Abstract
Plasma membrane Ca2+-ATPase (PMCA) and the Na+/Ca2+ exchanger participate in regulating cell function by maintaining proper intracellular Ca2+ concentrations ([Ca2+]i). In renal epithelial cells these proteins have been additionally implicated in cellular calcium absorption. The purpose of the present studies was to determine the Ca2+ extrusion mechanisms in cells derived from the proximal tubule. Homology-based RT-PCR was used to amplify PMCA transcripts from RNA isolated from mouse cell lines originating from the S1, S2, and S3 proximal tubule segments. S1, S2, and S3 cells exhibited only PMCA1 and PMCA4 products. PCR product identity was confirmed by sequence analysis. Northern analysis of proximal tubule cell RNAs revealed appropriate transcripts of 7.5 and 5.5 kb for PMCA1 and 8.5 and 7.5 kb for PMCA4, but were negative for PMCA2 and PMCA3. Western analysis with a monoclonal antibody to PMCA showed that all proximal cell lines expressed a reacting plasma membrane protein of 140 kD, the reported PMCA molecular mass. Na+/Ca2+ exchanger (NCX1) mRNA expression, analyzed by RT-PCR, protein expression by Western analysis, and functional exchange activity were uniformly absent from all proximal tubule cell lines. These observations support the idea that immortalized cells derived from the proximal tubule express PMCA1 and PMCA4, which may serve as the primary mechanism of cellular Ca2+ efflux.
Keywords: calcium transport, kidney, PMCA, Na/Ca exchange, Ca-ATPase
introduction
Cytoplasmic ionized calcium is tightly regulated by intracellular homeostatic mechanisms that quickly sequester calcium and, over the longer term, extrude calcium from the cell. Calcium efflux across plasma membranes is an energy-dependent process that may be mediated by Ca2+-ATPase and by various exchanger proteins including the Na+/Ca2+ exchanger (Philipson and Nicoll, 1992), Na+/Ca2+, K+ exchanger (Cervetto et al., 1989), and H+/Ca2+ exchanger (Tsukamoto et al., 1991). In polarized epithelial cells, such as those found in the intestine and the kidney, the situation is more complicated since these cells must regulate cytoplasmic calcium when challenged by rapid and large changes in the rate of transcellular calcium transport. Calcium absorption by epithelial cells is a two-step process wherein calcium entry is followed by efflux. The membrane-delimited proteins responsible for admitting calcium from the lumen are located in apical plasma membranes, whereas those responsible for calcium efflux are located in basolateral plasma membranes.
Calcium absorption occurs throughout the nephron. The majority of calcium is absorbed by proximal tubules, with smaller fractions recovered at more distal sites. In proximal tubules, most calcium is transported by a passive mechanism that is thought to proceed through the paracellular pathway, driven by osmotic solvent flow. A smaller fraction, 15–20 percent, occurs through a cellular pathway (Ullrich et al., 1976; Rouse et al., 1980; Bomsztyk et al., 1984). Although small by comparison with the paracellular flow, active cellular absorption by proximal tubules amounts to some 20 μmol/min, which is approximately twice that of the distal nephron, where calcium absorption is entirely cellular. The mammalian proximal tubule consists of three different segments: S1, S2, and S3. Calcium entry may involve voltage-dependent calcium channels (Almeida et al., 1992; Rose et al., 1994; Tanaka et al., 1995). Efflux is thought to be mediated by a Ca2+-ATPase or by Na+/ Ca2+ exchange, though the presence of these transport mechanisms in proximal tubules cells is controversial. The first objective of the present studies was to identify the potential macromolecules responsible for calcium efflux in proximal tubule cells. Studies were performed on immortalized lines of mouse proximal tubule cells that were derived from S1, S2, or S3 proximal tubules (Nesbitt et al., 1995, 1996).
The Ca2+-ATPase is a primary active transport mechanism. Plasma membrane Ca2+-ATPases (PMCAs)1 are P-type ATPases (Pedersen and Carafoli, 1987), encoded by four discrete genes, PMCA1–PMCA4. PMCA gene products are homologous isoforms of ∼140 kD (Stauffer et al., 1995) that vary in expression levels in a tissue-dependent manner. In humans and in the rat, PMCA1 and PMCA4 mRNA and protein are the dominant isoforms in virtually all tissues, including kidney (Stauffer et al., 1995), whereas PMCA2 and PMCA3 are expressed primarily in nervous tissue (Stauffer et al., 1993). In the kidney, PMCA2 transcripts are expressed in most cortical nephron segments (Magocsi et al., 1992). The glomerulus exhibits PMCA1 mRNA (Magocsi et al., 1992). These results apparently contrast with the aforementioned evidence that PMCA1 and PMCA4 are the primary renal isoforms.
Plasma membrane Na+/Ca2+ exchange is a secondary active transport mechanism, also capable of mediating cellular calcium efflux. The NCX1 Na+/Ca2+ exchanger has a wide tissue distribution, with transcripts expressed in heart (Nicoll et al., 1990), brain (Furman et al., 1993), kidney (Reilly and Shugrue, 1992), as well as in other tissues (Smith and Smith, 1995). NCX1 encodes a protein that is ∼125 kD in size and is highly conserved across species (Reilly et al., 1993). In renal epithelial cells the exchanger is localized to basolateral membranes (Reilly et al., 1993), where it couples the dissipative energy of Na+ entry to Ca2+ efflux with a 3:1 stoichiometry (Talor and Arruda, 1985). Attempts to localize the NCX1 Na+/Ca2+ exchanger to specific nephron segments have resulted in contradictory or ambiguous findings. The functional implications of differential expression of the PMCA isoforms and the Na+/Ca2+ exchanger in a single cell is unclear. A second aim of these studies was to determine whether expression of specific isoforms was related to the segmental origin of S1, S2, and S3 cells and if single cell types express multiple isoforms of particular proteins responsible for calcium efflux.
materials and methods
Cell Culture
Immortalized mouse proximal (S1, S2, or S3) and distal convoluted tubule (DCT) cell lines were established as detailed previously (Friedman et al., 1991; Nesbitt et al., 1995, 1996). The proximal tubule cells exhibit the phenotype of S1, S2, or S3 portions of the proximal tubule including: (a) functional Na:Pi cotransport; (b) formation of cAMP in response to parathyroid hormone or calcitonin; and, (c) alkaline phosphatase activity, appropriate for the segmental origin of each cell type (Nesbitt et al., 1995, 1996). DCT cells exhibit a distal convoluted tubule phenotype, as described elsewhere (Gesek and Friedman, 1992; Friedman et al., 1996). Distal tubule (DT) cells2 were isolated by a double-antibody immunodissection procedure described previously (Pizzonia et al., 1991) and maintained in primary culture. DCT and proximal tubule cell lines were grown on 100-mm dishes (Corning Glass Works, Corning, NY) in DMEM/Ham's F12 media (Sigma Chemical Co., St. Louis, MO) supplemented with 5% heat-inactivated (56°C for 20 min) FCS (Sigma) and PSN antibiotic mixture (50 μg penicillin, 50 μg streptomycin, 100 μg neomycin/100 ml media; Gibco BRL, Gaithersburg, MD) in a humidified atmosphere of 95% O2/5% CO2 at 37°C. Primary cultures of distal tubule cells were grown under the same conditions using 10% FCS. Cells were placed in serum-free DMEM/Ham's F12 media 16 h before use.
RNA Isolation
Culture dishes (100-mm diameter) of proximal tubule cells were washed twice with 5 ml 1× Ca2+/Mg2+-free Hank's balanced salt solution. Cells were solubilized and scraped in the presence of 1.0 ml 1 M GITC, layered onto a 1.5 ml CsCl gradient in 3 ml TL-100 centrifuge tubes (Beckman Instruments, Inc., Fullerton, CA) and overlaid with 0.15 ml of 20% sarkosyl. Gradients were centrifuged for 2 h at room temperature, pellets were washed with 70% ethanol, and resuspended in 100 μl sterile water. Quantitation of yield was determined by absorbance at 260 and 280 nm.
Reverse Transcriptase (RT)-PCR
One microgram of total RNA from proximal tubule cells or 250 ng of mRNA was reverse transcribed using MuMLV reverse transcriptase and random hexamers (GeneAmp RNA-PCR Kit; Perkin-Elmer, Foster City, CA) for 10 min at room temperature, then 15 min at 42°C, in the presence of 5 mM MgCl2. As a control for genomic DNA contamination of the RNA preparations, parallel samples were treated similarly but not reverse transcribed. The cDNA was then amplified with Taq polymerase. Mouse kidney mRNA (Clontech, Palo Alto, CA) was used as a positive control for appropriately-sized PCR products. Primer sequences and PCR annealing temperatures for each PMCA isoform-specific primer pair are given in Table I. PCR was performed at 94°C for 1 min, annealed at the specific temperature for each primer set (Table I) for 1 min, and extended for 2 min at 70°C for 35 cycles, with a final extension of 7 min. The products were electrophoresed on a 1% low-melting agarose gel (FMC Bioproducts, Rockland, ME) and stained with ethidium bromide.
Table I.
