Abstract
Gingival inflammation and alveolar bone resorption are hallmarks of adult periodontitis, elicited in response to oral micro-organisms such as Porphyromonas gingivalis. We hypothesized that omega (ω)-3 fatty acids (FA) dietary supplementation would modulate inflammatory reactions leading to periodontal disease in infected rats. Rats were fed fish oil (ω-3 FA) or corn oil (n-6 FA) diets for 22 weeks and were infected with P. gingivalis. Rats on the ω-3 FA diet exhibited elevated serum levels of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), documenting diet-induced changes. PCR analyses demonstrated that rats were orally colonized by P. gingivalis; increased IgG antibody levels substantiated this infection. P. gingivalis-infected rats treated with ω-3 FA had significantly less alveolar bone resorption. These results demonstrated the effectiveness of an ω-3 FA-supplemented diet in modulating alveolar bone resorption following P. gingivalis infection, and supported that ω-3 FA may be a useful adjunct in the treatment of periodontal disease. Abbreviations: PUFA, polyunsaturated fatty acid; EPA, eicosapentanoic acid; DHA, docosahexanoic acid; and PCR, polymerase chain-reaction.
Keywords: P. gingivalis, ω-3 PUFA, periodontal disease, alveolar bone loss, IgG antibody
INTRODUCTION
Periodontitis is a progressive loss of clinical attachment, and destruction of periodontal ligament and adjacent supporting alveolar bone induced by pathogenic biofilms containing several periodontal pathogens, including P. gingivalis. P. gingivalis is a major pathogen in severe forms of human periodontal disease, and possesses multiple metabolic properties and virulence factors consistent with its pathogenic role in the disease (Kuramitsu, 2003; Holt and Ebersole, 2005). Nevertheless, in vivo studies are considered as crucial steps in documenting specific bacterial pathogenesis. Several studies used both mouse and rat models to describe the virulence characteristics of oral micro-organisms and have facilitated experimental designs of short duration and the ability to control the complex microbiota. Generally, these studies have confirmed the potential of the pathogen to elicit inflammation and tissue destruction in the host (Madden and Caton, 1994).
Dietary fish oil has been demonstrated to: protect mice against infection with numerous extracellular bacterial pathogens, regulate serum triglycerides and cholesterol levels, inhibit synthesis of lipid mediators of inflammation (PGE2, arachidonic acid, cyclo-oxygenase, 5-lipoxygenase), alter cellular functions of polymorphonuclear leukocytes, modulate lymphocyte proliferation and cytokine production, and increase endogenous host anti-oxidant capacity, e.g., SOD and catalase (Alam et al., 1991; Blok et al., 1992; Fernandes and Venkataraman, 1993). These effects have been proposed to account for the potent anti-inflammatory properties of omega (ω)-3 fatty acids (FA) in human, non-human primate, and rodent disease models. While much of the attention over the last decades has focused on the beneficial effects of fish oil, particularly the ω-3 FA components, on a variety of chronic inflammatory diseases (cardiovascular disease, rheumatoid arthritis), few studies have examined its effects on the chronic immuno-inflammatory lesions of periodontal disease. In a recent study, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), principal ω-3 polyunsaturated fatty acids (PUFA) present in fish oil, were shown to decrease osteoclast activation in vitro (Sun et al., 2003). Campan et al. (1996, 1997) reported that human experimental gingivitis appeared to be modified by ω-3 FA, although no definitive conclusions were provided. It has also been reported that the n-6 PUFA levels in the serum are higher in periodontitis patients, suggesting that an imbalance between n-6 and n-3 fatty acids may contribute to susceptibility to oral bone loss (Requirand et al., 2000). Topical application of n-3 or n-6 fatty acids failed to inhibit the development of experimental gingivitis (Eberhard et al., 2002), although Rosenstein et al. (2003) suggested that dietary fatty acid supplementation in adult periodontitis correlated with an improvement in gingival inflammation. Treatment of rats with fish oil significantly reduced osteoclasts and pre-osteoclasts following pulp exposure (Indahyani et al., 2002), significantly reduced the gingival tissue levels of lipid inflammatory mediators in LPS-induced experimental periodontitis (Vardar et al., 2004), and reduced osteoclastic activity and alveolar bone resorption, suggesting that this model may be useful in exploring host-bacterial interactions leading to periodontitis (Iwami-Morimoto et al., 1999). The hypothesis tested in this investigation was that a fish-oil-supplemented diet would modulate the host response to oral P. gingivalis infection, determined by decreased alveolar bone resorption in rats.
