOI, multiplicity of infection; NFKB1, NF-κB p50; NHBE, normal human bronchial epithelial cell; Pam3CSK4, N-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2-RS)- propyl]-[R]-Cys-[S]-Ser-[S]-Lys4 trihydrochloride; PGN, peptidoglycan; PKR, dsRNA-dependent protein kinase; RELA, NF-κB p65; RIG-I, retinoic acid-inducible gene I; RV16, rhinovirus serotype 16; RXRA, retinoid × receptor A; siRNA, small interfering RNA; TSLPR, TSLP receptor.
Disclosures Dr. Schleimer has received honoraria from all major manufacturers of inhaled glucocorticoids, including GlaxoSmithKline, the manufacturer of fluticasone propionate. Dr. Avila has received honoraria and an investigator-initiated grant from GlaxoSmithKline.
References
- 1.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, Symington P, O’Toole S, Myint SH, Tyrrell DA, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avila PC. Interactions between allergic inflammation and respiratory viral infections. J. Allergy Clin. Immunol. 2000;106:829–831. doi: 10.1067/mai.2000.111027. [DOI] [PubMed] [Google Scholar]
- 4.Johnston SL. Overview of virus-induced airway disease. Proc. Am. Thorac. Soc. 2005;2:150–156. doi: 10.1513/pats.200502-018AW. [DOI] [PubMed] [Google Scholar]
- 5.Contoli M, Caramori G, Mallia P, Johnston S, Papi A. Mechanisms of respiratory virus-induced asthma exacerbations. Clin. Exp. Allergy. 2005;35:137–145. doi: 10.1111/j.1365-2222.2005.02163.x. [DOI] [PubMed] [Google Scholar]
- 6.Caramori G, Ito K, Contoli M, Di Stefano A, Johnston SL, Adcock IM, Papi A. Molecular mechanisms of respiratory virus-induced asthma and COPD exacerbations and pneumonia. Curr. Med. Chem. 2006;13:2267–2290. doi: 10.2174/092986706777935159. [DOI] [PubMed] [Google Scholar]
- 7.Hogg JC, Eggleston PA. Is asthma an epithelial disease? Am. Rev. Respir. Dis. 1984;129:207–208. [PubMed] [Google Scholar]
- 8.Cohn LA, Adler KB. Interactions between airway epithelium and mediators of inflammation. Exp. Lung Res. 1992;18:299–322. doi: 10.3109/01902149209031687. [DOI] [PubMed] [Google Scholar]
- 9.Rennard SI, Romberger DJ, Sisson JH, Von Essen SG, Rubinstein I, Robbins RA, Spurzem JR. Airway epithelial cells: functional roles in airway disease. Am. J. Respir. Crit. Care Med. 1994;150:S27–S30. doi: 10.1164/ajrccm/150.5_Pt_2.S27. [DOI] [PubMed] [Google Scholar]
- 10.Abreu MT, Fukata M, Arditi M. TLR signaling in the gut in health and disease. J. Immunol. 2005;174:4453–4460. doi: 10.4049/jimmunol.174.8.4453. [DOI] [PubMed] [Google Scholar]
- 11.Diamond G, Legarda D, Ryan LK. The innate immune response of the respiratory epithelium. Immunol. Rev. 2000;173:27–38. doi: 10.1034/j.1600-065x.2000.917304.x. [DOI] [PubMed] [Google Scholar]
- 12.Sha Q, Truong-Tran AQ, Plitt JR, Beck LA, Schleimer RP. Activation of airway epithelial cells by Toll-like receptor agonists. Am. J. Respir. Cell Mol. Biol. 2004;31:358–364. doi: 10.1165/rcmb.2003-0388OC. [DOI] [PubMed] [Google Scholar]
- 13.Einarsson O, Geba GP, Zhu Z, Landry M, Elias JA. Interleukin-11: stimulation in vivo and in vitro by respiratory viruses and induction of airways hyperresponsiveness. J. Clin. Invest. 1996;97:915–924. doi: 10.1172/JCI118514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gern JE, Vrtis R, Grindle KA, Swenson C, Busse WW. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am. J. Respir. Crit. Care Med. 2000;162:2226–2231. doi: 10.1164/ajrccm.162.6.2003019. [DOI] [PubMed] [Google Scholar]
