Abstract
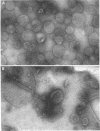
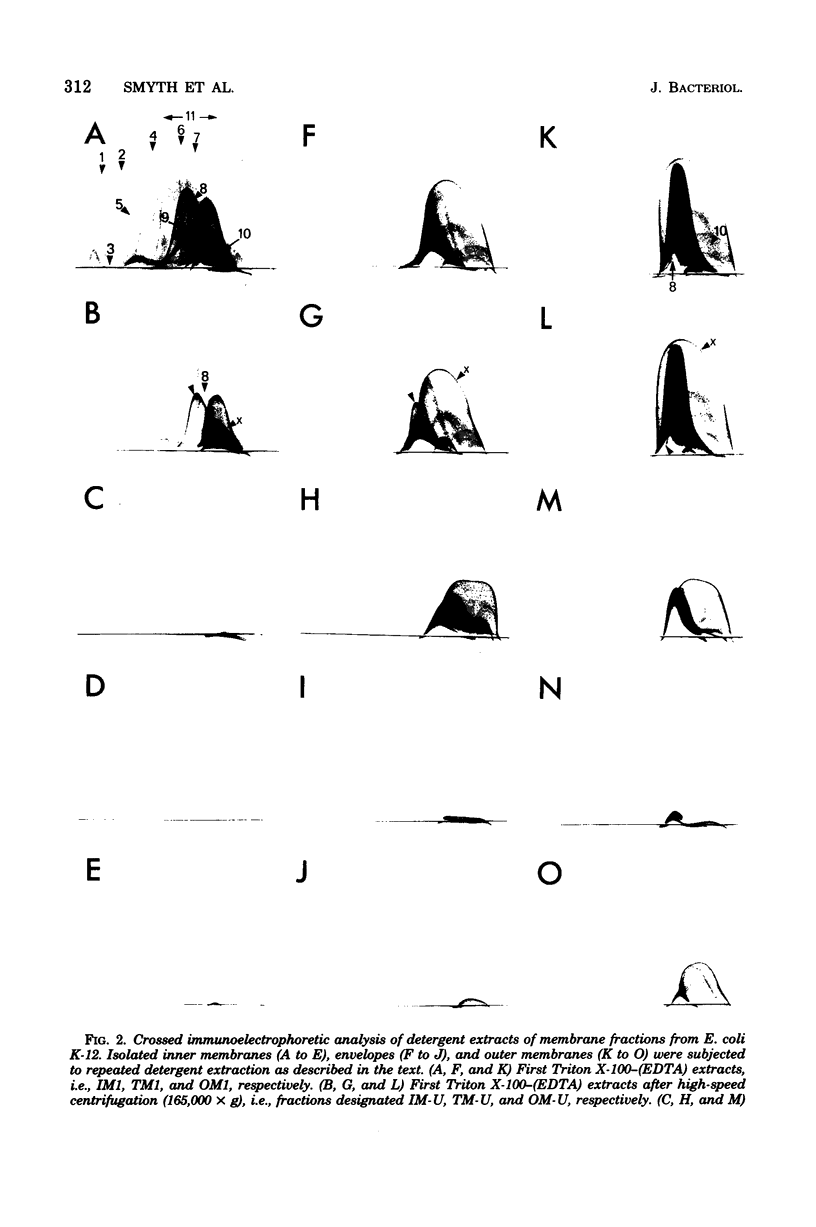
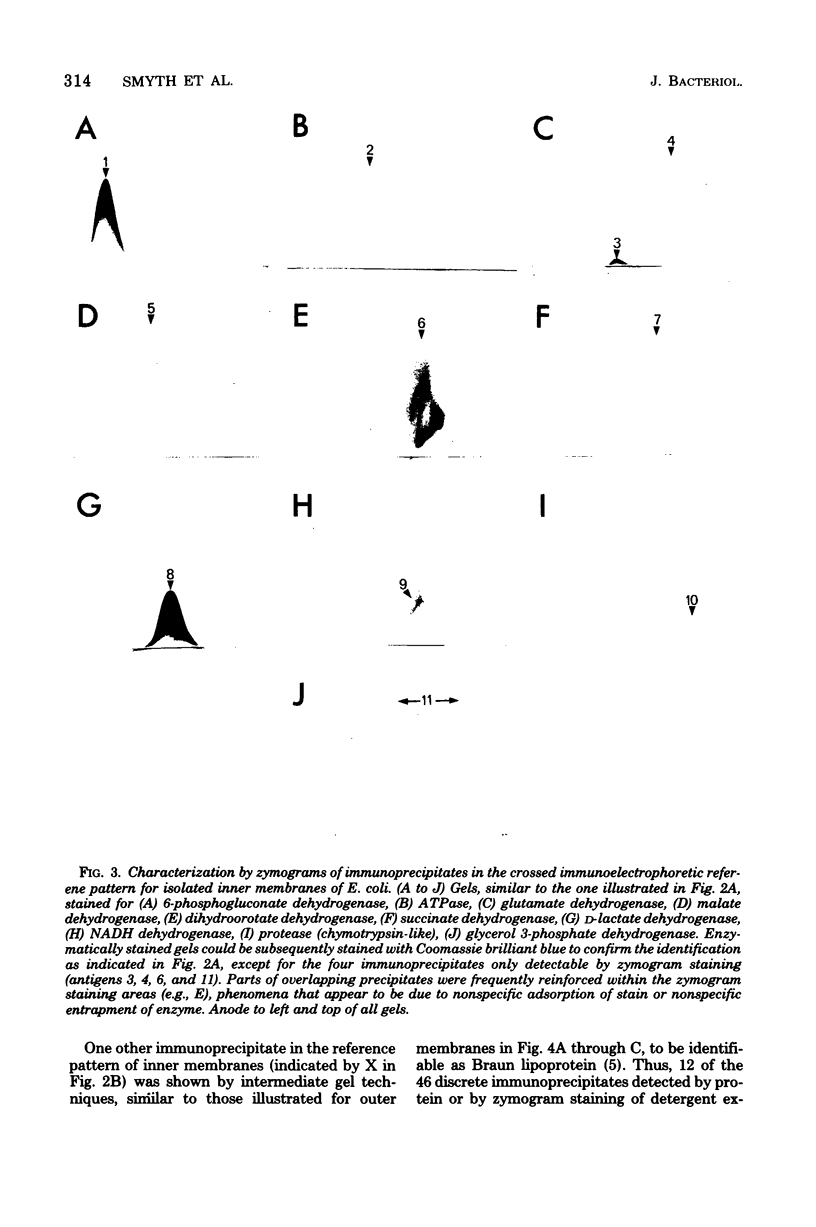
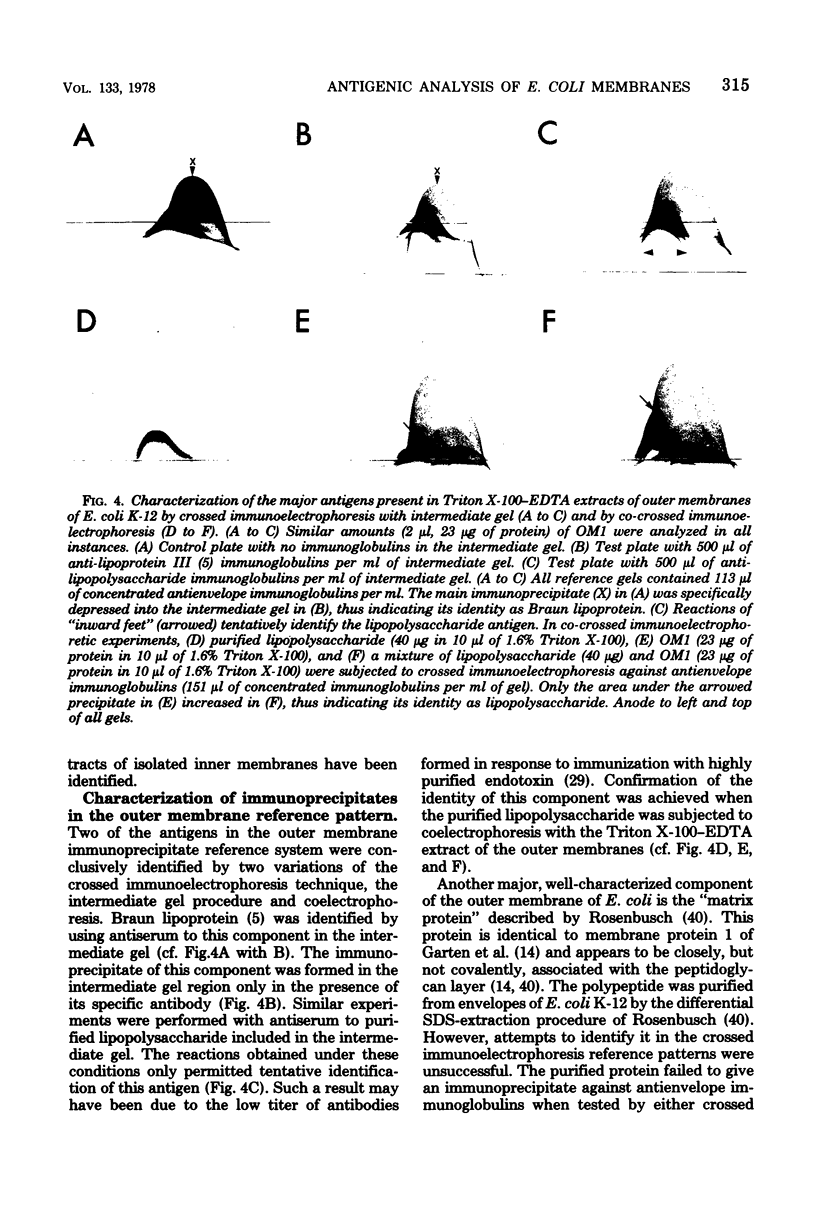
Isolated membrane fractions of Escherichia coli K-12 yielded complex immunoprecipitate patterns when Triton X-100 and sodium dodecyl sulfate extracts were examined by crossed immunoelectrophoresis with antienvelope immunoglobulins. Twelve of the 46 antigens in the immunoprecipitate patterns of inner (plasma) membranes were identified by zymograms and/or by the use of specific antisera. The following enzyme activities were detected in immunoprecipitates: 6-phosphogluconate dehydrogenase (EC 1.1.1.43); adenosine triphosphatase (EC 3.6.1.3); glutamate dehydrogenase (EC 1.4.1.4), two separate components; malate dehydrogenase (EC 1.1.1.37); dihydroorotate dehydrogenase (EC 1.3.3.1); succinate dehydrogenase (EC 1.3.99.1); lactate dehydrogeanse (EC 1.1.1.27); reduced nicotinamide adenine dinucleotide dehydrogenase (EC 1.6.99.3); protease (EC 3.4.21.1); and glycerol 3-phosphate dehydrogenase (EC 1.1.99.5). The corresponding immunoprecipitate pattern for isolated outer membranes consisted of at least 25 discrete antigens and differed strikingly from that obtained with inner membranes. Two major immunogens were identified as lipopolysaccharide and Braun lipoprotein. A protease-active immunoprecipitate was also detected in this fraction, but attempts to identify the Rosenbusch matrix protein in the crossed immunoelectrophoretic profile were unsuccessful.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg F., Raftell M. Enzyme polymorphism in rat-liver microsomes and plasma membranes. 1. An immunochemical study of multienzyme complexes and other enzyme-active antigens. Eur J Biochem. 1974 Nov 1;49(1):21–29. doi: 10.1111/j.1432-1033.1974.tb03807.x. [DOI] [PubMed] [Google Scholar]
