Abstract
To study the actions of Ca2+ on “early” stages of the transduction cascade, changes in cytoplasmic calcium concentration (Ca2+ i) were opposed by manipulating Ca2+ fluxes across the rod outer segment membrane immediately following a bright flash. If the outer segment was exposed to 0 Ca2+/0 Na+ solution for a brief period immediately after the flash, then the period of response saturation was prolonged in comparison with that in Ringer solution. But if the exposure to 0 Ca2+/0 Na+ solution instead came before or was delayed until 1 s after the flash then it had little effect. The degree of response prolongation increased with the duration of the exposure to 0 Ca2+/0 Na+ solution, revealing a time constant of 0.49 ± 0.03 s. By the time the response begins to recover from saturation, Ca2+ i seems likely to have fallen to a similar level in each case. Therefore the prolongation of the response when Ca2+ i was prevented from changing immediately after the flash seems likely to reflect the abolition of actions of the usual dynamic fall in Ca2+ i on an early stage in the transduction cascade at a site which is available for only a brief period after the flash. One possibility is that the observed time constant corresponds to the phosphorylation of photoisomerized rhodopsin.
Keywords: photoreceptor, retinal rod, light adaptation, calcium, rhodopsin
introduction
Photoreceptor light adaptation is believed to be largely if not exclusively controlled by cytoplasmic calcium concentration (Ca2+ i) (Matthews et al., 1988; Nakatani and Yau, 1988; Fain et al., 1989; Koutalos et al., 1995b ; Matthews, 1995). Both biochemical and electrophysiological evidence indicates that Ca2+ acts at a number of different sites in the transduction mechanism. These can broadly be subdivided into those associated with “early” stages in the transduction cascade, which are likely only to be accessible for a relatively brief period after the flash, and those “late” in transduction which will be available throughout the flash response (Koutalos et al., 1995b ; Matthews, 1996). The former are believed to involve actions of Ca2+ i on the production (Lagnado and Baylor, 1994; Jones, 1995) or the subsequent quenching via phosphorylation (Kawamura and Murakami, 1991; Kawamura, 1993; Chen et al., 1995) of photoisomerized rhodopsin (Rh*),1 whereas the latter include the inhibition of guanylyl cyclase (Lolley and Racz, 1982; Koch and Stryer, 1988; Koutalos et al., 1995a ) and modulation of the cGMP affinity of the outer segment conductance (Hsu and Molday, 1993; Nakatani et al., 1995) by Ca2+ i.
Photoreceptor Ca2+ i is governed by the balance between Ca2+ influx through the outer segment conductance (Yau and Nakatani, 1984a ; Hodgkin et al., 1985) and Ca2+ efflux via an Na+:Ca2+,K+ exchanger (Yau and Nakatani, 1984b ; Hodgkin et al., 1987; Cervetto et al., 1989). When the outer segment conductance is suppressed during the response to a bright flash, this balance is upset and Ca2+ i falls (Yau and Nakatani, 1985; McNaughton et al., 1986; Ratto et al., 1987; Gray-Keller and Detwiler, 1994; McCarthy et al., 1994) with a dominant time constant in salamander rods of 0.5–1 s (Yau and Nakatani, 1985; Hodgkin et al., 1987). If the flash is sufficiently bright to hold the response in saturation for several seconds, Ca2+ i is likely to fall sufficiently low that its actions late in transduction will be essentially complete by the time that recovery commences. However, this dynamic fall in Ca2+ i may also have a more modest effect on stages early in the transduction cascade during the period for which they remain accessible after the flash (Matthews, 1996).
