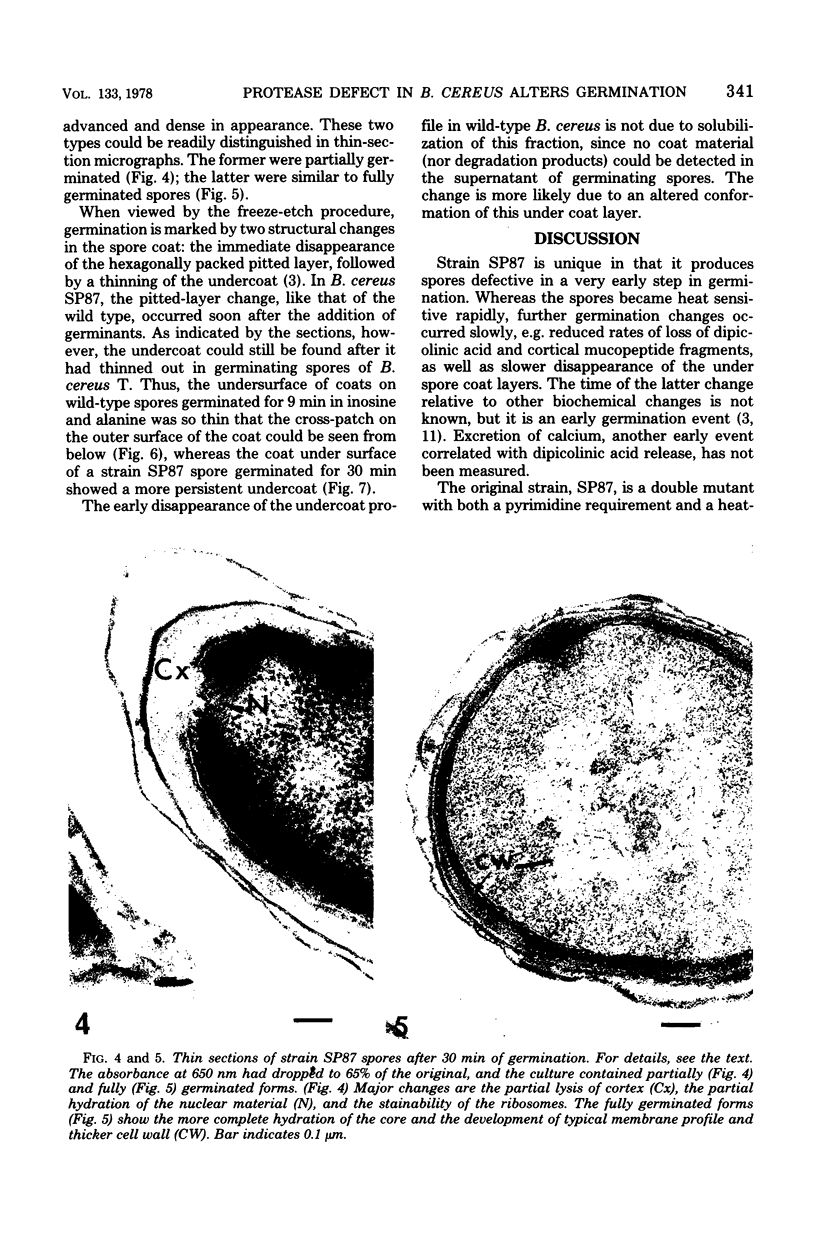
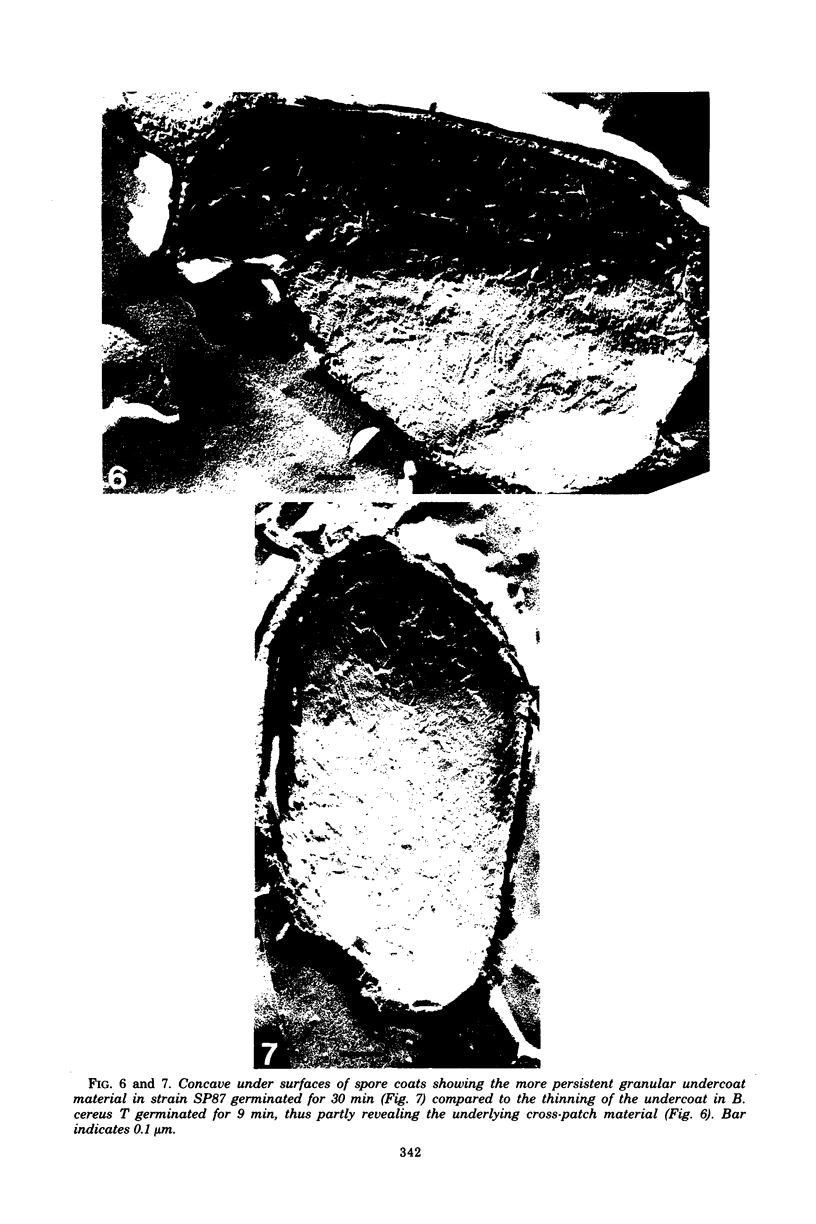
Abstract
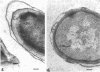
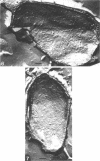
Temperature-sensitive sporulation mutants of Bacillus cereus were screened for intracellular protease activity that was more heat labile than that of the parental strain. One mutant grew as well as the wild type at 30 and 37 degrees C but sporulated poorly at 37 degrees C in an enriched or minimal medium. These spores germinated very slowly in response to alanine plus adenosine or calcium dipicolinate. During germination, spores produced by the mutant rapidly became heat sensitive, but released dipicolonic acid and mucopeptide fragments more slowly than the wild type and decreased only partially in density while remaining phase white (semirefractile). In freeze-etch electron micrographs, the mature spores were deficient in the outer cross-patched coat layer. During germination, the spore coat changes associated with wild-type germination occurred very slowly in this mutant. Although the original mutant was also a pyrimidine auxotroph, reversion to prototrophy did not alter any of the phenotypic properties discussed. Selection of revertants that germinated rapidly or sporulated well at 37 degrees C, however, resulted in restoratin of all wild-type properties (exclusive of the pyrimidine requirement) including heat-stable protease activity. The reversion frequency was consistent with an initial point mutation, indicating that a protease alteration resulted in production of spores defective in a very early stage of germination.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson A. I., Angelo N., Holt S. C. Regulation of extracellular protease production in Bacillus cereus T: characterization of mutants producing altered amounts of protease. J Bacteriol. 1971 Jun;106(3):1016–1025. doi: 10.1128/jb.106.3.1016-1025.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson A. I., Fitz-James P. C. Properties of Bacillus cereus spore coat mutants. J Bacteriol. 1975 Jul;123(1):354–365. doi: 10.1128/jb.123.1.354-365.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson A. I., Fitz-James P. Structure and morphogenesis of the bacterial spore coat. Bacteriol Rev. 1976 Jun;40(2):360–402. doi: 10.1128/br.40.2.360-402.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. S., Aronson A. I. Alterations of spore coat processing and protein turnover in a Bacillus cereus mutant with a defective postexponential intracellular protease. