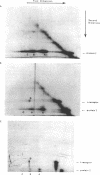
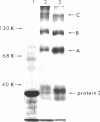
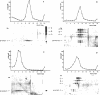
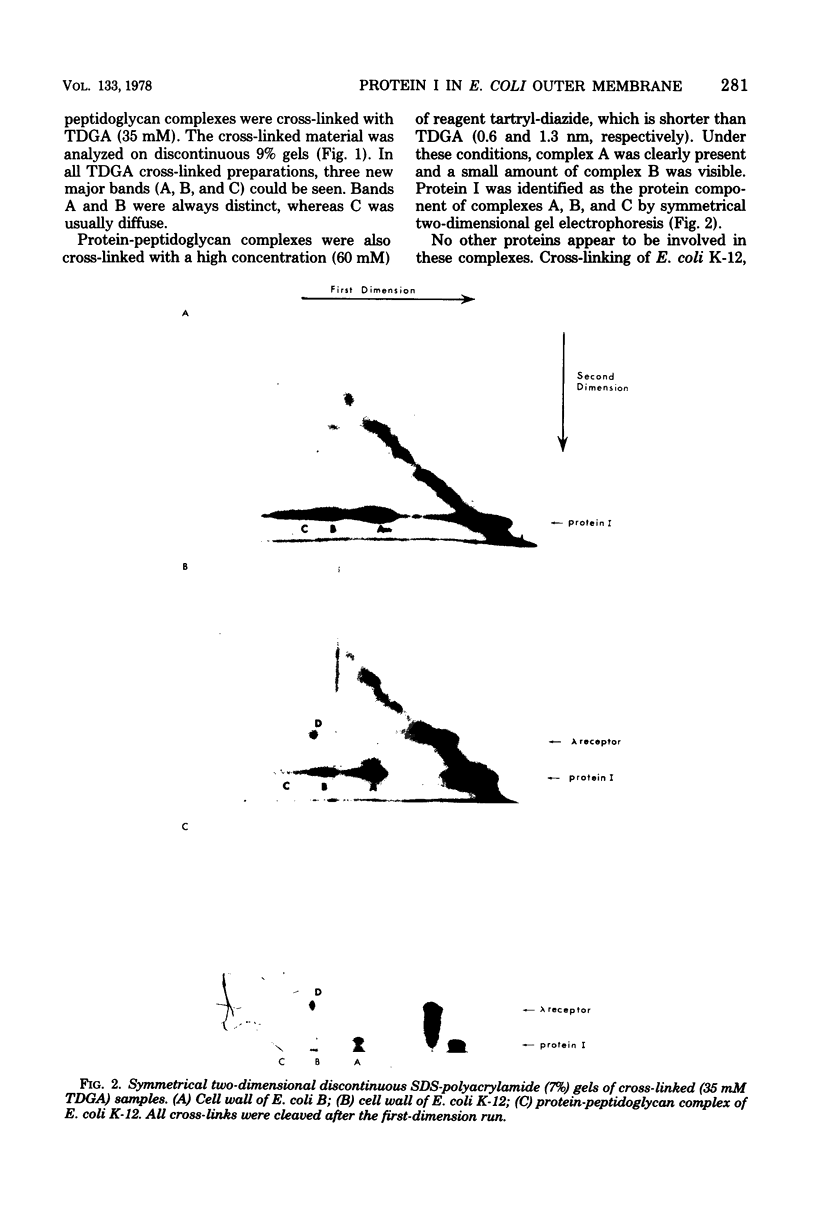
Abstract
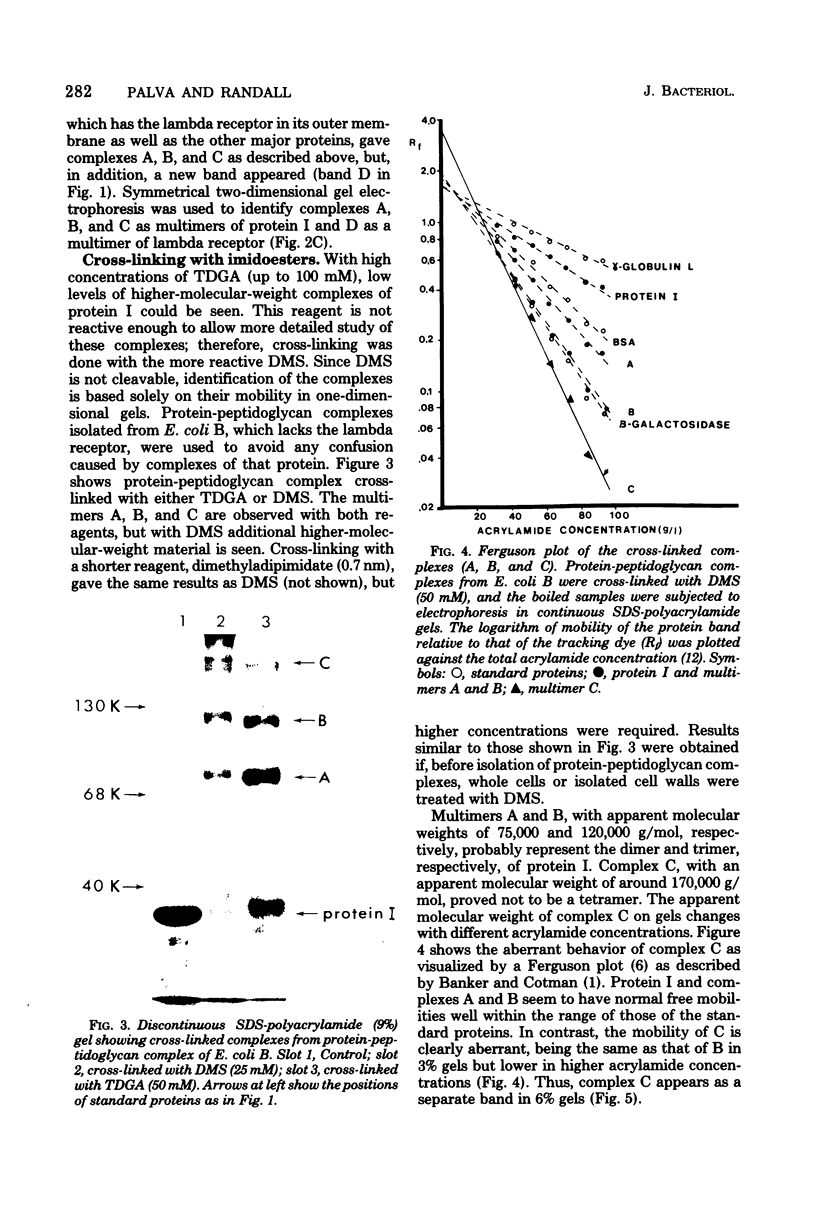
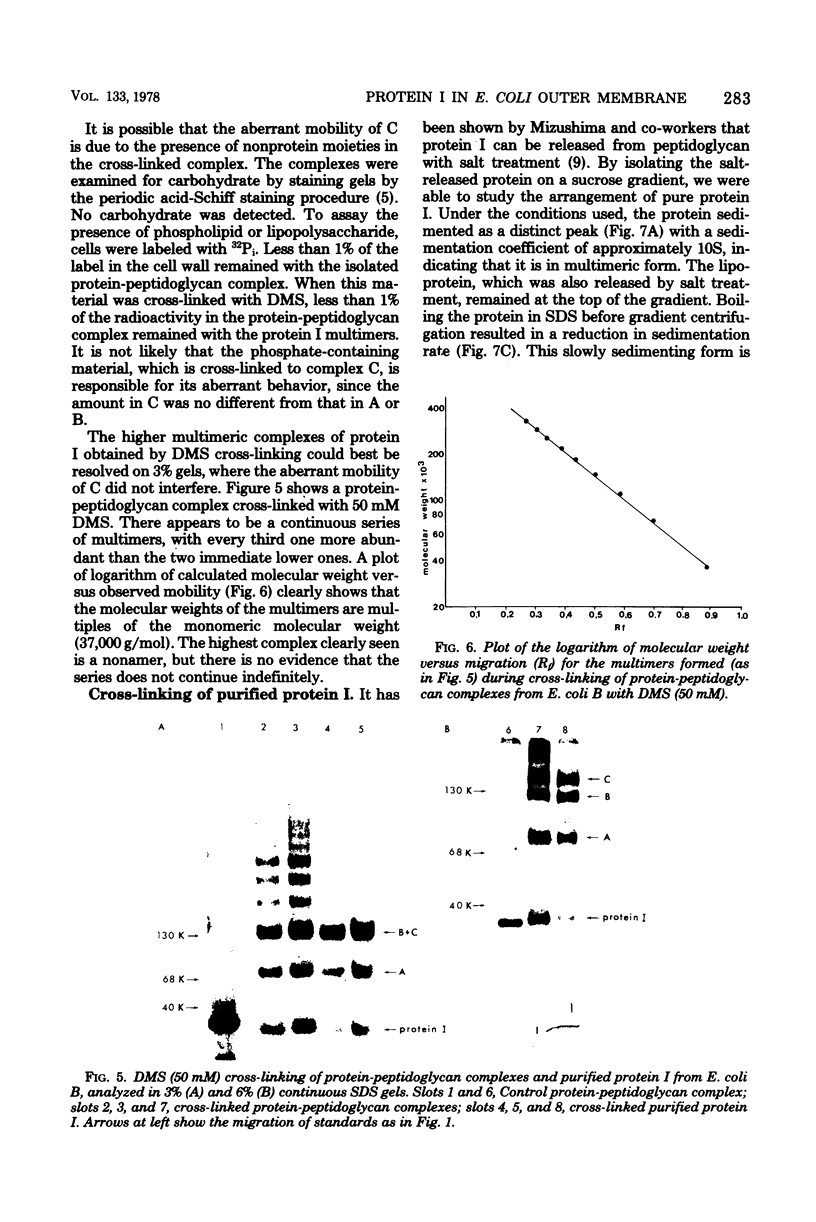
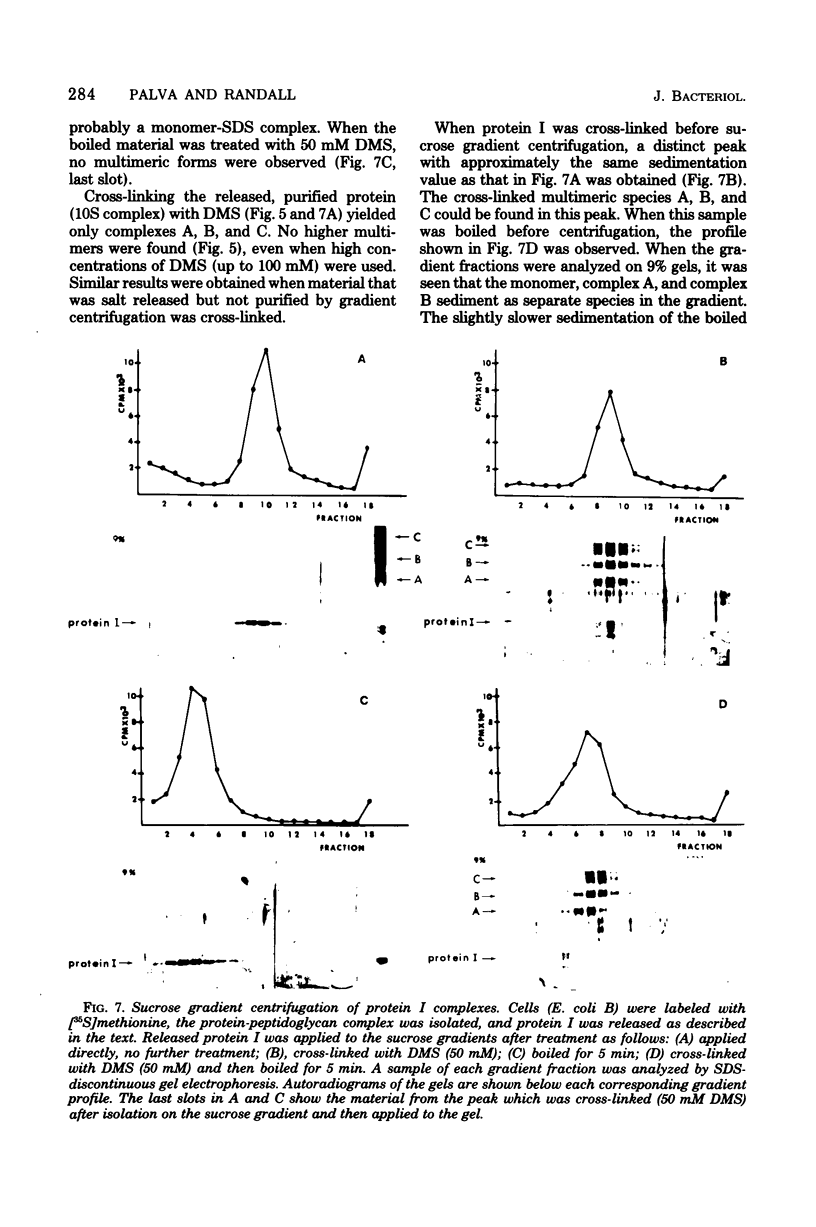
The arrangement of protein I in the outer membrane of Escherichia coli was investigated by cross-linking whole cells, isolated cell wall, protein-peptidoglycan complexes, and protein I released from peptidoglycan with NaCl. Both cleavable azide cross-linkers and imidoester reagents were used. The data presented suggest that protein I exists in the outer membrane as a trimer.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banker G. A., Cotman C. W. Measurement of free electrophoretic mobility and retardation coefficient of protein-sodium dodecyl sulfate complexes by gel electrophoresis. A method to validate molecular weight estimates. J Biol Chem. 1972 Sep 25;247(18):5856–5861. [PubMed] [Google Scholar]