- 15.Papadopoulos NG, Papi A, Meyer J, Stanciu LA, Salvi S, Holgate ST, Johnston SL. Rhinovirus infection up-regulates eotaxin and eotaxin-2 expression in bronchial epithelial cells. Clin. Exp. Allergy. 2001;31:1060–1066. doi: 10.1046/j.1365-2222.2001.01112.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Lau C, Wiehler S, Pow A, Mazzulli T, Gutierrez C, Proud D, Chow CW. Syk is downstream of intercellular adhesion molecule-1 and mediates human rhinovirus activation of p38 MAPK in airway epithelial cells. J. Immunol. 2006;177:6859–6870. doi: 10.4049/jimmunol.177.10.6859. [DOI] [PubMed] [Google Scholar]
- 17.Quentmeier H, Drexler HG, Fleckenstein D, Zaborski M, Armstrong A, Sims JE, Lyman SD. Cloning of human thymic stromal lymphopoietin (TSLP) and signaling mechanisms leading to proliferation. Leukemia. 2001;15:1286–1292. doi: 10.1038/sj.leu.2402175. [DOI] [PubMed] [Google Scholar]
- 18.Reche PA, Soumelis V, Gorman DM, Clifford T, Liu M, Travis M, Zurawski SM, Johnston J, Liu YJ, Spits H, et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J. Immunol. 2001;167:336–343. doi: 10.4049/jimmunol.167.1.336. [DOI] [PubMed] [Google Scholar]
- 19.Liu YJ, Soumelis V, Watanabe N, Ito T, Wang YH, Malefyt RD, Omori M, Zhou B, Ziegler SF. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu. Rev. Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 20.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, et al. Human epithelial cells trigger dendritic cell-mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 21.Tonozuka Y, Fujio K, Sugiyama T, Nosaka T, Hirai M, Kitamura T. Molecular cloning of a human novel type I cytokine receptor related to δ1/TSLPR. Cytogenet. Cell Genet. 2001;93:23–25. doi: 10.1159/000056941. [DOI] [PubMed] [Google Scholar]
- 22.Sims JE, Williams DE, Morrissey PJ, Garka K, Foxworthe D, Price V, Friend SL, Farr A, Bedell MA, Jenkins NA, et al. Molecular cloning and biological characterization of a novel murine lymphoid growth factor. J. Exp. Med. 2000;192:671–680. doi: 10.1084/jem.192.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levin SD, Koelling RM, Friend SL, Isaksen DE, Ziegler SF, Perlmutter RM, Farr AG. Thymic stromal lymphopoietin: a cytokine that promotes the development of IgM+ B cells in vitro and signals via a novel mechanism. J. Immunol. 1999;162:677–683. [PubMed] [Google Scholar]
- 24.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang YH, Ito T, Homey B, Watanabe N, Martin R, Barnes CJ, McIntyre BW, Gilliet M, Kumar R, Yao Z, Liu YJ. Maintenance and polarization of human TH2 central memory T cells by thymic stromal lymphopoietin-activated dendritic cells. Immunity. 2006;24:827–838. doi: 10.1016/j.immuni.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Yoo J, Omori M, Gyarmati D, Zhou B, Aye T, Brewer A, Comeau MR, Campbell DJ, Ziegler SF. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J. Exp. Med. 2005;202:541–549. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ying S, O’Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, Robinson D, Zhang G, Zhao J, Lee TH, Corrigan C. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J. Immunol. 2005;174:8183–8190. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 28.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J. Exp. Med. 2005;202:829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat. Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 30.Sethi SK, Bianco A, Allen JT, Knight RA, Spiteri MA. Interferon-γ (IFN-γ) down-regulates the rhinovirus-induced expression of intercellular adhesion molecule-1 (ICAM-1) on human airway epithelial cells. Clin. Exp. Immunol. 1997;110:362–369. doi: 10.1046/j.1365-2249.1997.4221440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato A, Truong-Tran AQ, Scott AL, Matsumoto K, Schleimer RP. Airway epithelial cells produce B cell-activating factor of TNF family by an IFN-β-dependent mechanism. J. Immunol. 2006;177:7164–7172. doi: 10.4049/jimmunol.177.10.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsukura S, Stellato C, Plitt JR, Bickel C, Miura K, Georas SN, Casolaro V, Schleimer RP. Activation of eotaxin gene transcription by NF-κB and STAT6 in human airway epithelial cells. J. Immunol. 1999;163:6876–6883. [PubMed] [Google Scholar]
- 33.Baldwin AS., Jr. The NF-κB and I κB proteins: new discoveries and insights. Annu. Rev. Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 34.Seidel HM, Milocco LH, Lamb P, Darnell JE, Jr, Stein RB, Rosen J. Spacing of palindromic half sites as a determinant of selective STAT (signal transducers and activators of transcription) DNA binding and transcriptional activity. Proc. Natl. Acad. Sci. USA. 1995;92:3041–3045. doi: 10.1073/pnas.92.7.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peppel K, Vinci JM, Baglioni C. The AU-rich sequences in the 3′ untranslated region mediate the increased turnover of interferon mRNA induced by glucocorticoids. J. Exp. Med. 1991;173:349–355. doi: 10.1084/jem.173.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stellato C, Matsukura S, Fal A, White J, Beck LA, Proud D, Schleimer RP. Differential regulation of epithelial-derived C-C chemokine expression by IL-4 and the glucocorticoid budesonide. J. Immunol. 1999;163:5624–5632. [PubMed] [Google Scholar]
- 37.Ying S, Robinson DS, Varney V, Meng Q, Tsicopoulos A, Moqbel R, Durham SR, Kay AB, Hamid Q. TNFα mRNA expression in allergic inflammation. Clin. Exp. Allergy. 1991;21:745–750. doi: 10.1111/j.1365-2222.1991.tb03205.x. [DOI] [PubMed] [Google Scholar]
- 38.Mukhopadhyay S, Hoidal JR, Mukherjee TK. Role of TNFα in pulmonary pathophysiology. Respir. Res. 2006;7:125. doi: 10.1186/1465-9921-7-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jahnke A, Johnson JP. Synergistic activation of intercellular adhesion molecule 1 (ICAM-1) by TNF-α and IFN-γ is mediated by p65/p50 and p65/c-Rel and interferon-responsive factor Stat1 α(p91) that can be activated by both IFN-γ and IFN-α. FEBS Lett. 1994;354:220–226. doi: 10.1016/0014-5793(94)01130-3. [DOI] [PubMed] [Google Scholar]
- 40.Schroder M, Bowie AG. TLR3 in antiviral immunity: key player or bystander? Trends Immunol. 2005;26:462–468. doi: 10.1016/j.it.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Meylan E, Tschopp J. Toll-like receptors and RNA helicases: two parallel ways to trigger antiviral responses. Mol. Cell. 2006;22:561–569. doi: 10.1016/j.molcel.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 42.Schleimer RP. Glucocorticoids: Part A: Mechanisms of action in allergic diseases. In: Yunginger JW, Adkinson NF Jr, Busse WW, Bochner BS, Holgate ST, Simons FER, editors. In Middleton’s Allergy: Principles and Practice. St. Louis: Mosby; [Google Scholar]