Primers Used for Amplification of Transcripts By RT-PCR
| Primer | Sequence | Reference | Annealing Temp. (°C) | |||
|---|---|---|---|---|---|---|
| PMCA1 Forward | 5′-TGGCAAACAACTCAGTTGCATATAGTGG-3′ | (Meszaros and Karin, 1993) | 65 | |||
| PMCA1 Reverse | 5′-TCCTGTTCAATTCGACTCTGCAAGCCTCG-3′ | (Meszaros and Karin, 1993) | ||||
| PMCA2 Forward | 5′-TCTGGTGAGGGTGTACTGAGGACA-3′ | (Abramowitz et al., 1995) | 62 | |||
| PCMA2 Reverse | 5′-GAGCGTCACGTCCTGTAGTGC-3′ | (Abramowitz et al., 1995) | ||||
| PMCA3 Forward | 5′-GAAGACCTCACCCACAGAGG-3′ | (Stauffer et al., 1993) | 60 | |||
| PMCA3 Reverse | 5′-TCTGCTCCTGCTCAATTCGG-3′ | (Stauffer et al., 1993) | ||||
| PMCA4 Forward | 5′-AAGAAGATGATGAAGGACAACAAC-3′ | (Abramowitz et al., 1995) | 65 | |||
| PMCA4 Reverse | 5′-GTTGCGTACCATATTGTCTCGGTC-3′ | (Abramowitz et al, 1995) | ||||
| β-Actin Forward | 5′-AACCGCGAGAAGATGACCCAGATCATGTTT-3′ | (Nakajima-Iijima et al., 1985) | 55 | |||
| β-Actin Reverse | 5′-AGCAGCCGTGGCCATCTCTTGCTCGAAGTC-3′ | (Nakajima-Iijima et al., 1985) | ||||
| NCX1 Forward | 5′-ATGCTGGGTCTGATTATGAGT-3′ | (White et al., 1996a ) | 60 | |||
| NCX1 Reverse | 5′-AGTGGCTGCTTGTCATCGTA-3′ | (White et al., 1996a ) |
The sequences of the oligodeoxynucleotide primers (listed from 5′ to 3′) used for RT-PCR analysis are shown. The optimized annealing temperature for the amplification of mouse transcripts by each primer pair is shown in the right hand column in the same row as the forward primer. The references in which the primer sequences were originally published are also listed.
β-Actin primers were designed from the human β-actin genomic sequence (Nakajima-Iijima et al., 1985). The primers span an intron, thereby differentiating between PCR products from mRNA and those from genomic DNA. RT-PCR was performed as described above. cDNA products arising from mRNA have a size of 372 bp, whereas products from genomic DNA have a size of 790 bp. The sequences for the forward and reverse primers and annealing temperature are given in Table I.
NCX1 Na+/Ca2+ exchanger primers were designed to conserved portions of the exchanger transcript spanning an alternatively spliced region (Kofuji et al., 1994) known to amplify a product of ∼600 bp from mouse kidney mRNA (White et al., 1996a ). RT-PCR was performed as above. The primer sequences are shown in Table I.
Resulting PCR products were cut from the gel and the cDNA eluted (Wizard Prep Kit; Promega Corp., Madison, WI). Products were subcloned into the blunt end SrfI site of Bluescript SK(+) with the PCRScript Kit (Stratagene Inc., La Jolla, CA). Plasmids were isolated by the Qiagen Mini-20 Plasmid Purification Kit (Qiagen Inc., Chatsworth, CA). 1 μg of purified plasmid was digested with the restriction enzymes BamHI and NotI (Gibco-BRL) and electrophoresed on a 1% agarose gel to confirm proper insert size.
DNA Sequencing
DNA sequencing was performed with the PRISM DyeDeoxy Sequencing Kit (Applied Biosystems, Inc., Foster City, CA) as described by the manufacturer. The cDNA products were sequenced on the Applied Biosystems Model 373A DNA Sequencing System using the T3 and T7 primer sites in pBluescript. Alternatively, products were cut from a low-melt agarose gel, the cDNA eluted as above, then 100 ng of each product was directly sequenced with 3.2 pmol of the forward or reverse PCR primers using the Prism Sequencing Kit and the Applied Biosystems Sequence Sequencing System. To control for nucleotide incorporation errors introduced by Taq polymerase, multiple RT-PCR reactions were performed and products from different reactions were sequenced. The cDNAs from at least two independent reactions were sequenced in both forward and reverse directions. The sequencing results for individual isoforms were the same, except for occasional indeterminate nucleotides. These templates were resequenced in both directions to identify the ambiguous nucleotide. Comparisons between subcloned cDNA products and previously identified PMCA sequences were carried out with GCG (Genetics Computer Group, Madison, WI) and GeneWorks (IntelliGenetics, Mountain View, CA) software.
Northern Analysis
2 μg of mouse kidney mRNA (Clontech) and 10 μg of total RNA from each proximal tubule cell line RNA were electrophoresed on a 1% agarose/formaldehyde gel and electrophoretically transferred overnight to GeneScreen Plus Membrane (Dupont NEN, Wilmington, DE). The blots were prehybridized in a solution of 1 M NaCl, 1% SDS, and 10% dextran sulfate for 60 min at 60°C, then probed with 2 × 106 cpm/ml of the randomly primed (Prime-it II Kit; Stratagene), [32P]dCTP-labeled (ICN Pharmaceuticals, Inc., Costa Mesa, CA) mouse PMCA1, 2, 3, or 4 PCR cDNA product. The blots were washed at high stringency with 50 ml 2× SSC (sodium chloride/sodium citrate), 0.1% SDS three times at room temperature, then with 0.1× SSC, 0.1% SDS three times at 60°C and exposed to X-AR film (Eastman Kodak Co., Rochester, NY) for 1–4 d at −70°C.
Free Intracellular Ca2+
Single S1, S2, S3, or DCT cells were analyzed for changes of free intracellular Ca2+ concentration, [Ca2+]i, with the Ca2+-sensitive dye, fura-2 AM as described previously (Bacskai and Friedman, 1990; White et al., 1996a ). The cell assay buffer consisted of: 140 mM Na+, 148 mM Cl−, 5 mM K+, 1 mM Ca2+, 1 mM Mg2+, 28 mM HEPES, 18 mM Tris, with 10 mM glucose at pH 7.4 and adjusted to 295 mosmol/kg H2O. The Na+-free assay buffer was prepared similarly except Na+ was replaced isosmotically by tetramethyl-ammonium (TMA). Proximal tubule cells on glass cover slips were incubated for 60 min at 37°C with 10 μM fura-2 AM (Molecular Probes, Inc., Eugene, OR). The cells were rinsed several times and placed in a temperature-controlled chamber set at 37°C and mounted on the stage of an inverted microscope (Nikon Diaphot; Nikon, Inc., Melville, NY). Emitted fluorescence was measured with a Nikon Photoscan-2. The experimental protocol consisted of an initial 2-min baseline period, 15-min incubation with the cell assay buffer, 5 min with Na+-free assay buffer, 10 min wash with control buffer, and finally 5 min in Na+-free, Ca2+-free assay buffer, followed by calibration as previously described (Gesek and Friedman, 1992). All experimental solutions contained ouabain (10−3 M) to inhibit the Na+/K+ ATPase and nifedipine (10−5 M) to block Ca2+ entry through Ca2+ channels.
Membrane Preparation and Western Analysis
Plasma membranes were isolated as follows: Six million proximal tubule cells or primary cultures of distal tubule cells were washed three times with 1× Hank's balanced salt solution in the presence of the protease inhibitors PMSF, EDTA, leupeptin, and pepstatin A (Sigma Chemical Co.). The cells were then sonicated for 10 s at 60% maximum output (Sonifier cell disrupter 450; Branson Ultrasonics Corp., Danbury, CT). The suspensions were transferred to 1.5 ml Eppendorf tubes and centrifuged at 500 g for 10 min. The supernatant was transferred to fresh tubes and centrifuged at 10,000 g for 20 min. The pellets were resuspended in 50 μl of 1× PBS buffer. A Lowry protein assay was performed on the resulting membranes using BSA as a standard.
50 μg of membranes from the mouse proximal tubule cell lines and primary cultures of distal tubule cells were electrophoresed at 20 mA (Hoefer Scientific, San Francisco, CA) on a 7.5% polyacrylamide gel (SDS-PAGE). Prestained markers (Bio-Rad Laboratories, Richmond, CA) were electrophoresed in parallel and used for protein mass determination. The protein was transferred to nitrocellulose (Bio-Rad Laboratories) in a transfer apparatus (Hoefer Scientific) for 1.5 h at 400 mA.
After blocking for 18 h in 5% BLOTTO (Carnation Instant Non-Fat Dry Milk in 1× Tris-buffered saline, pH 7.4) at 4°C, the blot was probed with a 1:1,000 dilution of an mAb raised to purified human PMCA, Clone 5F10 (Borke et al., 1989) (Affinity Bioreagents, Inc., Golden, CO) or a polyclonal antibody specific for the Na+/Ca2+ exchanger (Reilly et al., 1993), kindly provided by Dr. Robert Reilly (Yale University, New Haven, CT), in 1% BLOTTO for 90 min at room temperature. The PMCA blots were then incubated with a 1:3000 dilution of a horseradish peroxidase (HRP)-labeled goat anti–mouse IgG (Bio-Rad Laboratories). For the NCX1, the blots were incubated in a 1:500 dilution of HRP-labeled goat anti–guinea pig IgG in 1% BLOTTO for 60 min at room temperature. Blots were developed by enhanced chemiluminescence according to the manufacturer's (Amersham Corp., Arlington Heights, IL) instructions using Kodak X-OMAT (Eastman Kodak, Co.) film.