MATERIALS & METHODS
Bacterial Strains
P. gingivalis strains 381 and A7A1-28 (ATCC 53977) were used in this study, and were cultured and maintained for the animal infections as described previously (Kesavalu et al., 1992, 2003). P. gingivalis cells at 2 × 1010/mL were mixed with equal amounts of 2% carboxymethylcellulose (CMC; Sigma Chemical Co., St. Louis, MO, USA) and used to infect rats orally within 15 min of removal from the anaerobic environment. For use as whole-cell antigen, a P. gingivalis culture was grown in mycoplasma broth (Kesavalu et al., 2003). Serum IgG antibody to the micro-organism was determined by an ELISA as described previously (Kesavalu et al., 1992, 1999).
Animals and Diets
Female Sprague-Dawley rats (8–9 wks old, Harlan, Indianapolis, IN, USA) were maintained in groups (n = 2) housed under microisolator conditions and fed standard pelleted chow (Harlan Teklad) and H2O ad libitum during acclimation (1 wk). All procedures were performed in accordance with the approved guidelines set forth by the Institutional Animal Care and Use Committee at the University of Kentucky. The diets were prepared according to the American Institute of Nutrition diet AIN-76A (Rao et al., 2001; Dyets Inc., Bethlehem, PA, USA), with the addition of 17% menhaden/3% corn oil (fish oil diet) and 5% corn oil only (corn oil diet). An aliquot of the diets was also analyzed by Dyets Inc. for fatty acid composition. The total diet composition is available at www.dyets.com. The corn oil (CO) diet contained 60% n-6 linoleic acid, whereas the fish oil (FO) diet contained high levels (24.6%) of ω-3 PUFA (EPA and DHA). These diets were received in bulk, repackaged into small air-tight packets (1 packet/day/group), and stored at −20°C. All diets were supplemented with equal amounts of vitamin E to minimize peroxidative damage during storage. Following acclimation, rats were randomized into fish oil and corn oil groups and were provided the appropriate diet for 8 wks until antibiotic administration and P. gingivalis infection (Fig. 1). Groups of rats infected with P. gingivalis 381 (n = 21, FO; n = 20, CO) or strain A7A1-28 (n = 21, FO; n = 20, CO) were compared with control uninfected rats (n = 13). Rats were continued on fish and corn oil diets ad libitum for the duration of the experiment. Leftover diet from the previous day was discarded to minimize oxidation of lipids.
Figure 1.
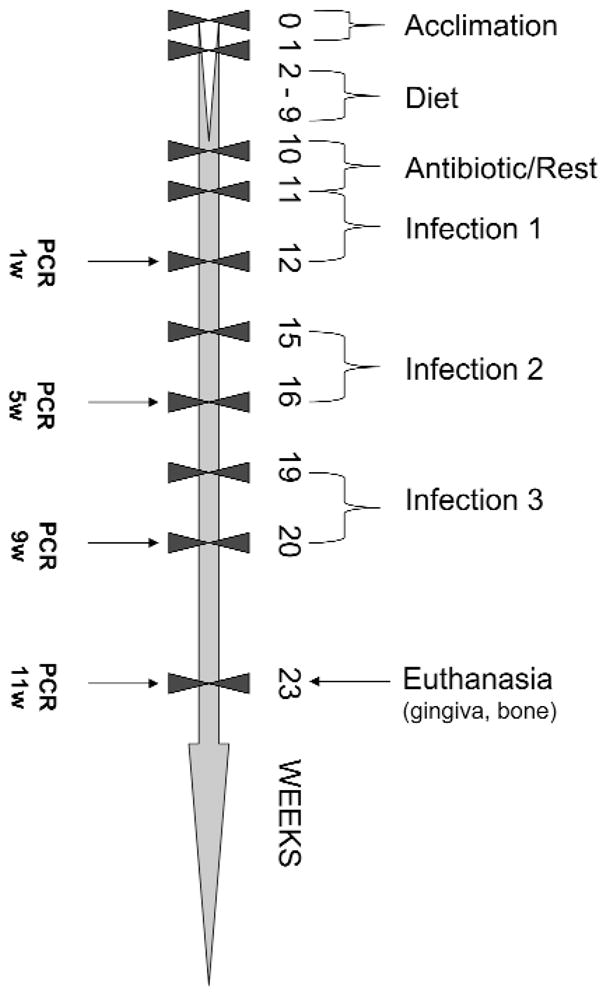
Schematic diagram illustrating experimental design, fish and corn oil diet feeding, P. gingivalis infections, plaque sample collection, PCR analysis, death, gingival tissue, and alveolar bone collection (detailed information in MATERIALS & METHODS).