- Bog-Hansen T. C. Crossed immuno-affinoelectrophoresis. An analytical method to predict the result of affinity chromatography. Anal Biochem. 1973 Dec;56(2):480–488. doi: 10.1016/0003-2697(73)90215-7. [DOI] [PubMed] [Google Scholar]
- Braun V., Bosch V., Klumpp E. R., Neff I., Mayer H., Schlecht S. Antigenic determinants of murein lipoprotein and its exposure at the surface of Enterobacteriaceae. Eur J Biochem. 1976 Mar 1;62(3):555–566. doi: 10.1111/j.1432-1033.1976.tb10190.x. [DOI] [PubMed] [Google Scholar]
- Braun V. Covalent lipoprotein from the outer membrane of Escherichia coli. Biochim Biophys Acta. 1975 Oct 31;415(3):335–377. doi: 10.1016/0304-4157(75)90013-1. [DOI] [PubMed] [Google Scholar]
- Burger M. M., Goldberg A. R. Identification of a tumor-specific determinant on neoplastic cell surfaces. Proc Natl Acad Sci U S A. 1967 Feb;57(2):359–366. doi: 10.1073/pnas.57.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowle A. J., Revis G. J., Jarrett K. Preparatory electroimmunodiffusion for making precipitins to selected native antigens. Immunol Commun. 1972;1(4):325–336. doi: 10.3109/08820137209022946. [DOI] [PubMed] [Google Scholar]
- Dancey G. F., Levine A. E., Shapiro B. M. The NADH dehydrogenase of the respiratory chain of Escherichia coli. I. Properties of the membrane-bound enzyme, its solubilization, and purification to near homogeneity. J Biol Chem. 1976 Oct 10;251(19):5911–5920. [PubMed] [Google Scholar]
- Dancey G. F., Shapiro B. M. The NADH dehydrogenase of the respiratory chain of Escherichia coli. II. Kinetics of the purified enzyme and the effects of antibodies elicited against it on membrane-bound and free enzyme. J Biol Chem. 1976 Oct 10;251(19):5921–5928. [PubMed] [Google Scholar]
- Dröge W., Lehmann V., Lüderitz O., Westphal O. Structural investigations on the 2-keto-3-deoxyoctonate region of lipopolysaccharides. Eur J Biochem. 1970 May 1;14(1):175–184. doi: 10.1111/j.1432-1033.1970.tb00276.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Garten W., Hindennach I., Henning U. The major proteins of the Escherichia coli outer cell-envelope membrane. Cyanogen bromide fragments of protein I, composition and order. Eur J Biochem. 1975 Dec 1;60(1):303–307. doi: 10.1111/j.1432-1033.1975.tb21004.x. [DOI] [PubMed] [Google Scholar]