results
Analysis of PMCA Transcripts in Proximal Tubule Cells
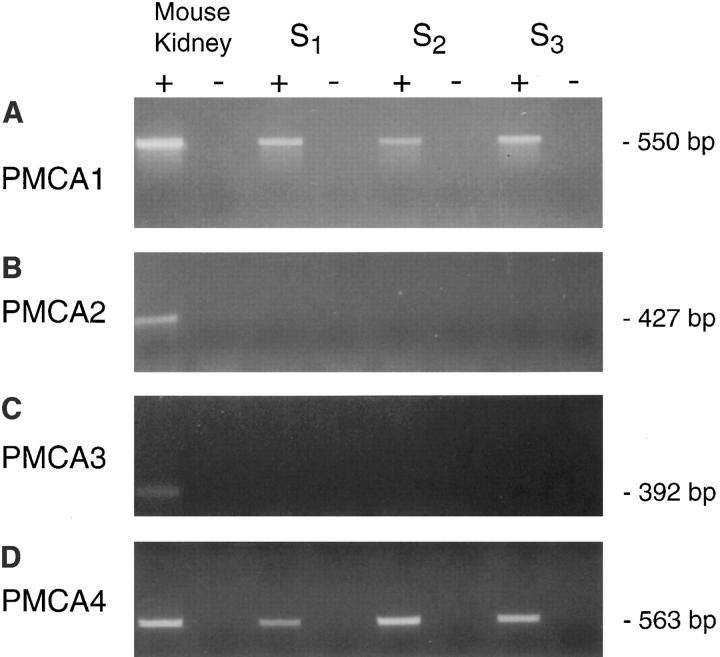
RNA isolated from the proximal tubule S1, S2, and S3 cell lines was reverse-transcribed and the resulting cDNA was amplified by PCR in separate experiments using oligodeoxynucleotide primers specific (Meszaros and Karin, 1993; Stauffer et al., 1993; Abramowitz et al., 1995) for the four PMCA isoforms (sequences given in Table I). RT-PCR was performed on at least two RNA samples for each primer set and yielded identical results. PMCA1 primers resulted in a product of ∼550 bp from S1, S2, and S3 cells and from mouse kidney, which served as a positive control (Fig. 1 A, +). Using the same RNA samples, different results were obtained with primers for PMCA2 and 3. The primers specific for PMCA2 (Fig. 1 B) and PMCA3 (Fig. 1 C) were uniformly negative for S1, S2, and S3 cells but the positive control revealed appropriately sized products from kidney of 427 and 392 bp, respectively. The primers specific for PMCA4 revealed an appropriately sized product of 563 bp in all proximal tubule cells, as well as in mouse kidney (Fig. 1 D). For all primer sets and RNAs, samples analyzed in the absence of RT showed no products (Fig. 1, A–D, −). These results support the view that cell lines derived from S1, S2, and S3 proximal tubule segments express transcripts encoding PMCA1 and PMCA4.
Figure 1.
Analysis of PMCA expression by RT-PCR in S1, S2, and S3 proximal tubule cells. RT-PCR was used to amplify specifically transcripts encoding each known PMCA isoform. For all PMCA primer sets, the + sign designates samples analyzed in the presence of reverse transcriptase; – indicates samples analyzed in the absence of RT. Product size is shown on the right. Appropriately sized products were amplified from mouse kidney RNA in the presence of RT by all primer sets and RNA analyzed in the absence of RT showed no products. Only products consistent with PMCA1 and PMCA4 were amplified from proximal tubule cells. Primer sequences are listed in Table I.
Sequence Analysis of Proximal Tubule PMCA PCR Products
To confirm the identity of the proximal tubule PMCA PCR products amplified by each primer pair, the cDNAs from at least two independent reactions (see materials and methods) were sequenced. The mouse partial clones were found to have high similarity to rat PMCAs in their respective regions, consistent with reports of high conservation of PMCA isoforms across species (Keeton and Shull, 1995). The amino acid sequence alignments for each mouse PCR product and its corresponding rat homologue is shown in Fig. 2. The mouse proximal tubule PMCA1 product was identical to the published (Shull and Greeb, 1988) rat PMCA1 sequence (Fig. 2 A). PMCA2 and PMCA3 products were 98 and 100% identical to rat PMCA2 and PMCA3 (Fig. 2, B and C) sequences, respectively, in their corresponding regions. In addition, the PMCA4 PCR product shares 92% similarity to the cloned rat PMCA4 cDNA sequence (Fig. 2 D). Therefore, sequence analysis confirms that the mouse PMCA isoforms are highly homologous to the rat isoforms within the amplified regions, and also demonstrates that the primers used for RT-PCR are specific for each isoform.
Figure 2.
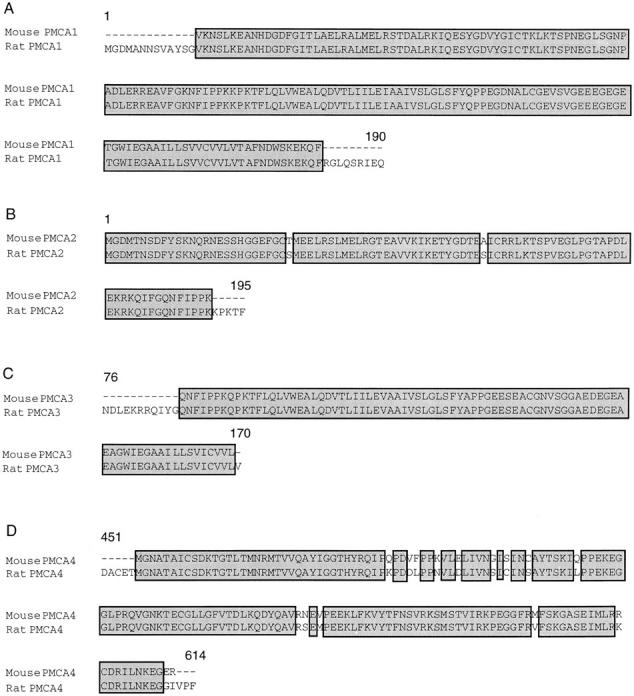
Sequence comparisons of mouse kidney PMCA RT-PCR products. The mouse proximal tubule PMCA RT-PCR products were sequenced. The cDNA sequence was translated and the one-letter amino acid code is shown. Each mouse PMCA isoform is compared with its previously identified rat homologue having the following GenBank accession numbers: J03753 (PMCA1) (Shull and Greeb, 1988), J03754 (PMCA2) (Shull and Greeb, 1988), J05087 (PMCA3) (Greeb and Shull, 1989), U15408 (PMCA4) (Keeton and Shull, 1995). The numbers at the beginning and at the end of each sequence pair indicate the amino acid positions of the corresponding residues within the cloned rat sequences. (A) PMCA1 shares 100% similarity with rat PMCA1 sequences; (B) PMCA2 shares 98% similarity; (C) PMCA3 100%; and (D) PMCA4 92%. Boxed regions indicate regions of exact similarity between mouse and rat sequences.
Northern Analysis of Proximal Tubule PMCA
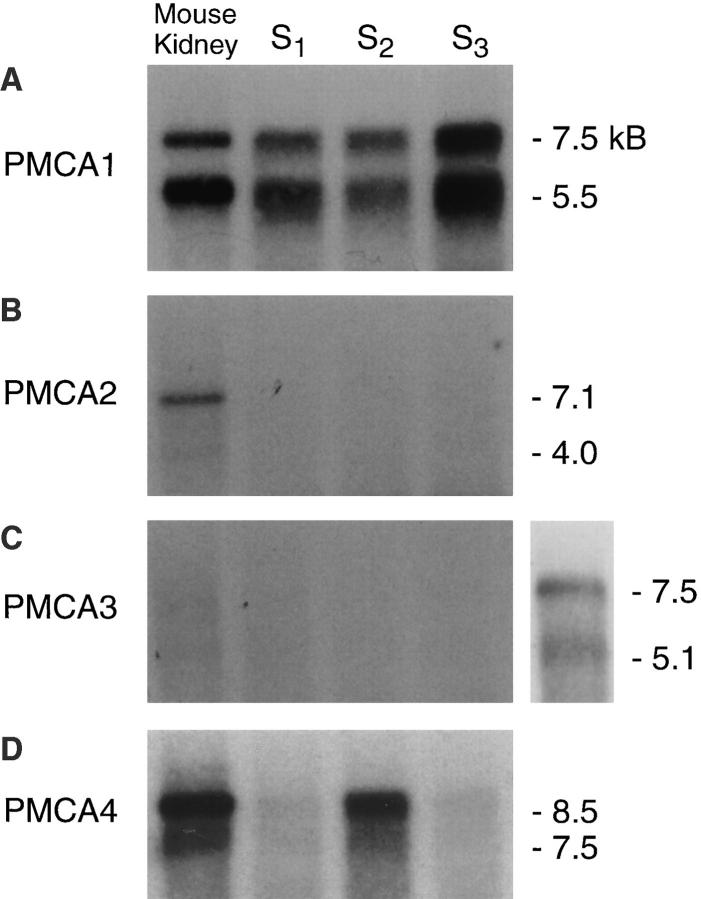
Northern analysis was performed to determine the sizes of the PMCA mRNAs in proximal tubule cells. The mouse PMCA1, 2, 3, and 4 PCR products were randomly primed, [32P]dCTP-labeled, and used as cDNA probes for the analysis of separate blots. The PMCA1 probe strongly hybridized with two distinct transcripts of 7.5 and 5.5 kb in mouse kidney mRNA (Fig. 3 A, Mouse Kidney) and with RNA from S1, S2, and S3 cell lines (Fig. 3 A, lanes S1 –S3). The probe specific for PMCA4 hybridized with 8.5 and 7.5 kb transcripts from both mouse kidney and the proximal cells (Fig. 3 D). Although the origin of multiple bands on the Northern blots is not known, these transcripts show doublets similar to those previously reported for PMCA1 and PMCA4 in other tissues (Kumar et al., 1993; Keeton and Shull, 1995). Neither PMCA2 nor PMCA3 was observed in the proximal tubule cell lines (Fig. 3, B and C). Mouse kidney mRNA exhibited PMCA2 transcripts of 7.1 and 4 kb (Fig. 3 B). PMCA3 was faintly detected in mouse kidney (Fig. 3 C). To assure that the PMCA3 cDNA probe was capable of hybridizing with PMCA3 transcripts, mouse brain mRNA was used as a positive control and revealed the presence of appropriately sized mRNAs of 7.5 and 5.1 kb (Fig. 3 C, lane 5), consistent with reported mRNA transcript sizes for rat skeletal muscle and brain (Burk et al., 1995). Therefore, molecular evidence provided by RT-PCR and Northern analysis support the idea that the dominant mRNAs in proximal tubule cells are PMCA1 and PMCA4.