P. gingivalis Oral Infection
All rats were administered kanamycin (20 mg) and ampicillin (20 mg) daily for 4 days in the drinking water, to suppress the oral microbiota (Fine et al., 2001). Rats were rested for 3 days and then randomized into the groups, followed by oral infection with approximately 1010 P. gingivalis for 5 consecutive days on 3 alternate wks during the study period (Fig. 1). Oral microbial samples from anesthetized rats were collected by means of sterile cotton swabs at 1, 5, 9, and 11 wks post-infection. The swabs were suspended in 300 μL of Tris-EDTA (TE) buffer. Blood was collected, and sera were stored at −20°C for IgG antibody analysis. Rats were killed; skulls were removed, autoclaved, and defleshed, and maxillae and mandibles were hemi-sected and trimmed for radiographic evaluation of alveolar bone loss.
P. gingivalis Identification
The oral microbial samples were boiled in TE buffer with an equal volume of 1 N NaOH, and DNA was dissolved in 40 μL of sterile distilled water. PCR was carried out in 50 μL volume with 10 pM of primer, 1 mM of each deoxynucleotide triphosphate, and 1.5 mM MgCl2. A two-unit quantity of Taq DNA polymerase (Invitrogen Life Technologies, Carlsbad, CA, USA) in the manufacturer’s buffer was used with a GeneAmp PCR System. The PCR oligonucleotide species-specific primers for both strains used 16S rDNA for P. gingivalis: (forward) 5′ GGT AAG TCA GCG GTG AAA CC 3′ and (reverse) 5′ ACG TCA TCC ACA CCT TCC TC 3′. After denaturation at 94°C for 5 min, 35 PCR cycles were performed, with each cycle consisting of 30 sec of denaturation at 94°C, 30 sec of annealing at 55°C, and 60 sec of polymerization at 72°C and final extension at 72°C for 7 min. PCR products were analyzed by 1.5% agarose gel electrophoresis and stained with ethidium bromide for detection of a 601-bp product.
Fatty Acid Composition of Serum
A 100-μL quantity of serum, collected at the end of the study period, was used for the extraction of total lipids, with oxidation prevented by the addition of butylated hydroxytoluene. The organic phase containing total lipid was dried under a stream of nitrogen, and the residue was methylated (Bhattacharya et al., 2003). Fatty acid methyl esters were separated by gas-liquid chromatography and identified and quantified by comparison of retention times with fatty acid methyl ester standards (68A; Nu Chek, Elysian, MN, USA) (Bhattacharya et al., 2003).
Radiographic Assessment of Alveolar Bone Resorption
We trimmed the hemisected maxillae and mandibles to reduce the bucco-lingual dimensions to allow the teeth to be close to the radiographic film. Each jaw was secured, by means of rope wax, to Kodak Ultra Speed size 2 films (Kodak, Rochester, NY, USA), and a Planmeca Prostyle Intra x-ray unit (Planmeca, Roselle, IL, USA) was placed at a right angle to the film. Each jaw was radiographed with an exposure time of 0.05 sec at a setting of 70 KvP and 8 mA. Radiographs were analyzed for alveolar bone height, with decreased bone level (i.e., resorption) as the primary outcome parameter of the study. We used radiographs projected at a 5x size to obtain linear measures from the CEJ to the bone height at mesial and distal interproximal surfaces (2 sites per tooth) of each of the 2 molars and 1 premolar in each quadrant (Reed and Polson, 1984). So that comparability of the results could be ensured, the measures were determined by investigators blinded as to the group designation and routinely calibrated with a set of standard radiographs from the rats. The summation of bone resorption in mm was tabulated and analyzed for intra- and inter-group differences.