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1254–1258. doi: 10.1073/pnas.74.3.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes I. W., Halvorson H. O. Temperature-sensitive mutants of Bacillus subtilis defective in spore outgrowth. Mol Gen Genet. 1974;131(2):147–157. doi: 10.1007/BF00266150. [DOI] [PubMed] [Google Scholar]
- Galizzi A., Gorrini F., Rollier A., Polsinelli M. Mutants of Bacillus subtilis temperature sensitive in the outgrowth phase of spore germination. J Bacteriol. 1973 Mar;113(3):1482–1490. doi: 10.1128/jb.113.3.1482-1490.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galizzi A., Siccardi A. G., Albertini A. M., Amileni A. R., Meneguzzi G., Polsinelli M. Properties of Bacillus subtilis mutants temperature sensitive in germination. J Bacteriol. 1975 Feb;121(2):450–454. doi: 10.1128/jb.121.2.450-454.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoo T., Conti S. F. Ultrastructural changes associated with activation and germination of Bacillus cereus T spores. J Bacteriol. 1971 Jan;105(1):361–368. doi: 10.1128/jb.105.1.361-368.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh L. K., Vary J. C. Germination and peptidoglycan solubilization in Bacillus megaterium spores. J Bacteriol. 1975 Aug;123(2):463–470. doi: 10.1128/jb.123.2.463-470.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Spizizen J. Increased rate of asporogenous mutations following treatment of Bacillus subtilis spores with ethyl methanesulfonate. Mutat Res. 1971 Sep;13(1):93–96. doi: 10.1016/0027-5107(71)90130-8. [DOI] [PubMed] [Google Scholar]
- JANSSEN F. W., LUND A. J., ANDERSON L. E. Colorimetric assay for dipicolinic acid in bacterial spores. Science. 1958 Jan 3;127(3288):26–27. doi: 10.1126/science.127.3288.26. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leighton T. J., Dor R. H., Warren R. A., Kelln R. A. The relationship of serine protease activity to RNA polymerase modification and sporulation in Bacillus subtilis. J Mol Biol. 1973 May 5;76(1):103–122. doi: 10.1016/0022-2836(73)90083-1. [DOI] [PubMed] [Google Scholar]
- Linn T. G., Greenleaf A. L., Shorenstein R. G., Losick R. Loss of the sigma activity of RNA polymerase of Bacillus subtilis during sporulation. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1865–1869. doi: 10.1073/pnas.70.6.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet J., Aubert J. P. Etude de la mégatériopeptidase, protéase exocellulaire de Bacillus megaterium. 3. Biosynthèse et rôle physiologique. Ann Inst Pasteur (Paris) 1969 Oct;117(4):461–473. [PubMed] [Google Scholar]