- Bell R. M. Mutants of Escherichia coli defective in membrane phospholipid synthesis: macromolecular synthesis in an sn-glycerol 3-phosphate acyltransferase Km mutant. J Bacteriol. 1974 Mar;117(3):1065–1076. doi: 10.1128/jb.117.3.1065-1076.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Bosch V. Repetitive sequences in the murein-lipoprotein of the cell wall of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Apr;69(4):970–974. doi: 10.1073/pnas.69.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Garten W., Henning U. Cell envelope and shape of Escherichia coli K12. Isolation and preliminary characterization of the major ghost-membrane proteins. Eur J Biochem. 1974 Sep 1;47(2):343–352. doi: 10.1111/j.1432-1033.1974.tb03699.x. [DOI] [PubMed] [Google Scholar]
- Haller I., Henning U. Cell envelope and shape of Escherichia coli K12. Crosslinking with dimethyl imidoesters of the whole cell wall. Proc Natl Acad Sci U S A. 1974 May;71(5):2018–2021. doi: 10.1073/pnas.71.5.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y., Yamada H., Mizushima S. Interactions of outer membrane proteins O-8 and O-9 with peptidoglycan sacculus of Escherichia coli K-12. J Biochem. 1976 Dec;80(6):1401–1409. doi: 10.1093/oxfordjournals.jbchem.a131413. [DOI] [PubMed] [Google Scholar]