Figure 3.
Northern analysis of proximal tubule cell RNA. The mouse kidney PMCA1-PMCA4 PCR products were randomly primed, [32P]dCTP-labeled, and used to probe 10 μg of mouse proximal tubule cell RNA and 2 μg of mouse kidney mRNA. For all Northern analyses, lane 1, mouse kidney mRNA; lane 2, S1 RNA; lane 3, S2 RNA; and lane 4, S3 RNA. (A) PMCA1 probe hybridizes with transcripts of 7.5 and 5.5 kb in all samples; (B) PMCA2 probes hybridize with a transcript of 7.1 and 4 kb in kidney mRNA; (C) PMCA3 probes hybridize with transcripts of 7.5 and 5.1 kb in mouse brain mRNA (lane 5); and (D) PMCA4 probes detect transcripts of 8.5 and 7.5 kb in all samples. Transcripts detected are appropriately sized for each PMCA isoform.
Analysis of NCX1 Na+/Ca2+ Exchanger Transcripts in Proximal Tubule Cells
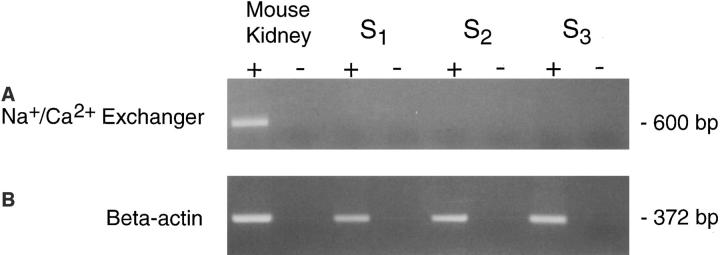
RT-PCR was performed on RNA isolated from the S1, S2, and S3 cell lines to determine if proximal tubule cells express transcripts encoding the NCX1 Na+/Ca2+ exchanger. The NCX1 primers target conserved portions of the rat exchanger transcript and are known to amplify a region of alternative splicing from mouse kidney mRNA (Kofuji et al., 1994; White et al., 1996a ). A band of the predicted size, 600 bp, was obtained from mouse kidney RNA analyzed in the presence of RT (Fig. 4, Mouse Kidney +). Mouse kidney RNA samples analyzed in the absence of RT (Fig. 4, −) failed to generate products indicating that the observed product arose from amplification of mRNA and not genomic DNA. All three proximal cell lines were negative for NCX1 (Fig. 4, S1 –S3). β-actin mRNA was amplified by RT-PCR as a control to verify that the RNA samples were intact. All proximal tubule RNA samples yielded an appropriately sized product of 372 bp (Fig. 4). Since the β-actin primers span an intron, the product was derived from RNA because genomic DNA would yield an expected product of 790 bp (Nakajima-Iijima et al., 1985). These results demonstrate that, within experimental error, the proximal tubule cell lines do not express the NCX1 transcript.
Figure 4.
Analysis of Na+/Ca2+ exchanger transcripts in proximal tubule cells. RT-PCR was used to assess exchanger transcript expression in proximal tubule cells as described in the text. The products were visualized on an ethidium bromide– stained agarose gel. (A)lane 1, mouse kidney mRNA; (+RT); lane 2, mouse kidney mRNA (−RT); lane 3, S1 RNA (+RT); lane 4, S1 RNA (−RT); lane 5, S2 RNA (−RT); lane 6, S2 RNA (−RT); lane 7, S3 RNA (+RT); lane 8, S3 RNA (−RT). Only mouse kidney displays a product of the appropriate size, 600 bp, whereas the proximal tubule cell lines are negative. (B) β-actin RT-PCR performed on the same samples used for analysis of exchanger expression. All samples yielded a product of the appropriate size, 372 bp, indicating that the RNA was intact.
Analysis of PMCA and Na+/Ca2+ Exchanger Protein Expression in Proximal Tubule Cells
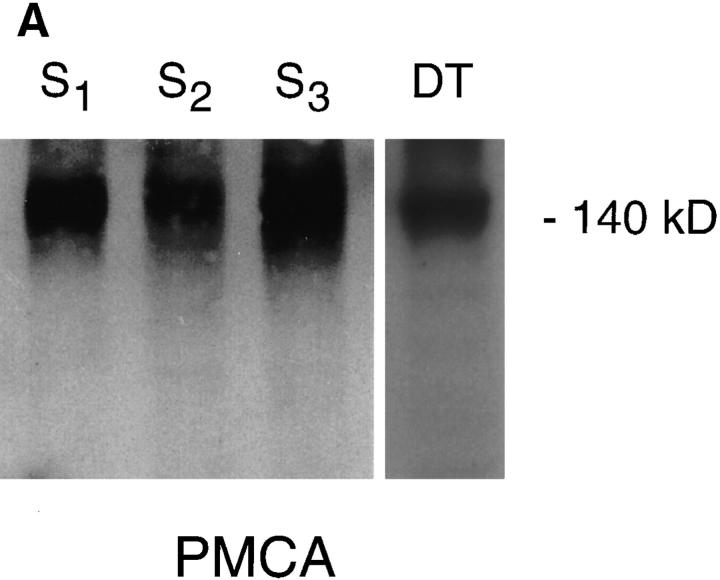
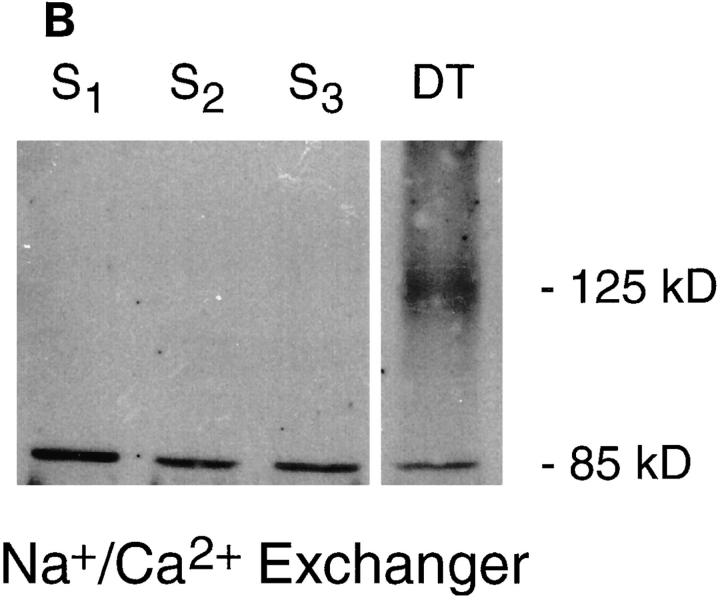
To assess protein expression of PMCA and Na+/Ca2+ exchanger Western analysis was performed on membrane preparations from the S1, S2, and S3 proximal tubule cell lines and primary cultures of mouse kidney distal tubule cells. PMCA was examined using an mAb that recognizes the highly conserved hinge region the enzyme (Borke et al., 1989), whereas the exchanger was assessed with an NCX1-specific polyclonal antibody, raised against the large intracellular loop of the rabbit kidney exchanger (Reilly et al., 1993). In agreement with the reported PMCA protein molecular mass (Borke et al., 1989), membrane preparations from the three mouse proximal tubule cell lines (Fig. 5 A, S1–S3) and primary cultures of mouse kidney distal tubule cells (Fig. 5 A, DT) show a single, strongly reacting band of 140 kD.
Figure 5.
Analysis of PMCA and Na+/Ca2+ exchanger protein expression in proximal tubule cells. Western analysis was performed on proximal tubule cell membrane preparations. Lane 1, S1 membranes; lane 2, S2; lane 3, S3; and lane 4, primary cultures of mouse distal tubule cells. The same preparations were probed with either: (A) an mAb against PMCA, which revealed a reacting protein of ∼140 kD; or (B) a polyclonal antibody raised against a fusion protein encoding the rabbit kidney exchanger. Bands of 125 and 85 kD, were seen in the positive control, distal tubule cells, whereas the proximal tubule cell membrane preparations revealed only an 85-kD band.
The same membrane preparations used for PMCA analysis were probed in parallel with an anti-Na+/Ca2+ exchanger antibody. The anti-exchanger antibody detected no reacting proteins in proximal tubule S1, S2 or S3 membrane preparations at 125 kD, the molecular mass of the mature form of the exchanger (Nicoll et al., 1990). A lower band of ∼85 kD was observed (Fig. 5 B, S1–S3). Membranes isolated from primary cultures of distal tubule cells, which are known to express the exchanger (White et al., 1996a ), were used as positive control. A strongly reacting protein was observed at ∼125 kD, the reported size of the mature exchanger protein (Reilly et al., 1993) (Fig. 5 B, DT), along with a lower band at 85 kD. The 85 kD band is hypothesized to be a proteolytic fragment of the mature exchanger protein (Nicoll et al., 1990) but its exact identity has yet to be determined. Anti-Na+/Ca2+ exchange pre-immune controls were shown previously to be negative for distal tubule cell membrane preparations (White et al., 1996a ). Because NCX1 transcripts were not detected in S1, S2, or S3 cells, it is unlikely that the lower weight band is a breakdown product of the NCX1 exchanger. Therefore, PMCA protein is expressed in plasma membranes of proximal tubule cells. However, within experimental error, proximal tubule cells do not express detectable levels of the mature NCX1 Na+/Ca2+ exchanger protein.