Statistical Analysis
Descriptive evaluation of the alveolar bone and antibody data are presented as mean ± SD. Statistically significant differences between the groups were determined by ANOVA and the Holm-Sidak post hoc multiple-comparisons test (SigmaStat 3.0, SYSTAT Software Inc., Chicago, IL, USA) for normally distributed data. Data determined to be non-normal were analyzed by the Kruskal-Wallis ANOVA on ranks, and multiple comparisons were adjusted by Dunn’s method (SigmaStat 3.0).
RESULTS
Effect of Fish Oil Diet on Serum Fatty Acid Content
The fish oil diet induced changes in serum fatty acid profiles, including a significant increase in the levels of EPA and DHA (Table 1). Moreover, the n-6/n-3 ratio was significantly decreased in the fish-oil-fed rats, consistent with an elevated peroxidizability index, and supporting the likelihood of a decreased oxidative stress/lower inflammatory environment in the treated animals.
Table 1.
Serum Fatty Acid Profiles of Rats on Corn Oil and Fish Oil Diets
| Fatty Acids, wt% | Corn Oil | Fish Oil |
|---|---|---|
| 14:0 Myristic | 0.58 ± 0.06a | 2.30 ± 0.12 |
| 16:0 Palmitic | 14.21 ± 0.64 | 21.91 ± 0.37 |
| 16:1 Palmitoleic n-9 | 1.54 ± 0.13 | 3.26 ± 0.14 |
| 18:0 Stearic | 14.78 ± 0.67 | 12.69 ± 0.73 |
| 18:1 Oleic n-9 | 10.82 ± 0.98 | 6.99 ± 0.12 |
| 18:2 Linoleic n-6 | 16.67 ± 0.76 | 9.76 ± 0.12 |
| 18:3 Linoleic n-3 | 0.16 ± 0.01 | 0.67 ± 0.02 |
| 20:3 Eicosatrienoic n-6 | 0.34 ± 0.01 | 0.30 ± 0.00 |
| 20:4 Arachidonic n-6 | 27.81 ± 1.91 | 7.86 ± 0.30 |
| 20:5 Eicosapentaenoic (EPA) n-3 | -- | 12.46 ± 0.37 |
| 22:5 Docosapentaenoic n-3 | -- | 1.16 ± 0.12 |
| 22:6 Docosahexaenoic (DHA) n-3 | 1.71 ± 0.16 | 9.61 ± 0.38 |
|
| ||
| Saturates | 30.16 ± 0.17 | 36.90 ± 0.96 |
| Mono-unsaturates | 14.30 ± 1.22 | 12.30 ± 0.28 |
| Polyunsaturates | 49.79 ± 1.40 | 42.51 ± 0.63 |
| P/S (Polyunsaturates/Saturates) | 1.65 ± 0.05 | 1.16 ± 0.05 |
| n-6 PUFA/n-3 PUFA | 18.91 ± 1.28 | 0.72 ± 0.03 |
| PI (Peroxidizability Index) | 181.41 ± 6.05 | 194.52 ± 3.79 |
Values are percentages of total fatty acids in serum from 5 rats from corn oil and fish oil diet groups at the end of the study period. Results are expressed as Mean ± SEM. Peroxidizability Index calculated as (monoenoic × 1) + (dienoic × 2) + (trienoic × 3) + (tetraenoic × 4) + (pentaenoic × 5) + (hexaenoic × 6). The bold numbers denote adjusted significant differences at least at p < 0.01.
P. gingivalis Colonization with Fish Oil Diets
A summary of the PCR evaluation of the oral microbial samples (Fig. 2) demonstrated that nearly all rats were infected with the strains of P. gingivalis during the experimental period. Moreover, the oral infection elicited significant levels of serum IgG antibody in all groups (homologous, Fig. 2) compared with uninfected control animals. As would be expected, there was also substantial cross-reactivity of antibody elicited by the 2 strains (x-reactive, Fig. 2).
Figure 2.