The light-induced fall in Ca2+ i can be opposed by superfusing the outer segment with a 0 Ca2+/0 Na+ solution designed to minimize simultaneously Ca2+ influx and efflux (Matthews et al., 1988; Nakatani and Yau, 1988; Fain et al., 1989). The removal of external Ca2+ minimizes Ca2+ influx through the outer segment conductance, while the removal of external Na+ prevents Ca2+ efflux through the Na+:Ca2+,K+ exchanger. I have investigated the actions of Ca2+ on early stages in transduction by briefly exposing the outer segment to this solution just after a bright flash, thereby delaying the onset of the usual dynamic fall in Ca2+ i.
methods
Preparation, Recording, and Light Stimuli
Rod photoreceptors were isolated mechanically under infrared illumination from the dark-adapted retina of the larval tiger salamander Ambystoma tigrinum after decapitation and pithing in dim red light. The circulating current was recorded by drawing the inner segment of the rod into a suction pipette, leaving the outer segment exposed to the superfusing solution. All experiments were carried out at room temperature (maintained at 20°C). The suction pipette current signal was low-pass filtered at 20 Hz (6-pole Bessel filter) and digitized continuously for subsequent analysis at a sampling rate of 100 Hz. Light stimuli were unpolarized 20-ms flashes of wavelength 500 nm. Light intensities were adjusted with neutral density filters and measured with a calibrated silicon photodiode (Graseby Optronics, Orlando, FL); they can be converted to isomerizations using a collecting area of 20 μm2.
Solutions and Solution Changes
