Abstract
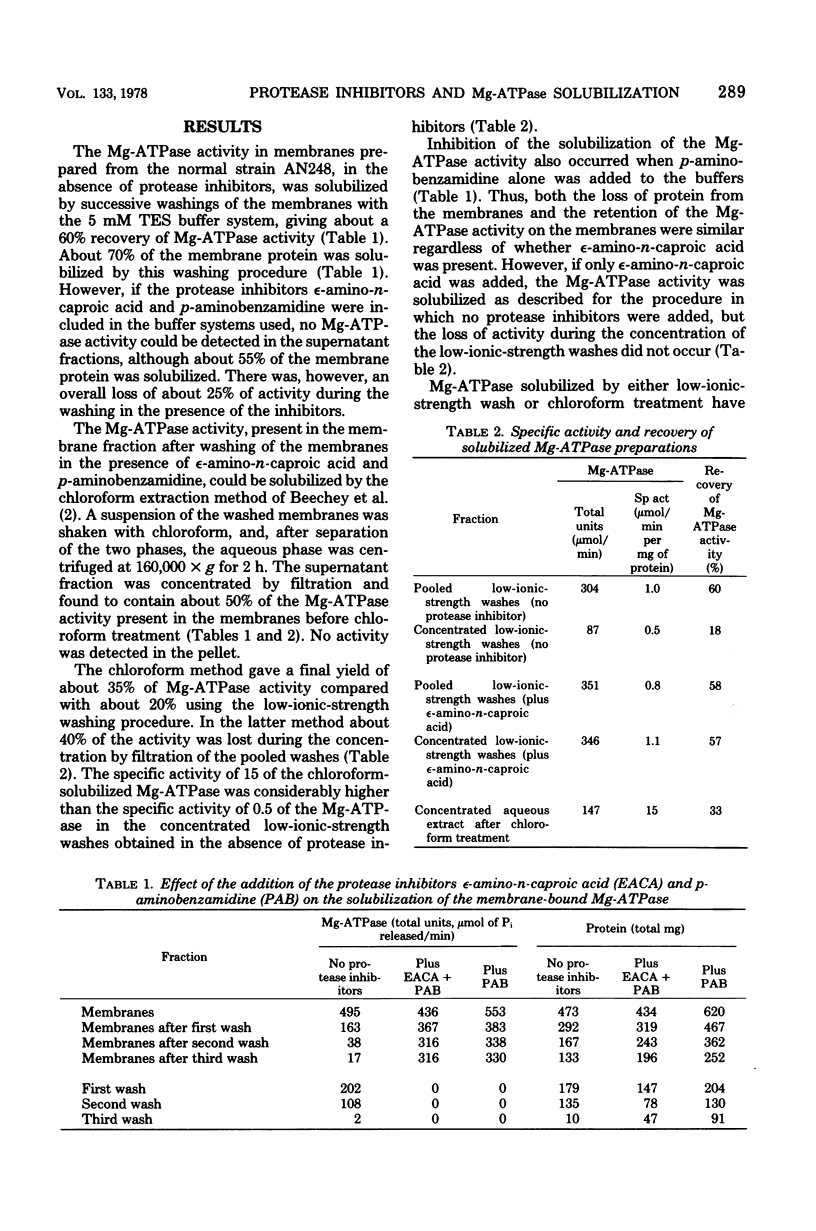
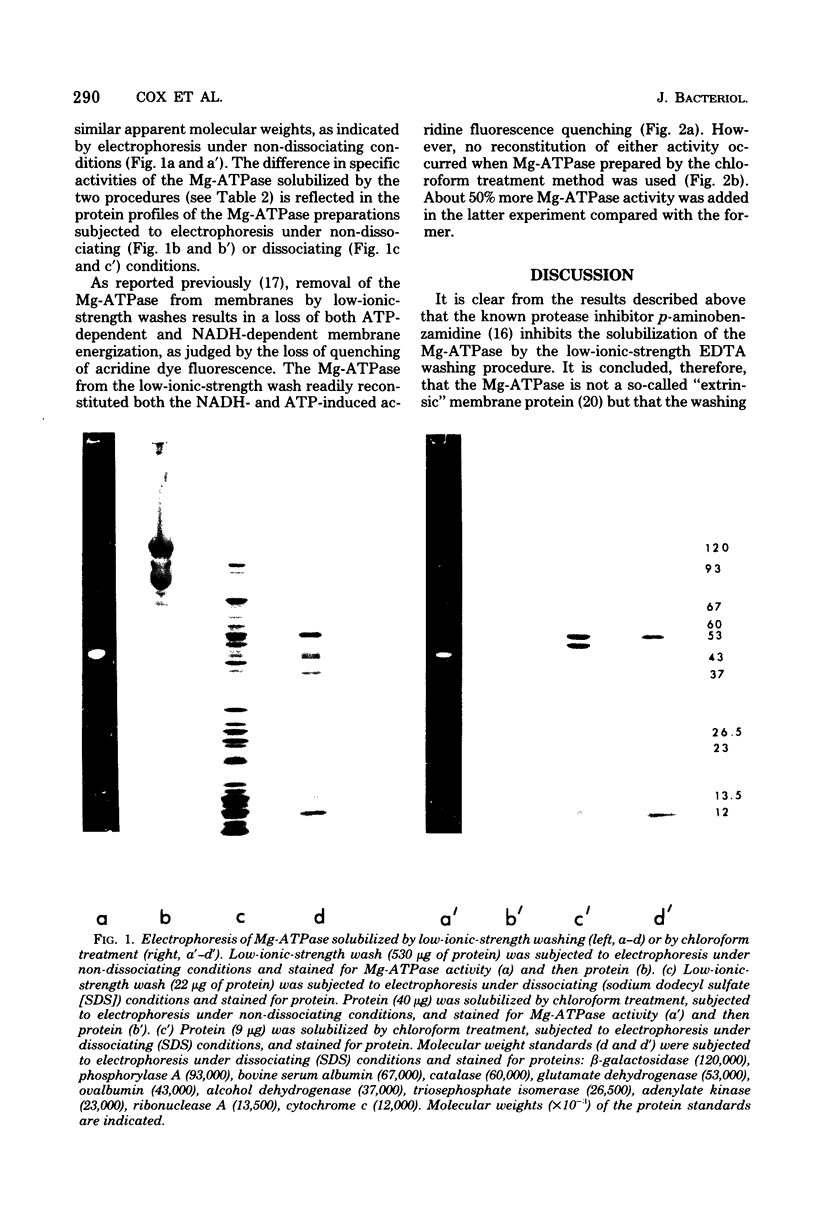
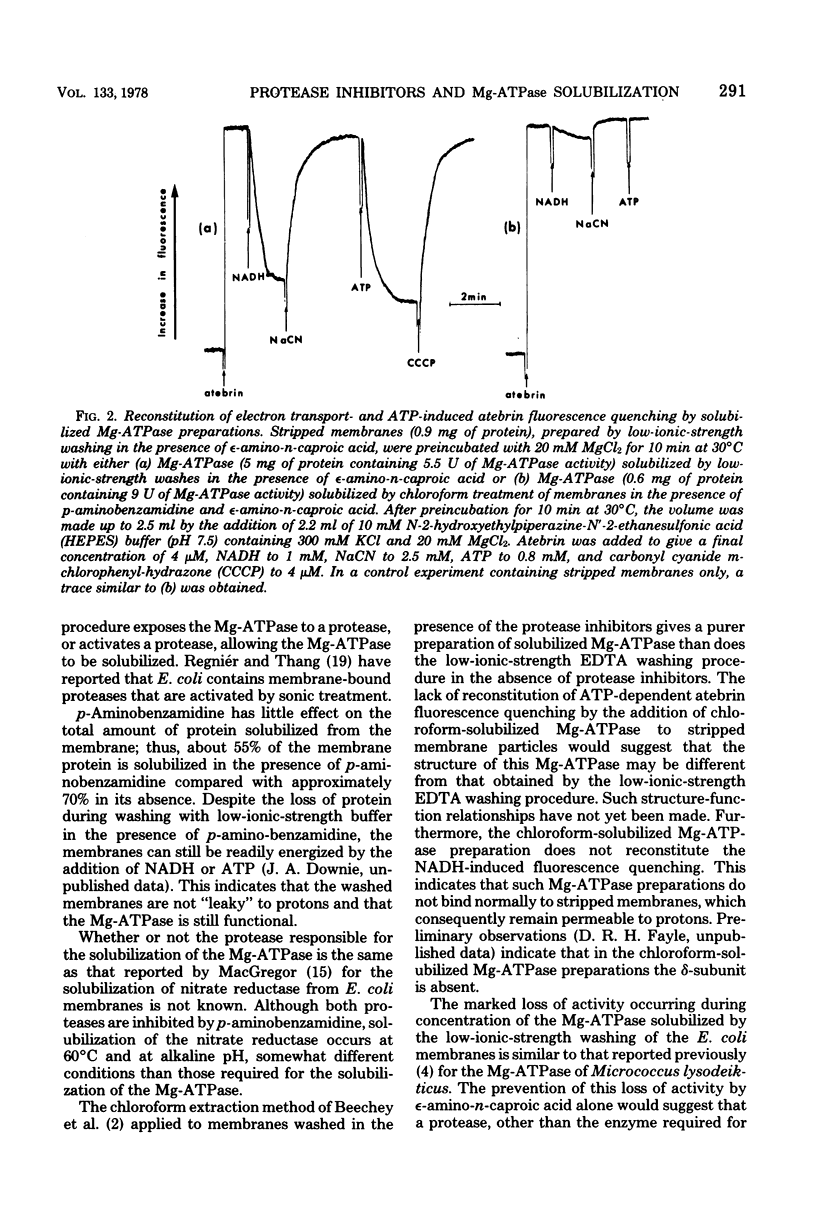
The effects of two protease inhibitors on the solubilization of the membrane-bound Mg2+-adenosine triphosphatase (Mg-ATPase) of Escherichia coli were investigated. p-Aminobenzamidine prevented the solubilization of the Mg-ATPase during treatment of membranes with low-ionic-strength buffers containing ethylenediaminetetraacetic acid. p-Aminobenzamidine did not prevent subsequent solubilization of the Mg-ATPase by treatment of the membranes with chloroform. This method of solubilization yielded a preparation of similar apparent molecular weight but with a 10-fold-increased specific activity as compared with the Mg-ATPase solubilized by washing with low-ionic-strength buffer. However, in contrast to the latter preparation, the chloroform-solubilized Mg-ATPase did not reconstitute ATP-dependent energization of stripped membranes, which were prepared by low-ionic-strength washing in the absence of p-aminobenzamidine. Another protease inhibitor, epsilon-amino-n-caproic acid, did not effect the solubilization of the Mg-ATPase, but did inhibit the loss of activity occurring during concentration, by ultrafiltration, of the Mg-ATPase solublized by the low-ionic-strength treatment.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams A. The release of bound adenosine triphosphatase from isolated bacterial membranes and the properties of the solubilized enzyme. J Biol Chem. 1965 Sep;240(9):3675–3681. [PubMed] [Google Scholar]