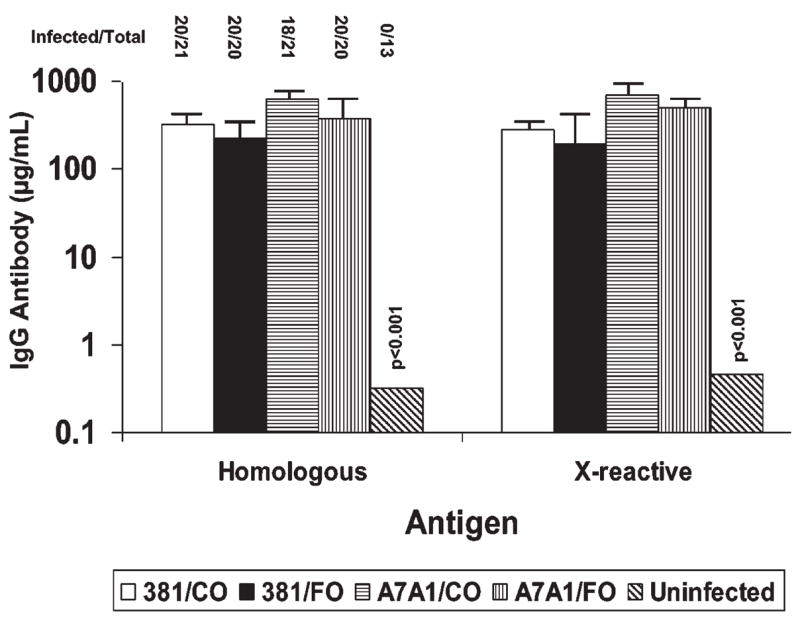
Effects of n-3 PUFA (FO) and corn oil (CO) diets on P. gingivalis 381 and A7A1-28-infection and induced IgG antibody levels in rats. Infected/total denotes number of rats positive for P. gingivalis infection by PCR, compared with total number of rats in the experimental group. Each bar represents the mean serum IgG antibody levels of 13–21 rats per group, and the vertical bars denote 1 standard deviation from the mean. Homologous denotes antibody responses in the rats to the identical infecting strain, and X-reactive denotes antibody levels to the heterologous P. gingivalis strain. IgG antibody levels in all infected groups were significantly greater than control uninfected rats (p < 0.001).
P. gingivalis-induced Alveolar Bone Loss Altered by Fish Oil
P. gingivalis 381 or A7A1-28 infection resulted in significantly increased alveolar bone resorption compared with that in uninfected rats (Table 2). P. gingivalis-infected rats fed a fish oil diet enriched in ω-3 FA demonstrated significantly decreased alveolar bone resorption around both mandiblular and maxillary teeth, compared with rats fed the corn oil diet, with alveolar bone levels in the fish-oil-treated animals being comparable with those in the control uninfected rats.
Table 2.
Alveolar Bone Resorption in Rats Infected with P. gingivalis Strains and Provided Corn Oil and Fish Oil Diets
| Σ of Alveolar Bone Resorption (Mean ± SD) | ||||
|---|---|---|---|---|
| Infection | Diet | Maxillary | Mandibular | Total |
| None | Standard | 4.89 ± 0.82a,g | 4.53 ± 0.65b,d,i | 9.43 ± 1.37c,k |
| 381 | Corn oil | 7.22 ± 0.76a,d | 7.66 ± 0.79b,e | 14.88 ± 1.18c,f |
| 381 | Fish oil | 5.02 ± 0.74d | 5.42 ± 1.11e | 10.44 ± 1.64i |
| A7A1-28 | Corn oil | 5.31 ± 0.93h* | 5.13 ± 0.81i,j | 10.44 ± 1.55k,l |
| A7A1-28 | Fish oil | 3.92 ± 0.55g*,h | 3.43 ± 0.48j | 7.75 ± 0.93l |
The values denote the alveolar bone resorption in mm, as a summation of 2 sites per tooth (mesial and distal) and 3 teeth in each quadrant, of 13–21 rats per group. Statistical differences were adjusted by Holm-Sidak’s or Dunn’s method* for multiple-comparison analysis and are denoted by matched letters:
p = 0.017;
p = 0.025; and
p < 0.050—e.g., a denotes a comparison between no infection and oral infection with P. gingivalis 381.