Ringer solution contained 111 mM NaCl, 2.5 mM KCl, 1.0 mM CaCl2, 1.6 mM MgCl2, and 3.0 mM HEPES, adjusted to pH 7.7 with NaOH, and 10 μM EDTA to chelate impurity heavy metals. The Ringer solution continuously perfusing the recording chamber also included 10 mM glucose. 0 Ca2+/0 Na+ solution was modified from Ringer solution by the equimolar substitution of choline chloride for NaCl, the omission of CaCl2 and MgCl2, the removal of EDTA, and the inclusion of 2 mM EGTA (Matthews, 1996). The normally inward dark current is inverted in this choline-substituted solution due to the efflux of K+ (Hodgkin et al., 1985; Matthews, 1995; Lyubarsky et al., 1996; Matthews, 1996). The removal of Mg2+ served to prevent the substantial Mg2+ influx which would otherwise have occurred under these conditions (Hodgkin et al., 1985) and which has been shown to retard response recovery (Matthews, 1995). Rapid external solution changes were effected by translating the boundary between two flowing streams of solution across the exposed outer segment (Hodgkin et al., 1985) using a piezo-electric actuator (Matthews, 1994). Recordings were corrected by subtraction of the junction current measured when the same solution changes were carried out during intense steady illumination at the end of the experiment, scaled for coincidence of saturating level before and after the solution change. The junction current, shown in Figs. 1 and 2 as the solution monitor, normally rose to 90% of its final value within 50 ms, suggesting a similar time course for the solution change. Times of solution changes in Figs. 2 and 3 are given as the half-rise times of the junction current.
Figure 1.
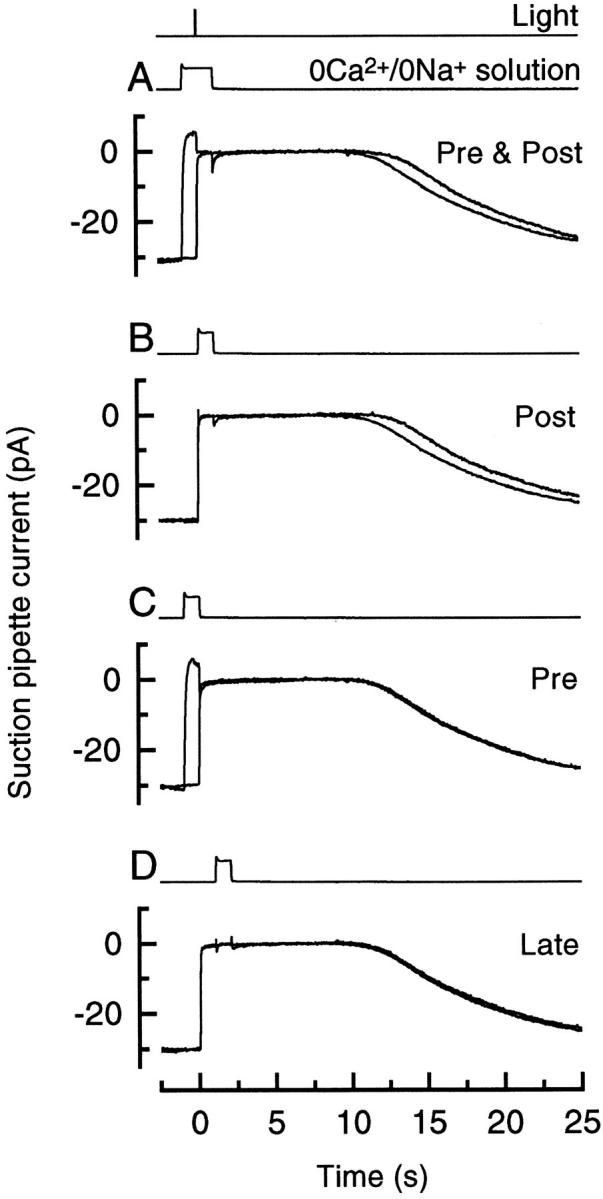