Functional Na+/Ca2+ Exchange Activity in Proximal Tubule Cells
Although analysis of NCX1 protein in proximal tubule cell plasma membranes, as described above, was negative for the mature form of the exchanger, functional Na+/Ca2+ exchange activity below the level of detection by Western blotting cannot be ruled out. Therefore we used an approach to analyze Na+/Ca2+ exchange in S1, S2, and S3 proximal tubule cells in a manner similar to that described elsewhere (Dai et al., 1996; White et al., 1996a ).
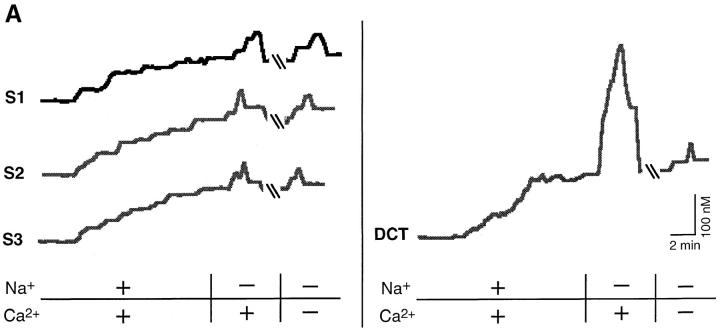
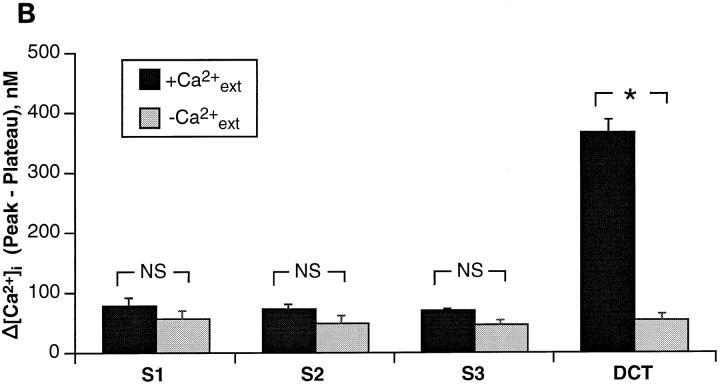
Under prevailing conditions of [Ca2+]i and membrane voltage basolateral Na+/Ca2+ exchange operates in the forward mode, coupling the influx of Na+ ions down their electrochemical gradient to the efflux of Ca2+ ions against a steep electrochemical barrier (Friedman and Gesek, 1995). The exchanger is reversible and can operate in the opposite mode, wherein sodium efflux energizes calcium uptake. This can be achieved experimentally by loading cells with Na+ and then isosmotically replacing extracellular Na+ (Goldman et al., 1994; Dai et al., 1996; White et al., 1996a ). Changes of [Ca2+]i in the presence and absence of extracellular Ca2+ were measured in paired experiments in single cells with the Ca2+-sensitive dye, fura-2. Representative traces from each cell type are shown in Fig. 6 A, and the results are summarized in Table II. Resting [Ca2+]i was ∼110 nM in all cell types. After a 15-min incubation with ouabain, to inhibit the Na+/K+ ATPase and thereby increase intracellular Na+, reversal of the Na+ gradient in the presence of extracellular Ca2+ caused 70–75 nM increases of [Ca2+]i in S1, S2, and S3 cells. In the absence of external Ca2+, [Ca2+]i increased by 50–58 nM. The elevation of [Ca2+]i was not significantly different in the presence or absence of external Ca2+ (Fig. 6 B). Thus, it is unlikely that the majority of the rise of [Ca2+]i in proximal cells was due to the entry of Ca2+ and presumably resulted from the release of calcium from cytoplasmic organelles (White et al., 1996a ). In contrast, DCT cells exhibited average rises of [Ca2+]i of 369 nM in the presence of extracellular Ca2+ and 54 nM in the absence of extracellular Ca2+ (Table II), as reported previously (White et al., 1996a ). These latter findings are consistent with the view that the majority of the rise of [Ca2+]i in DCT cells is due to Ca2+ entry from the extracellular buffer.
Figure 6.
Analysis of Na+/Ca2+ exchange in single S1, S2, and S3 cells. To assess Na+/Ca2+ exchange activity, proximal tubule cells were loaded with the Ca2+-sensitive dye fura-2 and single cells were assayed by measuring [Ca2+]i upon reversal of the electrochemical gradient for Na+ by isosmotic replacement of extracellular Na+ in the presence or absence of external Ca2+ in paired analysis on the same cell. DCT cells served as the positive controls. (A) Representative traces are shown for each cell type analyzed. Slash marks in the traces indicate a ten minute wash with control buffer. All proximal cells showed similar rises of [Ca2+]i in the presence and absence of external Ca2+. However, the DCT cell exhibited a large rise of [Ca2+]i in the presence of external Ca2+ but only a minimal rise in the absence of external Ca2+. (B) The difference between the peak and plateau [Ca2+]i in the presence or absence of external Ca2+ was not significantly different for all proximal lines. In contrast, the control DCT cell showed a large rise of [Ca2+]i in the presence of external Ca2+, and a rise similar to that of proximal cells in the absence of external Ca2+ (P < 0.01). Each bar represents the mean ± SEM of three independent experiments performed on each cell type.
Table II.
Na+-dependent increases of [Ca2+]i in the Presence and Absence of Extracellular Calcium
| nM | Δ[Ca2+]i, nM | |||||
| S1 | 113 ± 2 | 79 ± 12* | 54 ± 13 | |||
| S2 | 111 ± 1 | 75 ± 6* | 58 ± 12 | |||
| S3 | 119 ± 3 | 70 ± 4* | 50 ± 11 | |||
| DCT | 108 ± 9 | 369 ± 17‡ | 54 ± 11 | |||
[Ca2+]ext = 0mM
discussion
In this report experimental evidence is provided for the presence of multiple isoforms of the plasma membrane Ca2+-ATPases (PMCA1 and PMCA4) in S1, S2, and S3 proximal tubule cells. By contrast, we were unable to detect NCX1 Na+/Ca2+ exchanger transcripts, protein, or Na+/Ca2+ exchange activity in these proximal tubule cell lines.
PMCA in Proximal Tubule Cells
Proximal tubule cells express mRNA for multiple isoforms of PMCA. Using RT-PCR, we show that clonally expanded S1, S2, and S3 proximal tubule cell lines express transcripts encoding PMCA1 and PMCA4 (Fig. 1, A and D). Northern analysis performed on RNA isolated from the proximal cells using specific cDNA probes provides further evidence that multiple isoforms are present in these cells (Fig. 3). Appropriately sized transcripts for PMCA1 and PMCA4 were detected in all proximal tubule cell lines, however PMCA2 and PMCA3 were not detected in the same RNA samples, consistent with the RT-PCR experiments targeting individual PMCA isoforms (Fig. 3). The positive control, mouse kidney RNA, strongly hybridized with all cDNA probes except PMCA3, confirmed by mouse brain RNA (Fig. 3 C), indicating that the probes were capable of detecting specific transcripts. Therefore, molecular evidence provided by RT-PCR and Northern analysis support the idea that the dominant mRNAs in proximal tubule cells are PMCA1 and PMCA4. UMR-106 osteosarcoma cells, which express PMCA1, 2, and 4 (Abramowitz et al., 1995), and cultured pancreatic β cells, which contain PMCA1, 2, and 4 transcripts (Varadi et al., 1996), have also been demonstrated to exhibit multiple PMCA isoforms. The consequence of expressing multiple PMCA isoforms in a single cell type is not known. However by varying isoform structure in modifying domains, and thus varying sensitivities to calmodulin and phospholipids, the PMCA isoforms could be differentially regulated (Strehler, 1991), thereby affecting cellular calcium homeostasis or transcellular calcium transport.