- Beechey R. B., Hubbard S. A., Linnett P. E., Mitchell A. D., Munn E. A. A simple and rapid method for the preparation of adenosine triphosphatase from submitochondrial particles. Biochem J. 1975 Jun;148(3):533–537. doi: 10.1042/bj1480533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Purification of a factor for both aerobic-driven and ATP-driven energy-dependent transhydrogenases of Escherichia coli. FEBS Lett. 1972 Dec 15;28(3):309–312. doi: 10.1016/0014-5793(72)80738-5. [DOI] [PubMed] [Google Scholar]
- Carreira J., Andreu J. M., Nieto M., Muñoz E. Membrane adenosine triphosphatase of Micrococcus lysodeikticus. ISolation of two forms of the enzyme complex and correlation between ezymatic stability, latency and activity. Mol Cell Biochem. 1976 Feb 16;10(2):67–76. doi: 10.1007/BF01742200. [DOI] [PubMed] [Google Scholar]
- Cox G. B., Gibson F., McCann L. M., Butlin J. D., Crane F. L. Reconstitution of the energy-linked transhydrogenase activity in membranes from a mutant strain of Escherichia coli K12 lacking magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem J. 1973 Apr;132(4):689–695. doi: 10.1042/bj1320689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Newton N. A., Butlin J. D., Gibson F. The energy-linked transhydrogenase reaction in respiratory mutants of Escherichia coli K12. Biochem J. 1971 Nov;125(2):489–493. doi: 10.1042/bj1250489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Newton N. A., Gibson F., Snoswell A. M., Hamilton J. A. The function of ubiquinone in Escherichia coli. Biochem J. 1970 Apr;117(3):551–562. doi: 10.1042/bj1170551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F., Cox G. B., Downie J. A., Radik J. A mutation affecting a second component of the F0 portion of the magnesium ion-stimulated adenosine triphosphatase of Escherichia coli K12. The uncC424 allele. Biochem J. 1977 Apr 15;164(1):193–198. doi: 10.1042/bj1640193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Downie J. A. The reconstitution of functional respiratory chains in membranes from