- Harboe N., Ingild A. Immunization, isolation of immunoglobulins, estimation of antibody titre. Scand J Immunol Suppl. 1973;1:161–164. doi: 10.1111/j.1365-3083.1973.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Hill J. C., Weiss E. Protein fraction with immunogenic potential and low toxicity isolated from the cell wall of Neisseria meningitidis group B. Infect Immun. 1974 Sep;10(3):605–615. doi: 10.1128/iai.10.3.605-615.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M. A three-dimensional molecular assembly model of a lipoprotein from the Escherichia coli outer membrane. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2396–2400. doi: 10.1073/pnas.71.6.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson K. E., Blomqvist I., Hjertén S. Purification of membrane proteins from Acholeplasma laidlawii by agarose suspension electrophoresis in Tween 20 and polyacrylamide and dextran gel electrophoresis in detergent-free media. J Biol Chem. 1975 Apr 10;250(7):2463–2469. [PubMed] [Google Scholar]
- Johansson K. E., Hjertén S. Localization of the Tween 20-soluble membrane proteins of Acholeplasma laidlawii by crossed immunoelectrophoresis. J Mol Biol. 1974 Jun 25;86(2):341–348. doi: 10.1016/0022-2836(74)90023-0. [DOI] [PubMed] [Google Scholar]
- Kidroni G., Weinbaum G. Glucosamine-labelled envelope proteins of Escherichia coli K-12. I. Electrophoretic studies and partial fractionation of phenol-soluble proteins. Biochim Biophys Acta. 1975 Aug 13;399(2):428–446. doi: 10.1016/0304-4165(75)90271-8. [DOI] [PubMed] [Google Scholar]
- Kidroni G., Weinbaum G. Glucosamine-labelled envelope proteins of Escherichia coli K-12. II. Location in inner and outer membranes. Biochim Biophys Acta. 1975 Aug 13;399(2):447–459. doi: 10.1016/0304-4165(75)90272-x. [DOI] [PubMed] [Google Scholar]