- Henning U., Höhn B., Sonntag I. Cell envelope and shape of Escherichia coli K12. The ghost membrane. Eur J Biochem. 1973 Nov 1;39(1):27–36. doi: 10.1111/j.1432-1033.1973.tb03099.x. [DOI] [PubMed] [Google Scholar]
- Henning U., Rehn K., Hoehn B. Cell envelope and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2033–2036. doi: 10.1073/pnas.70.7.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter L. C., Ortanderl F., Fasold H. The use of a new series of cleavable protein-crosslinkers on the Escherichia coli ribosome. FEBS Lett. 1974 Nov 15;48(2):288–292. doi: 10.1016/0014-5793(74)80488-6. [DOI] [PubMed] [Google Scholar]
- McEwen C. R. Tables for estimating sedimentation through linear concentration gradients of sucrose solution. Anal Biochem. 1967 Jul;20(1):114–149. doi: 10.1016/0003-2697(67)90271-0. [DOI] [PubMed] [Google Scholar]
- Nakae T. Identification of the outer membrane protein of E. coli that produces transmembrane channels in reconstituted vesicle membranes. Biochem Biophys Res Commun. 1976 Aug 9;71(3):877–884. doi: 10.1016/0006-291x(76)90913-x. [DOI] [PubMed] [Google Scholar]