electron-transport-deficient mutants of Escherichia coli as demonstrated by quenching of atebrin fluorescence. Biochem J. 1974 Sep;142(3):703–706. doi: 10.1042/bj1420703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Jones C. W. Bacterial respiration. Bacteriol Rev. 1977 Mar;41(1):47–99. doi: 10.1128/br.41.1.47-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King E. J. The colorimetric determination of phosphorus. Biochem J. 1932;26(2):292–297. doi: 10.1042/bj0260292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LURIA S. E., BURROUS J. W. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957 Oct;74(4):461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARES-GUIA M., SHAW E. STUDIES ON THE ACTIVE CENTER OF TRYPSIN. THE BINDING OF AMIDINES AND GUANIDINES AS MODELS OF THE SUBSTRATE SIDE CHAIN. J Biol Chem. 1965 Apr;240:1579–1585. [PubMed] [Google Scholar]
- MacGregor C. H. Solubilization of Escherichia coli nitrate reductase by a membrane-bound protease. J Bacteriol. 1975 Mar;121(3):1102–1110. doi: 10.1128/jb.121.3.1102-1110.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis F. J., Kanner B. I., Gutnick D. L., Postma P. W., van Dam K. Energy conservation in membranes of mutants of Escherichia coli defective in oxidative phosphorylation. Biochim Biophys Acta. 1973 Oct 19;325(1):62–71. doi: 10.1016/0005-2728(73)90151-5. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Régnier P., Thang M. N. Membrane associated proteases in E. coli. FEBS Lett. 1973 Oct 1;36(1):31–33. doi: 10.1016/0014-5793(73)80330-8. [DOI] [PubMed] [Google Scholar]
- Vanderkooi G. Organisation of proteins in membranes with special reference to the cytochrome oxidase system. Biochim Biophys Acta. 1974 Dec 16;344(3-4):307–344. doi: 10.1016/0304-4157(74)90011-2. [DOI] [PubMed] [Google Scholar]