- Millet J. Caractérisation d'une endopeptidase cytoplasmique chez Bacillus megaterium en voie de sporulation. C R Acad Sci Hebd Seances Acad Sci D. 1971 Mar 29;272(13):1806–1809. [PubMed] [Google Scholar]
- Millet J., Larribe M., Aubert J. P. Mutant thermosensible de B. subtilis affecté dans la sporulation et la sérylprotéase extracellulaire. Biochimie. 1976;58(1-2):109–117. doi: 10.1016/s0300-9084(76)80361-6. [DOI] [PubMed] [Google Scholar]
- Prasad C., Diesterhaft M., Freese E. Initiation of spore germination in glycolytic mutants of Bacillus subtilis. J Bacteriol. 1972 Apr;110(1):321–328. doi: 10.1128/jb.110.1.321-328.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIEMANN H., ORDAL Z. J. Germination of bacterial endospores with calcium and dipicolinic acid. Science. 1961 May 26;133(3465):1703–1704. doi: 10.1126/science.133.3465.1703. [DOI] [PubMed] [Google Scholar]
- Reysset G., Millet J. Characterization of an intracellular protease in B. subtillus during sporulation. Biochem Biophys Res Commun. 1972 Oct 17;49(2):328–334. doi: 10.1016/0006-291x(72)90414-7. [DOI] [PubMed] [Google Scholar]
- Sadoff H. L., Celikkol E., Engelbrecht H. L. Conversion of bacterial aldolase from vegetative to spore form by a sporulation-specific protease. Proc Natl Acad Sci U S A. 1970 Jul;66(3):844–849. doi: 10.1073/pnas.66.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff H. L., Hitchins A. D., Celikkol E. Properties of fructose 1,6-diphosphate aldolases from spores and vegetative cells of Bacillus cereus. J Bacteriol. 1969 Jun;98(3):1208–1218. doi: 10.1128/jb.98.3.1208-1218.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P. Sporulation and the production of antibiotics, exoenzymes, and exotonins. Bacteriol Rev. 1969 Mar;33(1):48–71. doi: 10.1128/br.33.1.48-71.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowsdale J., Smith D. A. Isolation, characterization, and mapping of Bacillus subtilis 168 germination mutants. J Bacteriol. 1975 Jul;123(1):83–95. doi: 10.1128/jb.123.1.83-95.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vary J. C., Kornberg A. Biochemical studies of bacterial sporulation and germination. XXI. Temperature-sensitive mutants for initiation of germination. J Bacteriol. 1970 Jan;101(1):327–329. doi: 10.1128/jb.101.1.327-329.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]