Superimposed responses to bright flashes in Ringer solution and on exposure to 0 Ca2+/0 Na+ solution, (A) from 1 s before until 1 s after the flash, (B) from the time of the flash until 1 s after the flash, (C) from 1 s before the flash until the time of the flash, and (D) from 1 s after until 2 s after the flash. Each trace is the average of four responses; measurements in Ringer and 0 Ca2+/0 Na+ solution were bracketed symmetrically in time. Bright flash delivered 5,560 photons μm−2.
Figure 2.
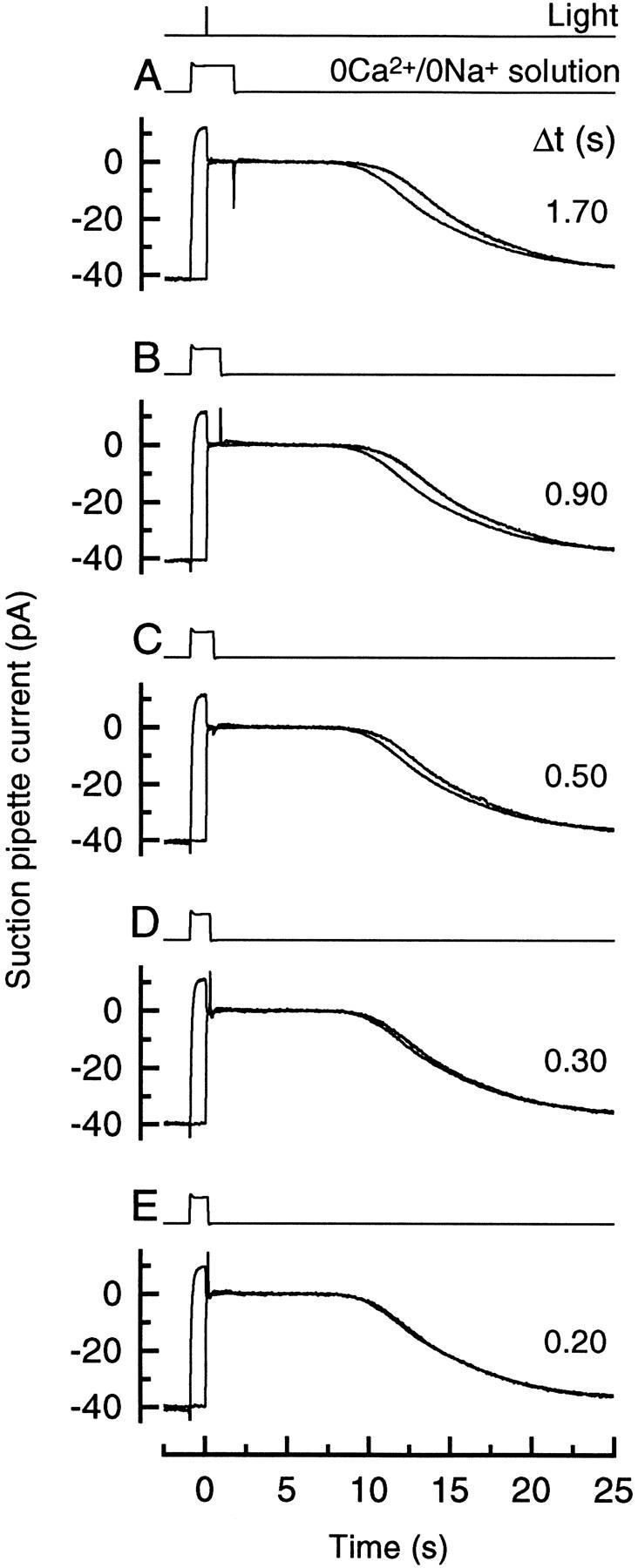
Superimposed responses to bright flashes in Ringer solution and on exposure to 0 Ca2+/0 Na+ solution from 1 s before the flash until the times (Δt) after the flash indicated beside each trace, measured from the half-relaxation time of the junction current. Each trace is the average of four responses; measurements in Ringer solution and 0 Ca2+/0 Na+ solution were bracketed symmetrically in time. Bright flash delivered 5,350 photons μm−2.
Figure 3.
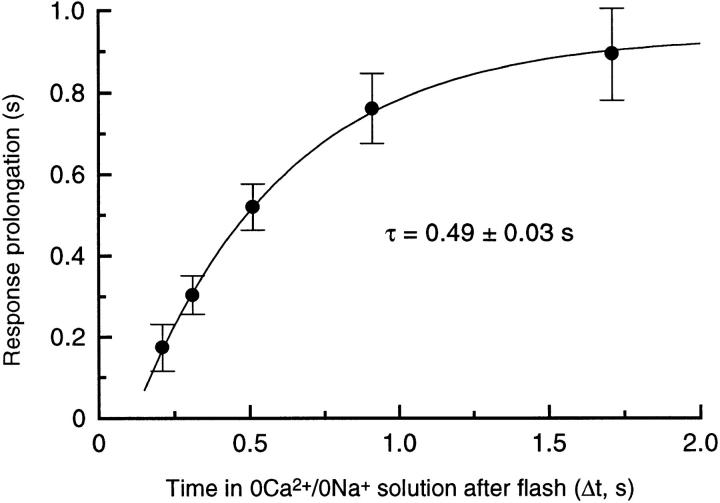
Collected data from 8 rods for the prolongation of the flash response by exposure to 0 Ca2+/0 Na+ solution as a function of the time spent in 0 Ca2+/0 Na+ solution after the flash, as in Fig. 2. Data represent the mean prolongation (mean ± SEM) of the time for 25% recovery of the original dark current plotted against the interval, Δt, between the flash and the half-relaxation time of the junction current on the return to Ringer solution. Values for Δt differed slightly between cells (total scatter ± 0.01 s), probably due to variation in the precise position of the interface between the two solution streams; mean values are plotted. Solid curve is a single exponential with time constant 0.49 ± 0.03 s offset from passing through the origin by 0.11 s, fitted using a weighted least squares algorithm. Bright flashes delivered 5,350 photons μm−2.