- Kohn L. D., Kaback H. R. Mechanisms of active transport in isolated bacterial membrane vesicles. XV. Purification and properties of the membrane-bound D-lactate dehydrogenase from Escherichia coli. J Biol Chem. 1973 Oct 25;248(20):7012–7017. [PubMed] [Google Scholar]
- Kowit J. D., Choy W. N., Champe S. P., Goldberg A. L. Role and location of "protease I" from Escherichia coli. J Bacteriol. 1976 Dec;128(3):776–784. doi: 10.1128/jb.128.3.776-784.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAURELL C. B. ANTIGEN-ANTIBODY CROSSED ELECTROPHORESIS. Anal Biochem. 1965 Feb;10:358–361. doi: 10.1016/0003-2697(65)90278-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazdunski A., Murgier M., Lazdunski C. Evidence for an aminoendopeptidase localized near the cell surface of Escherichia coli. Regulation of synthesis by inorganic phosphate. Eur J Biochem. 1975 Dec 15;60(2):349–355. doi: 10.1111/j.1432-1033.1975.tb21009.x. [DOI] [PubMed] [Google Scholar]
- Morris D. L. Quantitative Determination of Carbohydrates With Dreywood's Anthrone Reagent. Science. 1948 Mar 5;107(2775):254–255. doi: 10.1126/science.107.2775.254. [DOI] [PubMed] [Google Scholar]
- Nakae T. Identification of the outer membrane protein of E. coli that produces transmembrane channels in reconstituted vesicle membranes. Biochem Biophys Res Commun. 1976 Aug 9;71(3):877–884. doi: 10.1016/0006-291x(76)90913-x. [DOI] [PubMed] [Google Scholar]
- Nguyen-Distèche M., Pollock J. J., Ghuysen J. M., Puig J., Reynolds P., Perkins H. R., Coyette J., Salton M. R. Sensitivity to ampicillin and cephalothin of enzymes involved in wall peptide crosslinking in Escherichia coli K12, strain 44. Eur J Biochem. 1974 Feb 1;41(3):457–463. doi: 10.1111/j.1432-1033.1974.tb03287.x. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Munson R. Separation of the inner (cytoplasmic) and outer membranes of Gram-negative bacteria. Methods Enzymol. 1974;31:642–653. doi: 10.1016/0076-6879(74)31070-1. [DOI] [PubMed] [Google Scholar]