Studies of PMCA mRNA levels in human and rat kidney reveal that PMCA1 and PMCA4 are the dominant renal transcripts, whereas PMCA2 mRNA represents <2% of the total PMCA mRNA in the kidney; PMCA3 was virtually undetectable (Stauffer et al., 1993). Subsequent work substantiated these findings at the protein level using Western analysis with PMCA isoform-specific antibodies (Stauffer et al., 1995). The present findings are consistent with these earlier results (Stauffer et al., 1993). However, an analysis of microdissected rat nephron segments by RT-PCR concluded that PMCA2 was the dominant isoform in proximal convoluted tubules, cortical thick ascending limbs, distal convoluted tubules, and cortical collecting ducts (Magocsi et al., 1992). The mRNA for PMCA1 was present in glomeruli but absent in tubular segments; PMCA3 was not found. In the present study PMCA2 was undetectable in S1, S2, or S3 proximal tubule cells by RT-PCR or by Northern analysis (Figs. 1 and 3). Several reasons can be envisioned for the discrepancies regarding localization of PMCA isoform transcripts in proximal tubule cells between the present work and previous studies. These differences may stem, in part, from the experimental procedures employed. To localize PMCA isoforms in cortical nephron segments, Magocsi et al.(1992) performed RT-PCR with isoform specific primers on microdissected nephron segments. Since PMCAs are expressed ubiquitously in both tubular epithelial cells and in nonepithelial cells, the source of the specific mRNA amplified is critical. Contamination of excised tubule segments by adjoining nephron segments, nervous tissue, fibroblasts, and connective tissue may occur, thereby confounding interpretation. This is of particular concern with a procedure such as RT-PCR. Moreover, the cDNA products resulting from the RT-PCR reactions were not sequenced, thereby precluding verification of their identity. Instead, product specificity was based upon predicted sizes of PCR products and PCR Southern analysis. Because of the high degree of homology between PMCA isoforms, however, PCR products may be of similar sizes regardless of the isoforms amplified. Also, Southern analysis with cDNA probes may cross-hybridize between isoforms. This possibility was not ruled out by testing individual probes against cloned PMCA cDNAs as controls. Finally, the PMCA4 cDNA sequence was not identified at the time of the Magocsi publication, therefore confirmation of cross-reaction with this transcript could not be established. In the present work these problems were circumvented by: using primers known to be specific for individual PMCA isoforms; isolating RNA from characterized, clonal cell lines to avoid contamination; sequencing cDNA reaction products to provide absolute confirmation of their identity; and including appropriate control RNAs in Northern analysis to verify proper PMCA transcript presence and size. Thus, the absence of PMCA2 transcripts in proximal tubule cells is unlikely to involve failure of the PMCA2 primers to amplify the transcript because whole mouse kidney mRNA gave a product of the appropriate size (Fig. 1 B) and its identity was confirmed by sequence analysis (Fig. 2 B). In addition, mouse kidney was positive by Northern analysis for PMCA2, but negative in the cell lines (Fig. 2 B). The possibility exists, of course, that PMCA2 and PMCA3 transcripts may be expressed at levels in proximal cells below the limits of resolution by RT-PCR and Northern analysis. Furthermore, the absence of PMCA2 and PMCA3 transcripts by RT-PCR or Northern analysis is not attributable to compromised RNA integrity since β-actin mRNA was amplified from all samples and PMCA1 and PMCA4 were amplified from the same samples that were negative for PMCA2 and PMCA3. Selective downregulation of the PMCA2 transcript in cultured proximal tubule cell lines as a consequence of the transformation with SV40 cannot be excluded and could potentially contribute to or account for its apparent absence. Also, species-specific expression of the PMCA isoforms may account for the failure to find PMCA2 in mouse proximal tubule cells.
In addition to the molecular evidence supporting the presence of PMCA mRNA in proximal tubule cell lines, protein expression of PMCA was analyzed. Application of an mAb directed against the hinge region of the human PMCA (Borke et al., 1989) confirmed the presence of PMCA protein in plasma membranes of proximal tubule cells (Fig. 5). Membrane preparations from primary cultures of distal tubule cells, derived from nephron regions known to express PMCA (Borke et al., 1987), revealed a band of similar mass when analyzed in parallel with proximal cell lines (Fig. 5 A). This finding supports the view that the reacting protein is a PMCA. The antibody used in Western analysis in this report does not distinguish between the four PMCA isoforms (Borke et al., 1989). Although transcripts encoding two isoforms, PMCA1 and PMCA4 were detected, the appearance of a single band upon Western analysis is most likely due to the similar mass and migration of PMCA isoforms when separated by SDS-PAGE (Stauffer et al., 1995). The presence of PMCA protein in proximal tubule cells is not entirely consistent with other reports, where PMCA was immunolocalized only to distal portions of the human (Borke et al., 1987) and the rat (Borke et al., 1989) nephron. This apparent discrepancy may result from lower abundance of proximal tubule PMCA, below the level of detection by immunocytochemical methods. This possibility is supported by evidence showing reduced PMCA activity in proximal tubules compared to distal tubule segments in both rat and rabbit nephrons (Doucet and Katz, 1982; Ramachandran and Brunette, 1989; Ramachandran et al., 1991). Moreover, associated proteins that mask the antigenic epitope of PMCA in vivo may be present in proximal tubule cells, since intracellular proteins are known to interact with the enzymes (Enyedi et al., 1989). During SDS-PAGE the plasma membranes are denatured, and the antibody recognition sites could be revealed. Therefore, readily detectable amounts of PMCA protein are present in membrane preparations from the S1, S2, and S3 cell lines regardless of segment origin, supporting the molecular identification of PMCA transcripts in these cells.
Na+/Ca2+ Exchange in Proximal Tubule Cells
The presence of Na+/Ca2+ exchange in proximal tubules is uncertain and controversial. Evidence supporting the presence of Na+/Ca2+ exchange derives primarily, though not entirely, from functional studies (Ullrich et al., 1976; Gmaj et al., 1979; Lee et al., 1980; Friedman et al., 1981; Lorenzen et al., 1984; Yang et al., 1988; Dominguez et al., 1991, 1992), whereas molecular structural experiments (Ramachandran and Brunette, 1989; Yu et al., 1992; Bourdeau et al., 1993; Reilly et al., 1993) have generally failed to confirm its presence. In the present studies we applied both a structural and functional strategy to evaluate the presence of Na+/Ca2+ exchange in proximal tubule cells.
Examination of primary cultures of distal tubule cells using a characterized NCX1 exchanger polyclonal antibody (Reilly et al., 1993) revealed a protein of 125 kD, consistent with the molecular mass of the processed, mature exchanger protein (Nicoll et al., 1990; Reilly et al., 1993). In contrast, all three proximal tubule cell lines were negative for the mature exchanger (Fig. 5 B). The membrane preparations analyzed however, revealed a reacting protein of 85 kD (Fig. 5 B), similar in size to a reported proteolytic fragment (Philipson et al., 1988; Vemuri et al., 1990). Since proximal tubule S1, S2, and S3 cells apparently do not express NCX1 (Figs. 4–6), the smaller band is unlikely to be a breakdown product of the mature exchanger. By virtue of the fact that the anti-exchanger antibody is polyclonal, the 85 kD band could be a protein with a similar antigenic epitope to that present in the NCX1 exchanger since the protein does not appear to react with preimmune sera (White et al., 1996a ). Further analysis of the 85 kD protein will be required to make a definitive statement as to its identity. The results of the studies described herein support the idea that NCX1 is absent from mouse proximal tubule cells. These results (Fig. 5 B) and conclusion differ from studies where the protein was reported in proximal tubule preparations (Dominguez et al., 1992). The present results do not rule out the possibility of species-specific expression of NCX1 in proximal tubule cells.
Independent functional assessment of Na+/Ca2+ exchange using experimental protocols that definitively revealed Na+-dependent calcium transport in DCT cells (Fig. 6 and White et al., 1996a ) and UMR-106 osteoblast cells (White et al., 1996b ) failed to uncover significant exchange activity in S1, S2, or S3 cells (Fig. 6, Table II). Nonetheless, the rise of [Ca2+]i in the presence of external Ca2+ in proximal cells was slightly greater (∼15–20 nM) than in the absence of Ca2+ (Table II). This modest difference could be due to a slightly greater release of intracellular Ca2+ stores in the presence of external Ca2+. Alternatively, modest exchange activity may be present in proximal tubule cells but not readily demonstrable under the imposed experimental conditions. Nonetheless, the majority of the increase clearly was not due to calcium entry, consistent with the interpretation that the residual rise of [Ca2+]i is due to release from subcellular organelles and does not result from Na+/Ca2+ exchange.
Summary and Conclusion
Although an appreciable portion of proximal calcium absorption involves a cellular transport pathway, little is known of the mechanisms by which calcium enters or exits these cells. The present work focused on transport mechanisms in basolateral plasma membranes. In principle, either or both a PMCA and Na+/Ca2+ exchanger might mediate efflux. We provide evidence for the presence of two PMCA isoforms (PMCA1 and PMCA 4) in S1, S2, and S3 cells. Data showing that PMCA protein is expressed by these cells is also provided. Conversely, we were unable to find evidence for Na+/Ca2+ exchange, NCX1 exchanger transcripts or protein in proximal tubule cells. Apparent K ms for calcium for PMCA in kidney are 0.1–0.4 μM (Doucet and Katz, 1982; van Heeswijk et al., 1984; Ramachandran et al., 1991) and indistinguishable in proximal and distal tubule basolateral membranes (Doucet and Katz, 1982; Ramachandran et al., 1991). The Na+/Ca2+ exchanger has apparent K ms for calcium of 15–40 μM and of 1–2 μM (Philipson, 1985). In the kidney, the K m has been reported to be 0.1 μM (van Heeswijk et al., 1984). These findings would suggest that basal [Ca2+]i may be controlled, in part, by efflux mediated by PMCA1 or PMCA4, which exhibit K ms similar to the resting [Ca2+]i (Friedman and Gesek, 1995). Possible roles of Na+/ Ca2+ exchange in proximal tubule cells are more difficult to assign on the basis of the present work, which within experimental error, failed to adduce evidence for the expression of NCX1 transcripts or functional exchange activity in three proximal tubule cell lines. It could be argued that proximal tubules express Na+/ Ca2+ exchangers, but such expression and activity is downregulated in the immortalized cells studied herein. Although some evidence supporting functional Na+/Ca2+ exchange in proximal cells is compelling (Lee et al., 1980; Lorenzen et al., 1984), rigorous tests involving collapse of the Na+ gradient to determine if Ca2+ efflux was coupled directly to Na+ entry through the Na+/Ca2+ exchanger were not performed. Furthermore, the kinetic measurements would suggest that even if present in proximal tubules, Na+/Ca2+ exchange is unlikely to play a vital role in regulating resting or stimulated levels of [Ca2+]i. The present results, however, do not definitively rule out the possibility that basolateral Ca2+ efflux pathways are differentially expressed along the proximal nephron or that they may differ between species. Furthermore, cell transformation may affect the pattern of expression and might theoretically explain the failure to detect the NCX1 exchanger. These arguments notwithstanding, it is possible that proximal tubules express an alternate NCX gene product or a different form of exchanger, such as the Na+/Ca2+, K+ exchanger. Although to our knowledge this hypothesis has not been explicitly tested, Windhager (Milovanovic et al., 1991) noted that, when expressed in Xenopus oocytes, Ca2+ uptake mediated by the renal Na+/Ca2+ exchange was stimulated by K+.