results
Fig. 1 illustrates the effect of briefly exposing the outer segment to 0 Ca2+/0 Na+ solution either shortly before or shortly after a bright flash. If the outer segment was stepped into 0 Ca2+/0 Na+ solution 1 s before the flash and returned to Ringer solution 1 s thereafter (Fig. 1 A, Pre & Post), then the flash response recorded by the suction pipette recovered from saturation slightly later than when the outer segment remained in Ringer solution throughout the response. A similar prolongation of the response was also induced by a 1 s exposure to 0 Ca2+/0 Na+ solution immediately after the flash (Fig. 1 B, Post). Both of these procedures will have delayed the dynamic fall in Ca2+ i, which normally ensues when the circulating current is suppressed by the flash, until after the outer segment was returned to Ringer solution. These data were quantified by measuring the time for the response to recover to a criterion level (Pepperberg et al., 1992; Matthews, 1995, 1996). Since the shape of the initial recovery was virtually unaffected by these manipulations, measurements of the delay in recovery will be essentially independent of the criterion selected, which was taken here as 25% of the original dark current. 0 Ca2+/0 Na+ exposures as in Fig. 1, A and B, prolonged the bright flash response by 0.81 ± 0.15 s (11 cells) and 0.75 ± 0.10 s (10 cells) respectively (mean ± SEM; flash delivered 1,320 or 5,560 photons μm−2), which do not differ significantly at the 5% level (Student's t test, t = 0.38). The earlier recovery of the flash response in Ringer solution when Ca2+ i was allowed to fall can be contrasted with the much greater shortening of the response induced by nearly saturating steady light (5.0 ± 0.5 s, 8 cells) which should produce a static reduction in Ca2+ i of similar magnitude.
If, instead, the exposure to 0 Ca2+/0 Na+ solution took place immediately before the flash (Fig. 1 C, Pre) or was delayed until 1 s after the flash (Fig. 1 D, Late) then it had little effect on response recovery. These manipulations only prolonged the time for 25% recovery of the dark current by 0.02 ± 0.03 s (10 cells) and 0.05 ± 0.04 s (8 cells), respectively, neither of which differs significantly from zero. These observations indicate that the response was only prolonged when Ca2+ i was prevented from falling during a brief period immediately after the flash and not as the result of exposure to 0 Ca2+/0 Na+ solution per se.
The period during which the dynamic fall in Ca2+ i could exert this effect was explored in Fig. 2 by varying the time at which the outer segment was returned to Ringer solution after the flash. In each case the outer segment was stepped to 0 Ca2+/0 Na+ solution 1 s before the flash, and returned to Ringer solution at the time (Δt) after the flash indicated beside each trace. As the time spent in 0 Ca2+/0 Na+ solution after the flash decreased, the duration of the response progressively declined towards that in Ringer solution.
These data are quantified in Fig. 3 by once again measuring the time for recovery of 25% of the dark current in each case. The longest exposures to 0 Ca2+/0 Na+ solution prolonged the response by nearly 1 s in comparison with the response in Ringer, but this delay in recovery progressively decreased as the time spent in 0 Ca2+/0 Na+ solution after the flash was reduced. These data could be well fitted by a single exponential of time constant 0.49 ± 0.03 s (8 cells). The simplest interpretation of this result is that it reflects the progressive removal of a Ca2+-sensitive stage early in the transduction cascade. When the dynamic fall in Ca2+ i was delayed in this way an ever-smaller proportion of these sites would remain to respond to it. The small displacement of the fitted exponential curve from the origin probably arises largely from the delay between the flash and the complete suppression of the circulating current (Cobbs and Pugh, 1987) which initiates the dynamic fall in Ca2+ i. This delay will have been augmented by the finite flash duration, a 20-ms group delay from the Bessel low-pass filter, and the time taken for completion of the solution change.
discussion
Stepping the outer segment to 0 Ca2+/0 Na+ solution is believed greatly to retard changes in Ca2+ i, largely preventing the light-induced fall in Ca2+ i for some 10–15 s after the solution change (Fain et al., 1989; Lyubarsky et al., 1996). The short exposures used here therefore seem likely to have held Ca2+ i near to its pre-existing level before the solution change, and thereby to have delayed the subsequent dynamic fall in Ca2+ i until after the return to Ringer solution.
