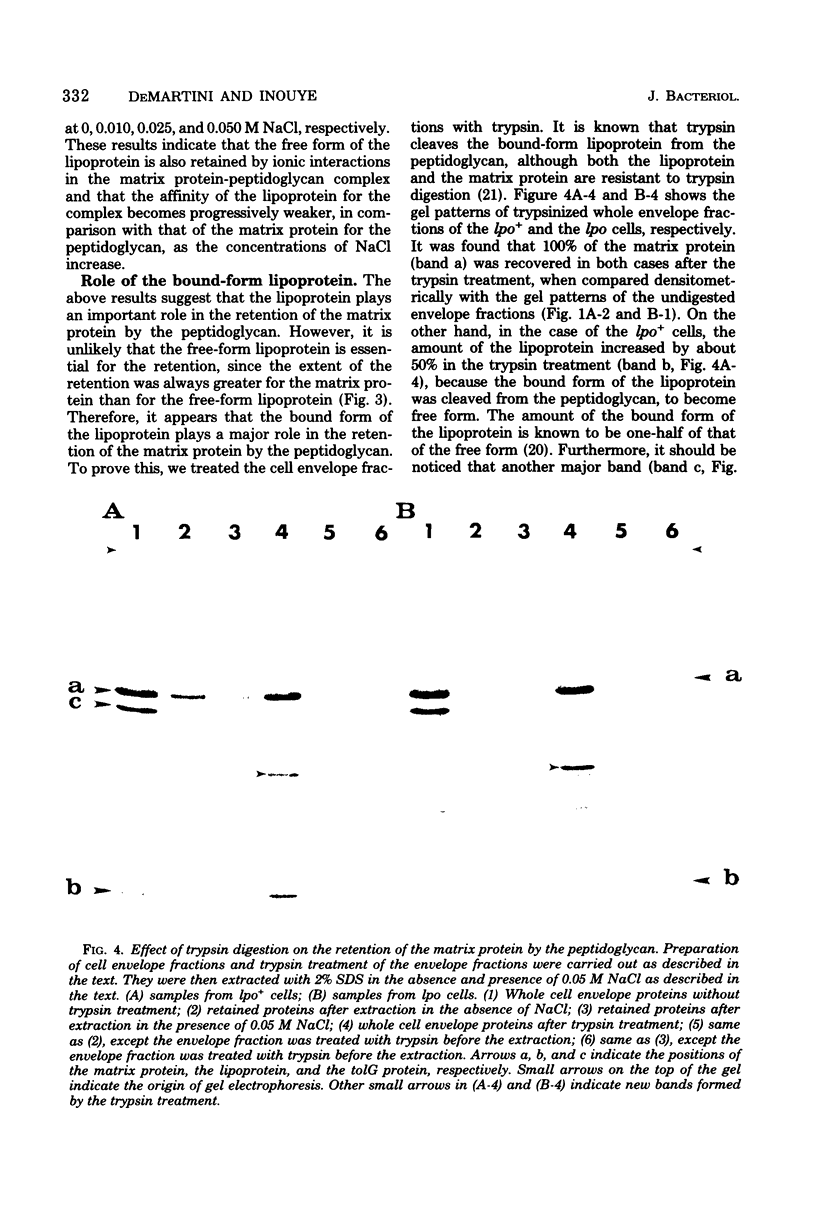
Abstract
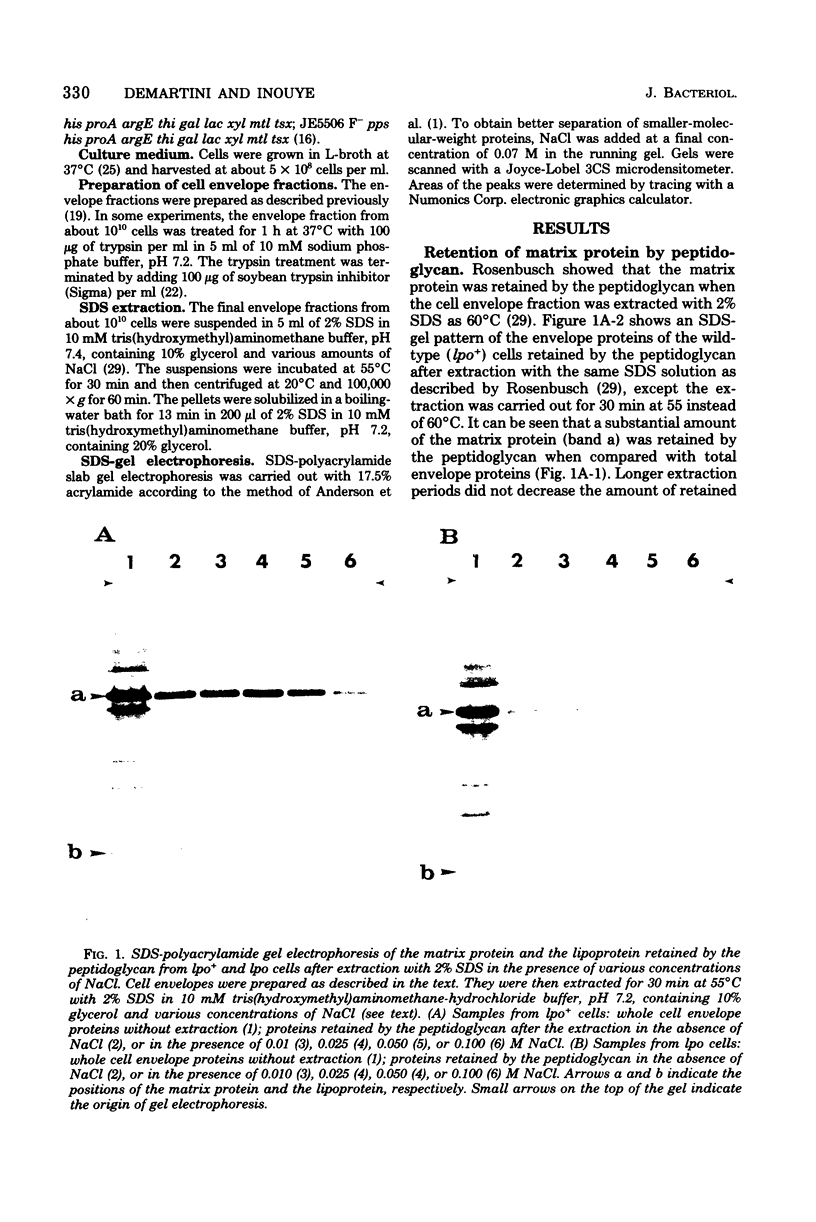
The affinity to the matrix protein, one of the major outer membrane proteins of Escherichia coli, for the peptidoglycan was examined of extracting the cell envelope complex at 55 degrees C and 2% sodium dodecyl sulfate containing different amounts of NaCl. It was found that the matrix protein was extracted from the peptidoglycan of a mutant strain (lpo) that lacks another major membrane protein, the lipoprotein, at a lower NaCl concentration than was the matrix protein of the wild-type cell (lpo+). When the envelope fraction of the wild-type strain was treated with trypsin, which is known to cleave the bound-form lipoprotein from the peptidoglycan, the affinity of the matrix protein for the peptidoglycan decreased to the same level as that of the affinity of the matrix protein for the peptidoglycan of the mutant strain. It was further shown that the free-form lipoprotein was also retained in the matrix protein-peptidoglycan complex, although the extent of retention of the free form of the lipoprotein was less than that of the matrix protein. These results indicate that both the free and the bound forms of the lipoprotein are closely associated with the matrix protein and that the bound form of the lipoprotein plays and important role in the association between the matrix protein and the peptidoglycan.