Acknowledgments
These studies were supported by National Institutes of Health grants R01 GM-34399, R01 ES-05860 (P.A. Friedman), T32 DK-07301 (K.E. White), and R01 AR-27032 (M.K. Drezner).
Footnotes
This work was submitted in partial fulfillment of the requirements for a Ph.D. by Kenneth E. White, who was a predoctoral Fellow of the Albert J. Ryan Foundation during the performance of these studies.
Abbreviations used in this paper: DCT, distal convoluted tubule; DT, distal tubule; PMCA, plasma membrane Ca2+-ATPase; RT, reverse transcriptase.
The term “distal tubule cells” is used to describe the mixed population of cortical ascending limb and distal convoluted tubule cells that are isolated on the basis of expression of surface Tamm-Horsfall protein (Pizzonia et al., 1991) and grown in primary cell culture.
references
- Abramowitz J, Gonzalez JM, Rouse D, Suki WN. Differential expression of plasma membrane calcium pump mRNA isoforms in rat osteoblast-like cells. Miner Electrolyte Metab. 1995;21:367–374. [PubMed] [Google Scholar]
- Almeida AR, Bunnachak D, Burnier M, Wetzels JF, Burke TJ, Schrier RW. Time-dependent protective effects of calcium channel blockers on anoxia- and hypoxia-induced proximal tubule injury. J Pharmacol Exp Ther. 1992;260:526–532. [PubMed] [Google Scholar]
- Bacskai BJ, Friedman PA. Activation of latent Ca2+channels in renal epithelial cells by parathyroid hormone. Nature (Lond) 1990;347:388–391. doi: 10.1038/347388a0. [DOI] [PubMed] [Google Scholar]
- Bomsztyk K, George JP, Wright FS. Effects of luminal fluid anions on calcium transport by proximal tubule. Am J Physiol. 1984;246:F600–F608. doi: 10.1152/ajprenal.1984.246.5.F600. [DOI] [PubMed] [Google Scholar]
- Borke JL, Caride A, Verma AK, Penniston JT, Kumar R. Plasma membrane calcium pump and 28-kDa calcium binding protein in cells of rat kidney distal tubules. Am J Physiol. 1989;257:F842–F849. doi: 10.1152/ajprenal.1989.257.5.F842. [DOI] [PubMed] [Google Scholar]
- Borke JL, Minami J, Verma A, Penniston JT, Kumar R. Monoclonal antibodies to human erythrocyte membrane Ca++-Mg++adenosine triphosphatase pump recognize an epitope in the basolateral membrane of human kidney distal tubule cells. J Clin Invest. 1987;80:1225–1231. doi: 10.1172/JCI113196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdeau JE, Taylor AN, Iacopino AM. Immunocytochemical localization of sodium-calcium exchanger in canine nephron. J Am Soc Nephrol. 1993;4:105–110. doi: 10.1681/ASN.V41105. [DOI] [PubMed] [Google Scholar]
- Burk SE, Menon AG, Shull GE. Analysis of the 5′ end of the rat plasma membrane Ca2+ATPase isoform 3 gene and identification of extensive trinucleotide repeat in the 5′ untranslated region. Biochim Biophys Acta. 1995;1240:119–124. doi: 10.1016/0005-2736(95)00217-0. [DOI] [PubMed] [Google Scholar]
- Cervetto L, Lagnado L, Perry RJ, Robinson DW, McNaughton PA. Extrusion of calcium from rod outer segments is driven by both sodium and potassium gradients. Nature (Lond) 1989;337:740–743. doi: 10.1038/337740a0. [DOI] [PubMed] [Google Scholar]
- Dai LJ, Ritchie G, Bapty B, Raymond L, Quamme GA. Na+/Ca2+exchanger in epithelial cells of the porcine cortical thick ascending limb. Am J Physiol. 1996;270:F411–F418. doi: 10.1152/ajprenal.1996.270.3.F411. [DOI] [PubMed] [Google Scholar]
- Dominguez JH, Juhaszova M, Kleiboeker SB, Hale CC, Feister HA. Na+-Ca2+exchanger of rat proximal tubule: gene expression and subcellular localization. Am J Physiol. 1992;263:F945–F950. doi: 10.1152/ajprenal.1992.263.5.F945. [DOI] [PubMed] [Google Scholar]
- Dominguez JH, Mann C, Rothrock JK, Bhati V. Na+-Ca2+ exchange and Ca2+depletion in rat proximal tubules. Am J Physiol. 1991;261:F328–F335. doi: 10.1152/ajprenal.1991.261.2.F328. [DOI] [PubMed] [Google Scholar]
- Doucet A, Katz AI. High-affinity Ca-Mg-ATPase along the rabbit nephron. Am J Physiol. 1982;242:F346–F352. doi: 10.1152/ajprenal.1982.242.4.F346. [DOI] [PubMed] [Google Scholar]
- Enyedi A, Vorherr T, James P, McCormick DJ, Filoteo AG, Carafoli E, Penniston JT. The calmodulin binding domain of the plasma membrane Ca2+pump interacts both with calmodulin and with another part of the pump. J Biol Chem. 1989;264:12313–12321. [PubMed] [Google Scholar]
- Friedman, P.A., B.A. Coutermarsh, J.S. Rhim, and F.A. Gesek. 1991. Characterization of immortalized mouse distal convoluted tubule cells. J. Am. Soc. Nephrol. 2:737. (Abstr.).
- Friedman PA, Coutermarsh BA, Kennedy SM, Gesek FA. Parathyroid hormone stimulation of calcium transport is mediated by dual signaling mechanisms involving protein kinase A and protein kinase C. Endocrinology. 1996;137:13–20. doi: 10.1210/endo.137.1.8536604. [DOI] [PubMed] [Google Scholar]
- Friedman PA, Figueiredo JF, Maack T, Windhager EE. Sodium-calcium interactions in the renal proximal convoluted tubule of the rabbit. Am J Physiol. 1981;240:F558–F568. doi: 10.1152/ajprenal.1981.240.6.F558. [DOI] [PubMed] [Google Scholar]
- Friedman PA, Gesek FA. Cellular calcium transport in renal epithelia: measurement, mechanisms, and regulation. Physiol Rev. 1995;75:429–471. doi: 10.1152/physrev.1995.75.3.429. [DOI] [PubMed] [Google Scholar]
- Furman I, Cook O, Kasir J, Rahamimoff H. Cloning of two isoforms of the rat brain Na+-Ca2+exchanger gene and their functional expression in HeLa cells. FEBS Lett. 1993;319:105–109. doi: 10.1016/0014-5793(93)80046-w. [DOI] [PubMed] [Google Scholar]
- Gesek FA, Friedman PA. Mechanism of calcium transport stimulated by chlorothiazide in mouse distal convoluted tubule cells. J Clin Invest. 1992;90:429–438. doi: 10.1172/JCI115878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmaj P, Murer H, Kinne R. Calcium ion transport across plasma membranes isolated from rat kidney cortex. Biochem J. 1979;178:549–557. doi: 10.1042/bj1780549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman WF, Yarowsky PJ, Juhaszova M, Krueger BK, Blaustein MP. Sodium/calcium exchange in rat cortical astrocytes. J Neurosci. 1994;14:5834–5843. doi: 10.1523/JNEUROSCI.14-10-05834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greeb J, Shull GE. Molecular cloning of a third isoform of the calmodulin-sensitive plasma membrane Ca2+-transporting ATPase that is expressed predominantly in brain and skeletal muscle. J Biol Chem. 1989;264:18569–18576. [PubMed] [Google Scholar]
- Keeton TP, Shull GE. Primary structure of rat plasma membrane Ca2+-ATPase isoform 4 and analysis of alternative splicing patterns at splice site A. Biochem J. 1995;306:779–785. doi: 10.1042/bj3060779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji P, Lederer WJ, Schulze DH. Mutually exclusive and cassette exons underlie alternatively spliced isoforms of the Na/Ca exchanger. J Biol Chem. 1994;269:5145–5149. [PubMed] [Google Scholar]
- Kumar R, Haugen JD, Penniston JT. Molecular cloning of a plasma membrane calcium pump from human osteoblasts. J Bone Miner Res. 1993;8:505–513. doi: 10.1002/jbmr.5650080415. [DOI] [PubMed] [Google Scholar]
- Lee CO, Taylor A, Windhager EE. Cytosolic calcium ion activity in epithelial cells of Necturuskidney. Nature (Lond) 1980;287:859–861. doi: 10.1038/287859a0. [DOI] [PubMed] [Google Scholar]
- Lorenzen M, Lee CO, Windhager EE. Cytosolic Ca2+ and Na+ activities in perfused proximal tubules of Necturuskidney. Am J Physiol. 1984;247:F93–F102. doi: 10.1152/ajprenal.1984.247.1.F93. [DOI] [PubMed] [Google Scholar]
- Magocsi M, Yamaki M, Penniston JT, Dousa TP. Localization of mRNAs coding for isozymes of plasma membrane Ca2+-ATPase pump in rat kidney. Am J Physiol. 1992;263:F7–F14. doi: 10.1152/ajprenal.1992.263.1.F7. [DOI] [PubMed] [Google Scholar]