After the return to Ringer solution the response to the bright flash remained in saturation for a period sufficient to allow Ca2+ i to fall substantially (Yau and Nakatani, 1985; Hodgkin et al., 1987), thereby presumably resulting in near maximal activation of guanylyl cyclase before the circulating current began to recover (Koch and Stryer, 1988). Therefore the prolongation of the response when the outer segment was exposed to 0 Ca2+/0 Na+ solution immediately after the flash seems likely to reflect instead the abolition of actions of the dynamic fall in Ca2+ i on an early stage in the transduction cascade which was only accessible to Ca2+ for a relatively brief period after the flash (Koutalos et al., 1995b ; Matthews, 1996). These changes in response duration are much smaller than those induced by near saturating steady light (Fain et al., 1989; Pepperberg et al., 1992; Matthews, 1995), which presumably reduces Ca2+ i to a static level similar to that ultimately attained after a bright flash. This observation implies that the site for Ca2+ early in transduction is likely to become inaccessible at least as rapidly as the dynamic fall in Ca2+ i induced by the flash, thereby limiting the effect on response duration. The time constant of 0.49 ± 0.03 s obtained in Fig. 3 from the dependence of the response prolongation on the duration of the exposure to 0 Ca2+/0 Na+ solution is most readily interpreted as reflecting the time course with which this early site for Ca2+ is removed after the flash. Indeed, this interpretation follows directly, irrespective of the precise time course of the stereotypical decline in Ca2+ i once the outer segment is returned to Ringer solution, if it is assumed that the early site decays stochastically and that reduced Ca2+ i accelerates its removal independent of time after the flash.
The strongest candidate for such a site of action for Ca2+ early in the transduction cascade is Rh* itself, whose inactivation via phosphorylation is known to be modulated by Ca2+ i (Kawamura, 1993; Chen et al., 1995). Suppose that Rh* were phosphorylated more rapidly when Ca2+ i was reduced, or that its ability to activate transducin were decreased. Under control conditions the dynamic fall in Ca2+ i would act on Rh* to lower the activation of transducin resulting from the flash. If instead the dynamic fall in Ca2+ i were delayed until long after the flash, by which time Rh* would have been completely phosphorylated, then this effect would be abolished, and the response to a bright flash prolonged. However, as the delay between the flash and the dynamic fall in Ca2+ i was reduced, the overlap between the lifetime of unphosphorylated Rh* and the dynamic fall in Ca2+ i would increase, thereby progressively shortening the flash response. Thus the time constant derived from these measurements may represent the effective lifetime of unphosphorylated Rh* in the dark-adapted rod.
Previous estimates of Rh* lifetime have been derived from the time constant of around 2 s which dominates the recovery of the flash response (Pepperberg et al., 1992, 1994). However, the insensitivity of this longer time constant to Ca2+ i (Lyubarsky et al., 1996; Matthews, 1996) and light (Pepperberg et al., 1992, 1994; Murnick and Lamb, 1996) is difficult to reconcile with biochemical evidence for the Ca2+ dependence of Rh* phosphorylation (Kawamura, 1993; Chen et al., 1995). If the more rapid time constant of about 0.5 s obtained here for the removal of the Ca2+-sensitive site early in transduction represents Rh* phosphorylation then this problem would be resolved, suggesting that the longer time constant governing response recovery might originate instead from subsequent events in Rh* inactivation or from the inactivation of later stages in the transduction cascade (Lyubarsky et al., 1996; Matthews, 1996; Murnick and Lamb, 1996). This value is also in close agreement with the shorter of the two time constants required to model the kinetics of the flash response when the light-induced fall in Ca2+ i is prevented (Lyubarsky et al., 1996).
Such rapid phosphorylation of Rh* even in darkness would have several functional implications for the light response. First, to account for the magnitude of the change in sensitivity induced by bright light (Pepperberg et al., 1994; Jones, 1995; Matthews, 1996) or greatly reduced Ca2+ i (Lagnado and Baylor, 1994; Koutalos et al., 1995b ), it seems possible that the time constant for Rh* phosphorylation might shorten further in the fully light-adapted rod by at least three- to fourfold from this already rapid value in darkness. Second, much Rh* phosphorylation appears to take place during the rising phase of even the dark-adapted dim flash response, before it has reached its peak. Such rapid phosphorylation of Rh* might thereby contribute to the ability of lowered Ca2+ i to reduce the apparent gain of the flash response rising phase (Lagnado and Baylor, 1994; Jones, 1995).
Acknowledgments
Supported by the Wellcome Trust.