Full text
PDF
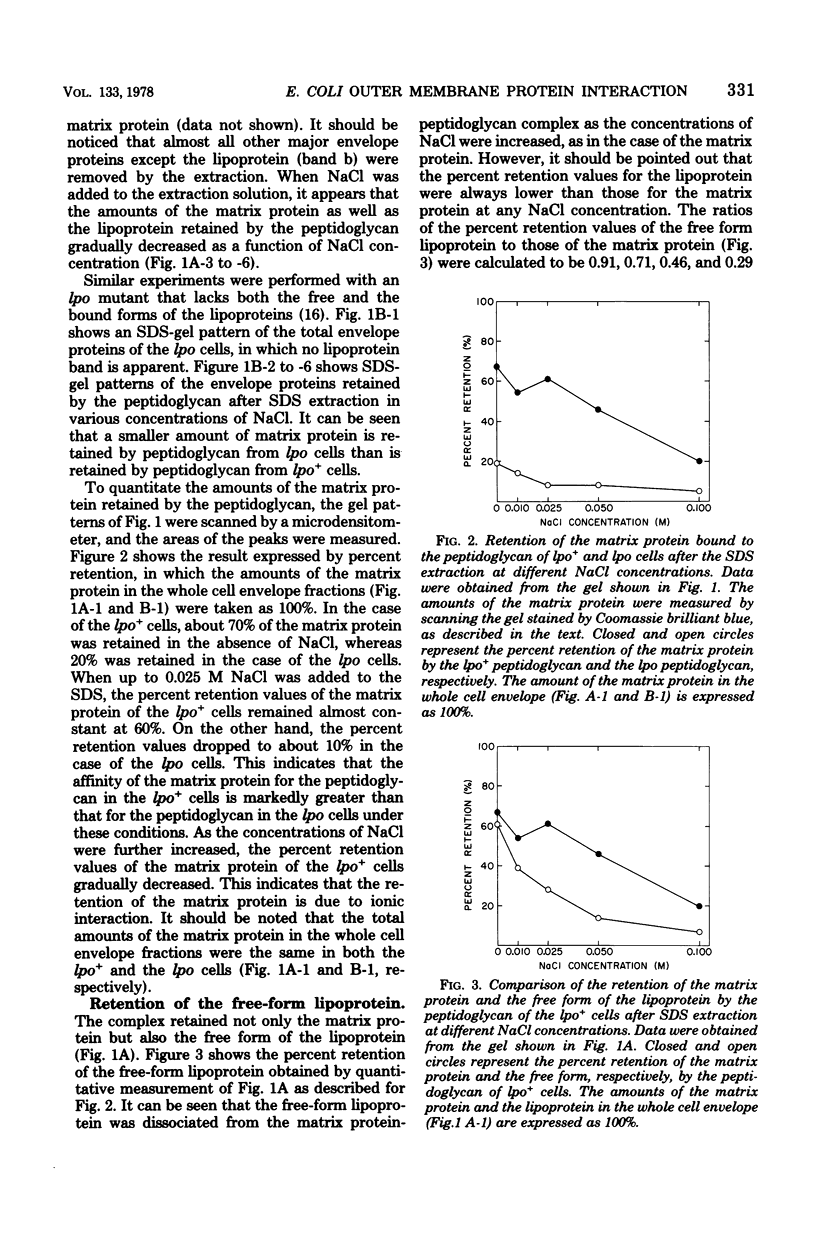



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Bosch V., Klumpp E. R., Neff I., Mayer H., Schlecht S. Antigenic determinants of murein lipoprotein and its exposure at the surface of Enterobacteriaceae. Eur J Biochem. 1976 Mar 1;62(3):555–566. doi: 10.1111/j.1432-1033.1976.tb10190.x. [DOI] [PubMed] [Google Scholar]
- Braun V., Bosch V. Repetitive sequences in the murein-lipoprotein of the cell wall of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Apr;69(4):970–974. doi: 10.1073/pnas.69.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V. Covalent lipoprotein from the outer membrane of Escherichia coli. Biochim Biophys Acta. 1975 Oct 31;415(3):335–377. doi: 10.1016/0304-4157(75)90013-1. [DOI] [PubMed] [Google Scholar]
- Braun V., Rotering H., Ohms J. P., Hagenmaier H. Conformational studies on murein-lipoprotein from the outer membrane of Escherichia coli. Eur J Biochem. 1976 Nov 15;70(2):601–610. doi: 10.1111/j.1432-1033.1976.tb11051.x. [DOI] [PubMed] [Google Scholar]
- DeMartini M., Inouye S., Inouye M. Ultrastructure of paracrystals of a lipoprotein from the outer membrane of Escherichia coli. J Bacteriol. 1976 Jul;127(1):564–571. doi: 10.1128/jb.127.1.564-571.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halegoua S., Hirashima A., Sekizawa J., Inouye M. Protein synthesis in toluene-treated Escherichia coli. Exclusive synthesis of membrane proteins. Eur J Biochem. 1976 Oct 1;69(1):163–167. doi: 10.1111/j.1432-1033.1976.tb10869.x. [DOI] [PubMed] [Google Scholar]
- Halegoua S., Sekizawa J., Inouye M. A new form of structural lipoprotein of outer membrane of Escherichia coli. J Biol Chem. 1977 Apr 10;252(7):2324–2330. [PubMed] [Google Scholar]
- Hantke K., Braun V. Covalent binding of lipid to protein. Diglyceride and amide-linked fatty acid at the N-terminal end of the murein-lipoprotein of the Escherichia coli outer membrane. Eur J Biochem. 1973 Apr;34(2):284–296. doi: 10.1111/j.1432-1033.1973.tb02757.x. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y., Yamada H., Mizushima S. Interactions of outer membrane proteins O-8 and O-9 with peptidoglycan sacculus of Escherichia coli K-12. J Biochem. 1976 Dec;80(6):1401–1409. doi: 10.1093/oxfordjournals.jbchem.a131413. [DOI] [PubMed] [Google Scholar]
- Henning U., Rehn K., Hoehn B. Cell envelope and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2033–2036. doi: 10.1073/pnas.70.7.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirashima A., Wang S., Inouye M. Cell-free synthesis of a specific lipoprotein of the Escherichia coli outer membrane directed by purified messenger RNA. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4149–4153. doi: 10.1073/pnas.71.10.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirashima A., Wu H. C., Venkateswaran P. S., Inouye M. Two forms of a structural lipoprotein in the envelope of Escherichia coli. Further characterization of the free form. J Biol Chem. 1973 Aug 25;248(16):5654–5659. [PubMed] [Google Scholar]