- Owen P., Oppenheim J. D., Nachbar M. S., Kessler R. E. The use of lectins in the quantitation and analysis of macromolecules by affinoelectrophoresis. Anal Biochem. 1977 Jun;80(2):446–457. doi: 10.1016/0003-2697(77)90667-4. [DOI] [PubMed] [Google Scholar]
- Owen P., Salton M. R. Antigenic and enzymatic architecture of Micrococcus lysodeikticus membranes established by crossed immunoelectrophoresis. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3711–3715. doi: 10.1073/pnas.72.9.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen P., Salton M. R. Isolation and characterization of a mannan from mesosomal membrane vesicles of Micrococcus lysodeikticus. Biochim Biophys Acta. 1975 Oct 6;406(2):214–234. doi: 10.1016/0005-2736(75)90006-1. [DOI] [PubMed] [Google Scholar]
- Pacaud M., Sibilli S., Bras G. Protease I from Escherichia coli. Some physicochemical properties and substrate specificity. Eur J Biochem. 1976 Oct 1;69(1):141–151. doi: 10.1111/j.1432-1033.1976.tb10867.x. [DOI] [PubMed] [Google Scholar]
- Pollock J. J., Nguyen-Distèche M., Ghuysen J. M., Coyette J., Linder R., Salton M. R., Kim K. S., Perkins H. R., Reynolds P. Fractionation of the DD-carboxypeptidase-transpeptidase activities solubilized from membranes of Escherichia coli K12, strain 44. Eur J Biochem. 1974 Feb 1;41(3):439–446. doi: 10.1111/j.1432-1033.1974.tb03285.x. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- SALTON M. R. J., HORNE R. W. Studies of the bacterial cell wall. II. Methods of preparation and some properties of cell walls. Biochim Biophys Acta. 1951 Jul;7(2):177–197. doi: 10.1016/0006-3002(51)90017-0. [DOI] [PubMed] [Google Scholar]
- Salton M. R., Owen P. Bacterial membrane structure. Annu Rev Microbiol. 1976;30:451–482. doi: 10.1146/annurev.mi.30.100176.002315. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Effect of ethylenediaminetetraacetic acid, Triton X-100, and lysozyme on the morphology and chemical composition of isolate cell walls of Escherichia coli. J Bacteriol. 