Abbreviation used in this paper
- Rh*
photoisomerized rhodopsin
Footnotes
I am grateful to Dr. G.L. Fain for helpful comments on the manuscript.
Preliminary results from this study have been presented to the Physiological Society (Matthews, H.R. 1996. Dynamic actions of Ca2+ i ‘early' in transduction in the adaptation of rods isolated from the tiger salamander. J. Physiol. (Lond.). 494:15P).
references
- Cervetto L, Lagnado L, Perry RJ, Robinson DW, McNaughton PA. Extrusion of calcium from rod outer segments is driven by both sodium and potassium gradients. Nature (Lond) 1989;337:740–743. doi: 10.1038/337740a0. [DOI] [PubMed] [Google Scholar]
- Chen CK, Inglese J, Lefkowitz RJ, Hurley JB. Ca2+-dependent interaction of recoverin with rhodopsin kinase. J Biol Chem. 1995;270:18060–18066. doi: 10.1074/jbc.270.30.18060. [DOI] [PubMed] [Google Scholar]
- Cobbs WH, Pugh EN. Kinetics and components of the flash photocurrent of isolated retinal rods of the larval salamander, Ambystoma tigrinum. . J Physiol (Lond) 1987;394:529–572. doi: 10.1113/jphysiol.1987.sp016884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain GL, Lamb TD, Matthews HR, Murphy RLW. Cytoplasmic calcium concentration as the messenger for light adaptation in salamander rods. J Physiol (Lond) 1989;416:215–243. doi: 10.1113/jphysiol.1989.sp017757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray-Keller MP, Detwiler PB. The calcium feedback signal in the phototransduction cascade of vertebrate rods. Neuron. 1994;13:849–861. doi: 10.1016/0896-6273(94)90251-8. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, McNaughton PA, Nunn BJ. The ionic selectivity and calcium dependence of the light-sensitive pathway in toad rods. J Physiol (Lond) 1985;358:447–468. doi: 10.1113/jphysiol.1985.sp015561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, McNaughton PA, Nunn BJ. Measurement of sodium-calcium exchange in salamander rods. J Physiol (Lond) 1987;391:347–370. doi: 10.1113/jphysiol.1987.sp016742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y-T, Molday RS. Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature (Lond) 1993;361:76–79. doi: 10.1038/361076a0. [DOI] [PubMed] [Google Scholar]
- Jones GJ. Light adaptation and the rising phase of the flash photocurrent of salamander retinal rods. J Physiol (Lond) 1995;487:441–451. doi: 10.1113/jphysiol.1995.sp020891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura S. Rhodopsin phosphorylation as a mechanism of cyclic GMP phosphodiesterase regulation by S-modulin. Nature (Lond) 1993;362:855–857. doi: 10.1038/362855a0. [DOI] [PubMed] [Google Scholar]
- Kawamura S, Murakami M. Calcium-dependent regulation of cyclic GMP phosphodiesterase by a protein from frog retinal rods. Nature (Lond) 1991;349:420–423. doi: 10.1038/349420a0. [DOI] [PubMed] [Google Scholar]
- Koch K-W, Stryer L. Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nature (Lond) 1988;334:64–66. doi: 10.1038/334064a0. [DOI] [PubMed] [Google Scholar]
- Koutalos Y, Nakatani K, Tamura T, Yau K-W. Characterization of guanylate cyclase activity in single retinal rod outer segments. J Gen Physiol. 1995a;106:863–890. doi: 10.1085/jgp.106.5.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutalos Y, Nakatani K, Yau K-W. The cGMP-phosphodiesterase and its contribution to sensitivity regulation in retinal rods. J Gen Physiol. 1995b;106:891–921. doi: 10.1085/jgp.106.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagnado L, Baylor DA. Calcium controls light-triggered formation of catalytically active rhodopsin. Nature (Lond) 1994;367:273–277. doi: 10.1038/367273a0. [DOI] [PubMed] [Google Scholar]
- Lolley RN, Racz E. Calcium modulation of cyclic GMP synthesis in rat visual cells. Vision Res. 1982;22:1481–1486. doi: 10.1016/0042-6989(82)90213-9. [DOI] [PubMed] [Google Scholar]
- Lyubarsky A, Nikonov S, Pugh EN. The kinetics of inactivation of the rod phototransduction cascade with constant Ca2+ i . J Gen Physiol. 1996;107:19–34. doi: 10.1085/jgp.107.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews, H.R. 1994. A piezo translator for effecting fast solution changes on isolated cells. J. Physiol. (Lond.). 480:3–4P. (Abstr.).