- Meszaros GJ, Karin NJ. Osteoblasts express the PMCA1b isoform of the plasma membrane Ca-ATPase. J Bone Miner Res. 1993;8:1235–1240. doi: 10.1002/jbmr.5650081011. [DOI] [PubMed] [Google Scholar]
- Milovanovic S, Frindt G, Tate SS, Windhager EE. Expression of renal Na+-Ca2+ exchange activity in Xenopus laevisoocytes. Am J Physiol. 1991;261:F207–F212. doi: 10.1152/ajprenal.1991.261.2.F207. [DOI] [PubMed] [Google Scholar]
- Nakajima-Iijima S, Hamada H, Reddy P, Kakunga T. Molecular structure of the human cytoplasmic β-actin gene: interspecies homology of sequences in the introns. Proc Natl Acad Sci USA. 1985;82:6133–6137. doi: 10.1073/pnas.82.18.6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt T, Byun JK, Drezner MK. Normal phosphate transport in cells from the S2 and S3 segments of Hyp -mouse proximal renal tubules. Endocrinology. 1996;137:943–948. doi: 10.1210/endo.137.3.8603607. [DOI] [PubMed] [Google Scholar]
- Nesbitt T, Econs MJ, Byun JK, Martel J, Tenenhouse HS, Drezner MK. Phosphate transport in immortalized cell cultures from the renal proximal tubule of normal and Hyp mice: evidence that the HYPgene locus product is an extrarenal factor. J Bone Miner Res. 1995;10:1327–1333. doi: 10.1002/jbmr.5650100909. [DOI] [PubMed] [Google Scholar]
- Nicoll DA, Longoni S, Philipson KD. Molecular cloning and functional expression of the cardiac sarcolemmal Na+-Ca2+exchanger. Science (Wash DC) 1990;250:562–565. doi: 10.1126/science.1700476. [DOI] [PubMed] [Google Scholar]
- Pedersen PL, Carafoli E. Ion motive ATPases. I. Ubiquity, properties, and significance to cell function. Trends Biochem Sci. 1987;12:146–150. [Google Scholar]
- Philipson KD. Sodium-calcium exchange in plasma membrane vesicles. Annu Rev Physiol. 1985;47:561–571. doi: 10.1146/annurev.ph.47.030185.003021. [DOI] [PubMed] [Google Scholar]
- Philipson KD, Longoni S, Ward R. Purification of the cardiac Na+-Ca2+exchange protein. Biochim Biophys Acta. 1988;945:298–306. doi: 10.1016/0005-2736(88)90492-0. [DOI] [PubMed] [Google Scholar]
- Philipson KD, Nicoll DA. Sodium-calcium exchange. Curr Opin Cell Biol. 1992;4:678–683. doi: 10.1016/0955-0674(92)90089-u. [DOI] [PubMed] [Google Scholar]
- Pizzonia JH, Gesek FA, Kennedy SM, Coutermarsh BA, Bacskai BJ, Friedman PA. Immunomagnetic separation, primary culture and characterization of cortical thick ascending limb plus distal convoluted tubule cells from mouse kidney. In Vitro Cell & Dev Biol. 1991;27A:409–416. doi: 10.1007/BF02630961. [DOI] [PubMed] [Google Scholar]
- Ramachandran C, Brunette MG. The renal Na+/Ca2+exchange system is located exclusively in the distal tubule. Biochem J. 1989;257:259–264. doi: 10.1042/bj2570259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran C, Chan M, Brunette MG. Characterization of ATP-dependent Ca2+transport in the basolateral membrane vesicles from proximal and distal tubules of the rabbit kidney. Biochem Cell Biol. 1991;69:109–114. doi: 10.1139/o91-017. [DOI] [PubMed] [Google Scholar]
- Reilly RF, Shugrue CA. cDNA cloning of a renal Na+-Ca2+exchanger. Am J Physiol. 1992;262:F1105–F1109. doi: 10.1152/ajprenal.1992.262.6.F1105. [DOI] [PubMed] [Google Scholar]
- Reilly RF, Shugrue CA, Lattanzi D, Biemesderfer D. Immunolocalization of the Na+/Ca2+exchanger in rabbit kidney. Am J Physiol. 1993;265:F327–F332. doi: 10.1152/ajprenal.1993.265.2.F327. [DOI] [PubMed] [Google Scholar]
- Rose UM, Bindels RJ, Jansen JW, van Os CH. Effects of Ca2+ channel blockers, low Ca2+ medium and glycine on cell Ca2+and injury in anoxic rabbit proximal tubules. Kidney Int. 1994;46:223–229. doi: 10.1038/ki.1994.263. [DOI] [PubMed] [Google Scholar]
- Rouse D, Ng RCK, Suki WN. Calcium transport in the pars recta and thin descending limb of Henle of rabbit perfused in vitro. J Clin Invest. 1980;65:37–42. doi: 10.1172/JCI109657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull GE, Greeb J. Molecular cloning of two isoforms of the plasma membrane Ca2+-transporting ATPase from rat brain. Structural and functional domains exhibit similarity to Na+,K+- and other cation transport ATPases. J Biol Chem. 1988;263:8646–8657. [PubMed] [Google Scholar]
- Smith L, Smith JB. Activation of adenylyl cyclase downregulates sodium/calcium exchanger of arterial myocytes. Am J Physiol. 1995;269:C1379–C1384. doi: 10.1152/ajpcell.1995.269.6.C1379. [DOI] [PubMed] [Google Scholar]
- Stauffer TP, Guerini D, Carafoli E. Tissue distribution of the four gene products of the plasma membrane Ca2+pump. A study using specific antibodies. J Biol Chem. 1995;270:12184–12190. doi: 10.1074/jbc.270.20.12184. [DOI] [PubMed] [Google Scholar]
- Stauffer TP, Hilfiker H, Carafoli E, Strehler EE. Quantitative analysis of alternative splicing options of human plasma membrane calcium pump genes. J Biol Chem. 1993;268:25993–26003. [PubMed] [Google Scholar]
- Strehler EE. Recent advances in the molecular characterization of plasma membrane Ca2+pumps. J Membr Biol. 1991;120:1–15. doi: 10.1007/BF01868586. [DOI] [PubMed] [Google Scholar]
- Talor Z, Arruda JAL. Partial purification and reconstitution of renal basolateral Na+/Ca2+exchanger into liposomes. J Biol Chem. 1985;260:15473–15476. [PubMed] [Google Scholar]
- Tanaka H, Smogorzewski M, Koss M, Massry SG. Pathways involved in PTH-induced rise in cytosolic Ca2+concentration of rat renal proximal tubule. Am J Physiol. 1995;268:F330–F337. doi: 10.1152/ajprenal.1995.268.2.F330. [DOI] [PubMed] [Google Scholar]
- Tsukamoto, Y., K. Sugimura, and W.N. Suki. 1991. Role of Ca2+/H+ antiporter in the kidney. Kidney Int. 40(Suppl. 33):S90–S94. [PubMed]
- Ullrich KJ, Rumrich G, Kloss S. Active Ca2+reabsorption in the proximal tubule of the rat kidney. Dependence on sodium- and buffer transport. Pflüg Arch. 1976;364:223–228. doi: 10.1007/BF00581759. [DOI] [PubMed] [Google Scholar]
- van Heeswijk MPE, Geertsen JAM, van Os CH. Kinetic properties of the ATP-dependent Ca2+ pump and Na+/ Ca2+exchange system in basolateral membranes from rat kidney cortex. J Membr Biol. 1984;79:19–31. doi: 10.1007/BF01868523. [DOI] [PubMed] [Google Scholar]
- Varadi A, Molnar E, Ashcroft SJH. A unique combination of plasma membrane Ca2+-ATPase isoforms is expressed in islets of Langerhans and pancreatic β-cell lines. Biochem J. 1996;314:663–669. doi: 10.1042/bj3140663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri R, Haberland ME, Fong D, Philipson KD. Identification of the cardiac sarcolemmal Na+-Ca2+exchanger using monoclonal antibodies. J Membr Biol. 1990;118:279–283. doi: 10.1007/BF01868612. [DOI] [PubMed] [Google Scholar]
- White KE, Gesek FA, Friedman PA. Structural and functional analysis of Na+/Ca2+exchange in distal convoluted tubule cells. Am J Physiol. 1996a;271:F560–F570. doi: 10.1152/ajprenal.1996.271.3.F560. [DOI] [PubMed] [Google Scholar]
- White KE, Gesek FA, Friedman PA. Na+/Ca2+exchange in rat osteoblast-like UMR-106 cells. J Bone Miner Res. 1996b;11:1666–1675. doi: 10.1002/jbmr.5650111110. [DOI] [PubMed] [Google Scholar]
- Yang JM, Lee CO, Windhager EE. Regulation of cytosolic free calcium in isolated perfused proximal tubules of Necturus. . Am J Physiol. 1988;255:F787–F799. doi: 10.1152/ajprenal.1988.255.4.F787. [DOI] [PubMed] [Google Scholar]
- Yu ASL, Hebert SC, Lee S-L, Brenner BM, Lytton J. Identification and localization of renal Na+-Ca2+exchanger by polymerase chain reaction. Am J Physiol. 1992;263:F680–F685. doi: 10.1152/ajprenal.1992.263.4.F680. [DOI] [PubMed] [Google Scholar]