- 43.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu. Rev. Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 44.Bogiatzi SI, Fernandez I, Bichet JC, Marloie-Provost MA, Volpe E, Sastre X, Soumelis V. Cutting edge: proinflammatory and Th2 cytokines synergize to induce thymic stromal lymphopoietin production by human skin keratinocytes. J. Immunol. 2007;178:3373–3377. doi: 10.4049/jimmunol.178.6.3373. [DOI] [PubMed] [Google Scholar]
- 45.Schulz C, Farkas L, Wolf K, Kratzel K, Eissner G, Pfeifer M. Differences in LPS-induced activation of bronchial epithelial cells (BEAS-2B) and type II-like pneumocytes (A-549) Scand. J. Immunol. 2002;56:294–302. doi: 10.1046/j.1365-3083.2002.01137.x. [DOI] [PubMed] [Google Scholar]
- 46.Homma T, Kato A, Hashimoto N, Batchelor J, Yoshikawa M, Imai S, Wakiguchi H, Saito H, Matsumoto K. Corticosteroid and cytokines synergistically enhance Toll-like receptor 2 expression in respiratory epithelial cells. Am. J. Respir. Cell Mol. Biol. 2004;31:463–469. doi: 10.1165/rcmb.2004-0161OC. [DOI] [PubMed] [Google Scholar]
- 47.Akira S, Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 48.Lee HC, Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFκB. Proc. Natl. Acad. Sci. USA. 2007;104:914–919. doi: 10.1073/pnas.0607305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, Paquette N, Ziegler SF, Sarfati M, Delespesse G. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J. Exp. Med. 2007;204:253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu W, He B, Chiu A, Chadburn A, Shan M, Buldys M, Ding A, Knowles DM, Santini PA, Cerutti A. Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nat. Immunol. 2007;8:294–303. doi: 10.1038/ni1434. [DOI] [PubMed] [Google Scholar]
- 51.Niimi K, Asano K, Shiraishi Y, Nakajima T, Wakaki M, Kagyo J, Takihara T, Suzuki Y, Fukunaga K, Shiomi T, et al. TLR3-mediated synthesis and release of eotaxin-1/CCL11 from human bronchial smooth muscle cells stimulated with double-stranded RNA. J. Immunol. 2007;178:489–495. doi: 10.4049/jimmunol.178.1.489. [DOI] [PubMed] [Google Scholar]
- 52.Tsuji K, Yamamoto S, Ou W, Nishi N, Kobayashi I, Zaitsu M, Muro E, Sadakane Y, Ichimaru T, Hamasaki Y. dsRNA enhances eotaxin-3 production through interleukin-4 receptor upregulation in airway epithelial cells. Eur. Respir. J. 2005;26:795–803. doi: 10.1183/09031936.05.00010805. [DOI] [PubMed] [Google Scholar]
- 53.Jiang Z, Mak TW, Sen G, Li X. Toll-like receptor 3-mediated activation of NF-κB and IRF3 diverges at Toll-IL-1 receptor domain-containing adapter inducing IFN-β. Proc. Natl. Acad. Sci. USA. 2004;101:3533–3538. doi: 10.1073/pnas.0308496101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 55.Chow EK, Castrillo A, Shahangian A, Pei L, O’Connell M, Modlin RL, Tontonoz P, Cheng G. A role for IRF3-dependent RXRα repression in hepatotoxicity associated with viral infections. J. Exp. Med. 2006;203:2589–2602. doi: 10.1084/jem.20060929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li M, Hener P, Zhang Z, Kato S, Metzger D, Chambon P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc. Natl. Acad. Sci. USA. 2006;103:11736–11741. doi: 10.1073/pnas.0604575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu MC, Proud D, Lichtenstein LM, Hubbard WC, Bochner BS, Stealey BA, Breslin L, Xiao H, Freidhoff LR, Schroeder JT, Schleimer RP. Effects of prednisone on the cellular responses and release of cytokines and mediators after segmental allergen challenge of asthmatic subjects. J. Allergy Clin. Immunol. 2001;108:29–38. doi: 10.1067/mai.2001.116004. [DOI] [PubMed] [Google Scholar]