- Matthews HR. Effects of lowered cytoplasmic calcium concentration and light on the responses of salamander rod photoreceptors. J Physiol (Lond) 1995;484:267–286. doi: 10.1113/jphysiol.1995.sp020664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews HR. Static and dynamic actions of cytoplasmic Ca2+in the adaptation of responses to saturating flashes in salamander rods. J Physiol (Lond) 1996;490:1–15. doi: 10.1113/jphysiol.1996.sp021123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews HR, Murphy RLW, Fain GL, Lamb TD. Photoreceptor light adaptation is mediated by cytoplasmic calcium concentration. Nature (Lond) 1988;334:67–69. doi: 10.1038/334067a0. [DOI] [PubMed] [Google Scholar]
- McCarthy ST, Younger JP, Owen WG. Free calcium concentrations in bullfrog rods determined in the presence of multiple forms of fura-2. Biophys J. 1994;67:2076–2089. doi: 10.1016/S0006-3495(94)80691-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton PA, Cervetto L, Nunn BJ. Measurement of the intracellular free calcium concentration in salamander rods. Nature (Lond) 1986;322:261–263. doi: 10.1038/322261a0. [DOI] [PubMed] [Google Scholar]
- Murnick JG, Lamb TD. Kinetics of desensitization induced by saturating flashes in toad and salamander rods. J Physiol (Lond) 1996;495:1–14. doi: 10.1113/jphysiol.1996.sp021569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani K, Koutalos Y, Yau KW. Ca2+modulation of the cGMP-gated channel of bullfrog retinal rod photoreceptors. J Physiol (Lond) 1995;484:69–76. doi: 10.1113/jphysiol.1995.sp020648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani K, Yau K-W. Calcium and light adaptation in retinal rods and cones. Nature (Lond) 1988;334:69–71. doi: 10.1038/334069a0. [DOI] [PubMed] [Google Scholar]
- Pepperberg DR, Cornwall MC, Kahlert M, Hofmann KP, Jin J, Jones GJ, Ripps H. Light-dependent delay in the falling phase of the retinal rod photoresponse. Vis Neurosci. 1992;8:9–18. doi: 10.1017/s0952523800006441. [DOI] [PubMed] [Google Scholar]
- Pepperberg DR, Jin J, Jones GJ. Modulation of transduction gain in light adaptation of retinal rods. Vis Neurosci. 1994;11:53–62. doi: 10.1017/s095252380001110x. [DOI] [PubMed] [Google Scholar]
- Ratto GM, Payne R, Owen WG, Tsien RY. The concentration of cytosolic free calcium in vertebrate rod outer segments measured with fura-2. J Neurosci. 1987;8:3240–3246. doi: 10.1523/JNEUROSCI.08-09-03240.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K-W, Nakatani K. Cation selectivity of light-sensitive conductance in retinal rods. Nature (Lond) 1984a;309:352–354. doi: 10.1038/309352a0. [DOI] [PubMed] [Google Scholar]
- Yau K-W, Nakatani K. Electrogenic Na-Ca exchange in retinal rod outer segment. Nature (Lond) 1984b;311:661–663. doi: 10.1038/311661a0. [DOI] [PubMed] [Google Scholar]
- Yau K-W, Nakatani K. Light-induced reduction of cytoplasmic free calcium in retinal rod outer segment. Nature (Lond) 1985;313:579–582. doi: 10.1038/313579a0. [DOI] [PubMed] [Google Scholar]