DISCUSSION
The results demonstrated that an extended ω-3 FA dietary supplementation altered the serum fatty acid profile of rats, with increased levels of EPA and DHA, both having been shown to possess anti-inflammatory properties (Geusens et al., 1994). The literature is replete with evidence supporting that EPA/DHA composition in serum reflects changes, in the membranes of most cells (Alam et al., 1991; Kitajka et al., 2002), which alter their functional properties. As examples, EPA, DHA, fish oil, and linoleic acid have been shown to regulate/activate several genes—such as fatty acid synthase, hormone-sensitive lipase, lipoprotein-lipase, phosphoenol-pyruvate carboxy-kinase, and CCAAT/enhancer binding protein αand leptin mRNA levels—in rat adipose tissues (Raclot et al., 1997), genes controlling synaptic plasticity, and cytoskeleton and signal transduction in the rat hippocampus (Kitajka et al., 2002), and increased transthyretin transcription in the rat hippocampus (Puskas et al., 2003). Thus, it might be expected that both resident cells and infiltrating inflammatory/immune cells in gingival tissues would also reflect functional alterations.
This study demonstrated our ability to colonize rats orally with 2 strains of P. gingivalis for an extended 12-week interval and determined the resulting alveolar bone resorption. The microbial plaque samples showed oral infection with this pathogen. Four observations were derived from serum IgG antibody levels to P. gingivalis: (1) Significant increases in serum IgG antibody provided additional evidence of oral infection; (2) a systemic response to the oral infection confirmed a challenge to the systemic immune apparatus; (3) the IgG antibodies demonstrated some cross-reactivity for each of these strains, as would be expected based upon previous immune response data (Kesavalu et al., 1992); and (4) IgG antibody levels in the fish-oil-fed rats demonstrated a consistent trend toward lower levels than in the corn oil groups. These findings suggest that the fish-oil-fed animals were more resistant to colonization and/or expansion of P. gingivalis in the oral cavity, or that decreasing the localized inflammatory response to infection dampened the resulting acquired immune response to the infection. The non-quantitative PCR procedure used to document P. gingivalis colonization/infection from plaque samples in this study cannot address variations in the level of P. gingivalis infection; however, future studies incorporating real-time quantitative PCR could potentially discriminate and quantitate the bacteria detected in the oral microbial samples. Moreover, extension of these studies will provide evidence to demonstrate the characteristics of host response regulation in gingival tissues (Ebersole et al., 2005).
Analysis of the data clearly demonstrates that, in rats, a fish-oil-enriched diet inhibited alveolar bone resorption resulting from P. gingivalis infection, when compared with rats on a corn oil diet. Moreover, this dietary supplementation strategy appeared to have a magnitude of effect such that the ω-3 FA groups had alveolar bone levels comparable with those in the uninfected animals. There have been numerous studies in animal models and humans suggesting that fish oil/ω-3 FA dietary supplements could be used to modulate chronic inflammatory responses and lesions in other diseases, e.g., rheumatoid arthritis (Geusens et al., 1994), and during osteoporosis in both ovariectomized and autoimmune-disease-prone mice (Sun et al., 2003; Bhattacharya et al., 2005). Data are rather limited with regard to anti-inflammatory molecules affecting chronic inflammation in periodontal disease, although a recent study showed that hyperlipidemia may be associated with periodontitis (Moeintaghavi et al. 2005), and a review of hyperlipidemia and periodontal disease in rats suggested that dietary lipids can modulate immune/inflammatory responses (Cutler and Iacopino, 2003). The ability for investigators to identify various dietary supplements that could modulate destructive localized gingival inflammatory responses would be an important contribution to the repertoire of therapies used to manage chronic periodontal disease. This report provides initial data suggesting an important utility for a dietary omega-3 FA treatment strategy in human periodontal disease. Further studies are required to determine critical anti-/pro-inflammatory mediators in gingival tissues that are modulated by the omega-3 FA-enriched fish oil supplements.
Acknowledgments
This investigation was supported by USPHS Research Grant DE-014896 from the National Institute of Dental and Craniofacial Research (LK) and Grant AG-23648 from the National Institute of Aging (GF), National Institutes of Health, Bethesda, MD 20892.