- Nakae T., Nikaido H. Outer membrane as a diffusion barrier in Salmonella typhimurium. Penetration of oligo- and polysaccharides into isolated outer membrane vesicles and cells with degraded peptidoglycan layer. J Biol Chem. 1975 Sep 25;250(18):7359–7365. [PubMed] [Google Scholar]
- Nikaido H., Song S. A., Shaltiel L., Nurminen M. Outer membrane of Salmonella XIV. Reduced transmembrane diffusion rates in porin-deficient mutants. Biochem Biophys Res Commun. 1976 May 23;76(2):324–330. doi: 10.1016/0006-291x(77)90728-8. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Palva E. T., Randall L. L. Nearest-neighbor analysis of Escherichia coli outer membrane proteins using cleavable cross-links. J Bacteriol. 1976 Sep;127(3):1558–1560. doi: 10.1128/jb.127.3.1558-1560.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reithmeier R. A., Bragg P. D. Cross-linking of the proteins in the outer membrane of Escherichia coli. Biochim Biophys Acta. 1977 Apr 18;466(2):245–256. doi: 10.1016/0005-2736(77)90222-x. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Schmitges C. J., Henning U. The major proteins of the Escherichia coli outer cell-envelope membrane. Heterogeneity of protein I. Eur J Biochem. 1976 Mar 16;63(1):47–52. doi: 10.1111/j.1432-1033.1976.tb10205.x. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Smith D., de Salsas M. F. Temperate Bacteriophage Which Causes the Production of a New Major Outer Membrane Protein by Escherichia coli. J Virol. 1975 May;15(5):1121–1130. doi: 10.1128/jvi.15.5.1121-1130.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. Expression phénotypique et localisation génétique de mutations affectant le métabolisme du maltose chez Escherichia coli K 12. Ann Inst Pasteur (Paris) 1967 Jun;112(6):673–698. [PubMed] [Google Scholar]
- Steven A. C., Heggeler B., Müller R., Kistler J., Rosenbusch J. P. Ultrastructure of a periodic protein layer in the outer membrane of Escherichia coli. J Cell Biol. 1977 Feb;72(2):292–301. doi: 10.1083/jcb.72.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Mizushima S. Stimulation by lipopolysaccharide of the binding of outer membrane proteins O-8 and O-9 to the peptidoglycan layer of Escherichia coli K--12. Biochem Biophys Res Commun. 1977 Feb 21;74(4):1397–1402. doi: 10.1016/0006-291x(77)90597-6. [DOI] [PubMed] [Google Scholar]