- 58.Robinson D, Hamid Q, Ying S, Bentley A, Assoufi B, Durham S, Kay AB. Prednisolone treatment in asthma is associated with modulation of bronchoalveolar lavage cell interleukin-4, interleukin-5, and interferon-γ cytokine gene expression. Am. Rev. Respir. Dis. 1993;148:401–406. doi: 10.1164/ajrccm/148.2.401. [DOI] [PubMed] [Google Scholar]
- 59.Ghaffar O, Laberge S, Jacobson MR, Lowhagen O, Rak S, Durham SR, Hamid Q. IL-13 mRNA and immunoreactivity in allergen-induced rhinitis: comparison with IL-4 expression and modulation by topical glucocorticoid therapy. Am. J. Respir. Cell Mol. Biol. 1997;17:17–24. doi: 10.1165/ajrcmb.17.1.2696. [DOI] [PubMed] [Google Scholar]
- 60.Matsukura S, Kokubu F, Kurokawa M, Kawaguchi M, Kuga H, Ieki K, Odaka M, Suzuki S, Watanabe S, Takeuchi H, et al. Molecular mechanisms of repression of eotaxin expression with fluticasone propionate in airway epithelial cells. Int. Arch. Allergy Immunol. 2004;134 Suppl. 1:12–20. doi: 10.1159/000077787. [DOI] [PubMed] [Google Scholar]
- 61.Barends M, Boelen A, de Rond L, Kwakkel J, Bestebroer T, Dormans J, Neijens H, Kimman T. Influence of respiratory syncytial virus infection on cytokine and inflammatory responses in allergic mice. Clin. Exp. Allergy. 2002;32:463–471. doi: 10.1046/j.1365-2222.2002.01317.x. [DOI] [PubMed] [Google Scholar]
- 62.Barends M, Van Oosten M, De Rond CG, Dormans JA, Osterhaus AD, Neijens HJ, Kimman TG. Timing of infection and prior immunization with respiratory syncytial virus (RSV) in RSV-enhanced allergic inflammation. J. Infect. Dis. 2004;189:1866–1872. doi: 10.1086/386341. [DOI] [PubMed] [Google Scholar]
- 63.Bianco A, Sethi SK, Allen JT, Knight RA, Spiteri MA. Th2 cytokines exert a dominant influence on epithelial cell expression of the major group human rhinovirus receptor, ICAM-1. Eur. Respir. J. 1998;12:619–626. doi: 10.1183/09031936.98.12030619. [DOI] [PubMed] [Google Scholar]
- 64.Behera AK, Matsuse H, Kumar M, Kong X, Lockey RF, Mohapatra SS. Blocking intercellular adhesion molecule-1 on human epithelial cells decreases respiratory syncytial virus infection. Biochem. Biophys. Res. Commun. 2001;280:188–195. doi: 10.1006/bbrc.2000.4093. [DOI] [PubMed] [Google Scholar]
- 65.Bentley AM, Durham SR, Robinson DS, Menz G, Storz C, Cromwell O, Kay AB, Wardlaw AJ. Expression of endothelial and leukocyte adhesion molecules intercellular adhesion molecule-1, E-selectin, and vascular cell adhesion molecule-1 in the bronchial mucosa in steady-state and allergen-induced asthma. J. Allergy Clin. Immunol. 1993;92:857–868. doi: 10.1016/0091-6749(93)90064-m. [DOI] [PubMed] [Google Scholar]
- 66.Bianco A, Whiteman SC, Sethi SK, Allen JT, Knight RA, Spiteri MA. Expression of intercellular adhesion molecule-1 (ICAM-1) in nasal epithelial cells of atopic subjects: a mechanism for increased rhinovirus infection? Clin. Exp. Immunol. 2000;121:339–345. doi: 10.1046/j.1365-2249.2000.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, Kebadze T, Mallia P, Stanciu LA, Parker HL, et al. Role of deficient type III interferon-λ production in asthma exacerbations. Nat. Med. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]