References
- Alam SQ, Bergens BM, Alam BS. Arachidonic acid, prostaglandin E2 and leukotriene C4 levels in gingival and submandibular salivary glands of rats fed diets containing n-3 fatty acids. Lipids. 1991;26:895–900. doi: 10.1007/BF02535974. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Lawrence RA, Krishnan A, Zaman K, Sun D, Fernandes G. Effect of dietary n-3 and n-6 oils and without food restriction on activity of antioxidant enzymes and lipid peroxidation in livers of cyclophosphamide treated autoimmune-prone NZB/W female mice. J Am Coll Nutr. 2003;22:388–399. doi: 10.1080/07315724.2003.10719322. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Rahman M, Banu J, Lawrence RA, McGuff HS, Garrett IR, et al. Inhibition of osteoporosis in autoimmune disease prone MRL-Mpj-Fas (lpr) mice by N-3 fatty acids. J Am Coll Nutr. 2005;24:200–209. doi: 10.1080/07315724.2005.10719466. [DOI] [PubMed] [Google Scholar]
- Blok WL, Vogels MT, Curfs JH, Eling WM, Buurman WA, van der Meer JW. Dietary fish-oil supplementation in experimental Gram-negative infection and in cerebral malaria in mice. J Infect Dis. 1992;165:898–903. doi: 10.1093/infdis/165.5.898. [DOI] [PubMed] [Google Scholar]
- Campan P, Planchand PO, Duran D. Polyunsaturated omega-3 fatty acids in the treatment of experimental human gingivitis. Bull Group Int Rech Sci Stomatol Odontol. 1996;39:25–31. [article in French] [PubMed] [Google Scholar]
- Campan P, Planchand PO, Duran D. Pilot study of n-3 polyunsaturated fatty acids in the treatment of human experimental gingivitis. J Clin Periodontol. 1997;24:907–913. doi: 10.1111/j.1600-051x.1997.tb01210.x. [DOI] [PubMed] [Google Scholar]
- Cutler CW, Iacopino AM. Periodontal disease: links with serum lipid/triglyceride levels? Review and new data. J Int Acad Periodontol. 2003;5:47–51. [PubMed] [Google Scholar]
- Eberhard J, Heilmann F, Acil Y, Albers HK, Jepsen S. Local application of n-3 or n-6 polyunsaturated fatty acids in the treatment of human experimental gingivitis. J Clin Periodontol. 2002;29:364–369. doi: 10.1034/j.1600-051x.2002.290413.x. [DOI] [PubMed] [Google Scholar]
- Ebersole JL, Meka A, Stromberg A, Saunders C, Kesavalu L. Host gene expression in local tissues in response to periodontal pathogens. Oral Biosci Med. 2005;2:175–184. [Google Scholar]
- Fernandes G, Venkataraman J. Role of omega-3 fatty acids in health and disease. Nutr Res. 1993;13:S19–S45. [Google Scholar]
- Fine DH, Goncharoff P, Schreiner H, Chang KM, Furgang D, Figurski D. Colonization and persistence of rough and smooth colony variants of Actinobacillus actinomycetemcomitans in the mouths of rats. Arch Oral Biol. 2001;46:1065–1078. doi: 10.1016/s0003-9969(01)00067-x. [DOI] [PubMed] [Google Scholar]
- Geusens P, Wouters C, Nijs J, Jiang Y, Dequeker J. Long-term effect of omega-3 fatty acid supplementation in active rheumatoid arthritis. A 12-month, double-blind, controlled study. Arthritis Rheum. 1994;37:824–829. doi: 10.1002/art.1780370608. [DOI] [PubMed] [Google Scholar]
- Holt SC, Ebersole JL. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000. 2005;38:72–122. doi: 10.1111/j.1600-0757.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- Indahyani DE, Pudyani PS, Santoso AL, Jonarta AL, Sosroseno W. The effect of fish oil on bone resorption following pulp exposure in rats. Dent Traumatol. 2002;18:206–211. doi: 10.1034/j.1600-9657.2002.00082.x. [DOI] [PubMed] [Google Scholar]