1971 Oct;108(1):553–563. doi: 10.1128/jb.108.1.553-563.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol. 1971 Oct;108(1):545–552. doi: 10.1128/jb.108.1.545-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short S. A., Kaback H. R., Hawkins T., Kohn L. D. Immunochemical properties of the membrane-bound D-lactate dehydrogenase from Escherichia coli. J Biol Chem. 1975 Jun 10;250(11):4285–4290. [PubMed] [Google Scholar]
- Smith J. B., Sternweis P. C., Heppel L. A. Partial purification of active delta and epsilon subunits of the membrane ATPase from escherichia coli. J Supramol Struct. 1975;3(3):248–255. doi: 10.1002/jss.400030307. [DOI] [PubMed] [Google Scholar]
- Smith R. P., Shapiro B. M. Immunological properties of membrane fractions from wild type and dnaA mutants of Escherichia coli. Biochim Biophys Acta. 1974 Aug 9;356(3):331–349. doi: 10.1016/0005-2736(74)90273-9. [DOI] [PubMed] [Google Scholar]
- Smyth C. J., Friedman-Kien A. E., Salton M. R. Antigenic analysis of Neisseria gonorrhoeae by crossed immunoelectrophoresis. Infect Immun. 1976 Apr;13(4):1273–1288. doi: 10.1128/iai.13.4.1273-1288.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So L. L., Goldstein I. J. Protein-carbohydrate interaction. IX. Application of the quantitative hapten inhibition technique to polysaccharide-concanavalin A interaction. Some comments on the forces involved n concanavalin A-polysaccharide interaction. J Immunol. 1967 Jul;99(1):158–163. [PubMed] [Google Scholar]
- Weiner J. H. The localization of glycerol-3-phosphate dehydrogenase in Escherichia coli. J Membr Biol. 1974;15(1):1–14. doi: 10.1007/BF01870078. [DOI] [PubMed] [Google Scholar]
- Yamato I., Anraku Y., Hirosawa K. Cytoplasmic membrane vesicles of Escherichia coli. A simple method for preparing the cytoplasmic and outer membranes. J Biochem. 1975 Apr;77(4):705–718. doi: 10.1093/oxfordjournals.jbchem.a130774. [DOI] [PubMed] [Google Scholar]