- Hirota Y., Suzuki H., Nishimura Y., Yasuda S. On the process of cellular division in Escherichia coli: a mutant of E. coli lacking a murein-lipoprotein. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1417–1420. doi: 10.1073/pnas.74.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M. A three-dimensional molecular assembly model of a lipoprotein from the Escherichia coli outer membrane. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2396–2400. doi: 10.1073/pnas.71.6.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Guthrie J. P. A mutation which changes a membrane protein of E. coli. Proc Natl Acad Sci U S A. 1969 Nov;64(3):957–961. doi: 10.1073/pnas.64.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Shaw J., Shen C. The assembly of a structural lipoprotein in the envelope of Escherichia coli. J Biol Chem. 1972 Dec 25;247(24):8154–8159. [PubMed] [Google Scholar]
- Inouye S., Wang S., Sekizawa J., Halegoua S., Inouye M. Amino acid sequence for the peptide extension on the prolipoprotein of the Escherichia coli outer membrane. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1004–1008. doi: 10.1073/pnas.74.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium. Identification of proteins exposed on cell surface. Biochim Biophys Acta. 1977 Feb 4;464(3):589–601. doi: 10.1016/0005-2736(77)90033-5. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Lee N., Cheng E., Inouye M. Optical properties of an outer membrane lipoprotein from Escherichia coli. Biochim Biophys Acta. 1977 Mar 17;465(3):650–656. doi: 10.1016/0005-2736(77)90280-2. [DOI] [PubMed] [Google Scholar]
- MURRAY R. G., STEED P., ELSON H. E. THE LOCATION OF THE MUCOPEPTIDE IN SECTIONS OF THE CELL WALL OF ESCHERICHIA COLI AND OTHER GRAM-NEGATIVE BACTERIA. Can J Microbiol. 1965 Jun;11:547–560. doi: 10.1139/m65-072. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Song S. A., Shaltiel L., Nurminen M. Outer membrane of Salmonella XIV. Reduced transmembrane diffusion rates in porin-deficient mutants. Biochem Biophys Res Commun. 1976 May 23;76(2):324–330. doi: 10.1016/0006-291x(77)90728-8. [DOI] [PubMed] [Google Scholar]
- Reithmeier R. A., Bragg P. D. Cross-linking of the proteins in the outer membrane of Escherichia coli. Biochim Biophys Acta. 1977 Apr 18;466(2):245–256. doi: 10.1016/0005-2736(77)90222-x. [DOI] [PubMed] [Google Scholar]
- Schmitges C. J., Henning U. The major proteins of the Escherichia coli outer cell-envelope membrane. Heterogeneity of protein I. Eur J Biochem. 1976 Mar 16;63(1):47–52. doi: 10.1111/j.1432-1033.1976.tb10205.x. [DOI] [PubMed] [Google Scholar]
- Steven A. C., Heggeler B., Müller R., Kistler J., Rosenbusch J. P. Ultrastructure of a periodic protein layer in the outer membrane of Escherichia coli. J Cell Biol. 1977 Feb;72(2):292–301. doi: 10.1083/jcb.72.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Mizushima S. Stimulation by lipopolysaccharide of the binding of outer membrane proteins O-8 and O-9 to the peptidoglycan layer of Escherichia coli K--12. Biochem Biophys Res Commun. 1977 Feb 21;74(4):1397–1402. doi: 10.1016/0006-291x(77)90597-6. [DOI] [PubMed] [Google Scholar]