- Iwami-Morimoto Y, Yamaguchi K, Tanne K. Influence of dietary n-3 polyunsaturated fatty acid on experimental tooth movement in rats. Angle Orthod. 1999;69:365–371. doi: 10.1043/0003-3219(1999)069<0365:IODNPF>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- Kesavalu L, Ebersole JL, Machen RL, Holt SC. Porphyromonas gingivalis virulence in mice: induction of immunity to bacterial components. Infect Immun. 1992;60:1455–1464. doi: 10.1128/iai.60.4.1455-1464.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavalu L, Holt SC, Ebersole JL. Lack of humoral immune protection against Treponema denticola virulence in a murine model. Infect Immun. 1999;67:5736–5746. doi: 10.1128/iai.67.11.5736-5746.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavalu L, Holt SC, Ebersole JL. In vitro environmental regulation of Porphyromonas gingivalis growth and virulence. Oral Microbiol Immunol. 2003;18:226–233. doi: 10.1034/j.1399-302x.2003.00071.x. [DOI] [PubMed] [Google Scholar]
- Kitajka K, Puskas LG, Zvara A, Hackler L, Jr, Barcelo-Coblijn G, Yeo YK, et al. The role of n-3 polyunsaturated fatty acids in brain: modulation of rat brain gene expression by dietary n-3 fatty acids. Proc Natl Acad Sci USA. 2002;99:2619–2624. doi: 10.1073/pnas.042698699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu HK. Molecular genetic analysis of the virulence of oral bacterial pathogens: an historical perspective. Crit Rev Oral Biol Med. 2003;14:331–344. doi: 10.1177/154411130301400504. [DOI] [PubMed] [Google Scholar]
- Madden TE, Caton JG. Animal models for periodontal disease. Methods Enzymol. 1994;235:106–119. doi: 10.1016/0076-6879(94)35135-x. [DOI] [PubMed] [Google Scholar]
- Moeintaghavi A, Haerian-Ardakani A, Talebi-Ardakani M, Tabatabaie I. Hyperlipidemia in patients with periodontitis. J Contemp Dent Pract. 2005;6:78–85. [PubMed] [Google Scholar]
- Puskas LG, Kitajka K, Nyakas C, Barcelo-Coblijn G, Farkas T. Short-term administration of omega 3 fatty acids from fish oil results in increased transthyretin transcription in old rat hippocampus. Proc Natl Acad Sci USA. 2003;100:1580–1585. doi: 10.1073/pnas.0337683100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raclot T, Groscolas R, Langin D, Ferre P. Site-specific regulation of gene expression by n-3 polyunsaturated fatty acids in rat white adipose tissues. J Lipid Res. 1997;38:1963–1972. [PubMed] [Google Scholar]
- Rao CV, Hirose Y, Indranie C, Reddy BS. Modulation of experimental colon tumorigenesis by types and amounts of dietary fatty acids. Cancer Res. 2001;61:1927–1933. [PubMed] [Google Scholar]
- Reed BE, Polson AM. Relationships between bitewing and periapical radiographs in assessing crestal alveolar bone levels. J Periodontol. 1984;55:22–27. doi: 10.1902/jop.1984.55.1.22. [DOI] [PubMed] [Google Scholar]
- Requirand P, Gilbert P, Tramini P, Crystol JP, Descomps B. Serum fatty acid imbalance in bone loss: example with periodontal disease. Clin Nutr. 2000;19:271–276. doi: 10.1054/clnu.2000.0107. [DOI] [PubMed] [Google Scholar]
- Rosenstein ED, Kushner LJ, Kramer N, Kazandjian G. Pilot study of dietary fatty acid supplementation in the treatment of adult periodontitis. Prostaglandins Leukot Essen Fatty Acids. 2003;68:213–218. doi: 10.1016/s0952-3278(02)00272-7. [DOI] [PubMed] [Google Scholar]
- Sun D, Krishnan A, Zaman K, Lawrence R, Bhattacharya A, Fernandes G. Dietary n-3 fatty acids decrease osteoclastogenesis and loss of bone mass in ovariectomized mice. J Bone Miner Res. 2003;18:1206–1216. doi: 10.1359/jbmr.2003.18.7.1206. [DOI] [PubMed] [Google Scholar]
- Vardar S, Buduneli E, Turkoglu O, Berdeli AH, Baylas H, Baskesen A, et al. Therapeutic versus prophylactic plus therapeutic administration of omega-3 fatty acid on endotoxin-induced periodontitis in rats. J Periodontol. 2004;75:1640–1646. doi: 10.1902/jop.2004.75.12.1640. [DOI] [PubMed] [Google Scholar]


