Summary
VDAC is the major permeability pathway in the mitochondrial outer membrane and can control the flow of metabolites and ions. Therefore Ca2+ flux across the outer membrane occurs mainly through VDAC. Since both Ca2+ fluxes and VDAC are involved in apoptosis, we examined whether Ca2+ is required for channel formation by VDAC isolated from rat liver. The voltage gating of VDAC does not require Ca2+ and it functions normally with or without Ca2+. Additionally, VDAC generally shows a higher permeability to Ca2+ in the closed states (states with lower permeability to metabolites) than that in the open state. Thus VDAC closure, which induces cell death, also favors Ca2+ flux into mitochondria, which can also lead to permeability transition and cell death. These results are consistent with the view that VDAC closure is a pro-apoptotic signal.
Keywords: PTP, mitochondria, apoptosis, voltage gating, swelling, planar membrane
Introduction
Mitochondria are the governors of both cell life (e.g. energy generation) and cell death. Some regulation of both of these functions occurs at the level of the outer membrane in that it controls the flow of metabolites and the release of intermembrane space proteins into the cytosol. These two functions are connected in that a drastic reduction in metabolite flow through the outer membrane, associated with VDAC closure*, can lead to protein release and apoptosis [1–2]. However, Ca2+ induced mitochondrial swelling and subsequent cell death may require an increase in Ca2+ flux and thus an increase in outer membrane permeability. Perhaps these apparently conflicting changes in outer membrane permeability may be understood if VDAC closure is irrelevant to a small ion such as Ca2+ and the Ca2+-induced mitochondrial swelling is strictly an inner-membrane phenomenon.
It is generally believed that mitochondrial swelling is caused by the opening of PTP (permeability transition pores). The pore has a molecular mass cut-off of 1500 Da. Many have proposed that PTP involves both the inner and outer membrane through the participation of VDAC. However, knockout of VDAC1 does not seem to affect the formation of PTP [3], making the participation of VDAC in PTP questionable. Nevertheless, it is generally believed that Ca2+ should flow easily through VDAC channels because VDAC shows only a weak selectivity for small mono-valent ions [4–5]. Indeed, in a recent study [6], the authors concluded that VDAC opening promotes calcium flux into mitochondria followed by PTP and mitochondrial swelling, i.e., VDAC opening induces cell death.
This conclusion is consistent with the report that anti-apoptotic Bcl-2 family proteins close VDAC channels [7]. It is also in agreement with studies on gelosin, hexokinase, RuR, DIDS etc. [8–11] that closure of VDAC is an antiapoptotic signal but in sharp contrast to other findings that VDAC closure is a pro-apoptotic signal. For example, the anti-apoptotic protein, Bcl-xL, favors the opening of VDAC channels [12–13], while the pro-apoptotic oligonucleotide, G3139 closes VDAC [2, 14–15]. In addition, removal of required growth factors from cell lines leads to decreased MOM permeability to metabolites, such as phosphocreatine, and subsequent apoptosis. This is also consistent with VDAC closure. It is possible that apoptosis induced by VDAC closure is through a different pathway from calcium induced PTP.
In addition to the role of Ca+2 in apoptosis, Ca2+ is an important intracellular second messenger. At sub-micromolar concentration, it can also stimulate mitochondrial respiration and phosphorylation by the activation of dehydrogenases [16–17]. The flux of Ca2+ into mitochondria seems come from the SR/ER-mitochondria contacts [18–19], which propagate calcium signaling into the mitochondria. The permeability of the mitochondrial outer membrane would thus have implications in other Ca2+ signaling besides mitochondrial swelling and cell apoptosis.
To clarify the role of VDAC gating in Ca2+ flux through the outer membrane, we examined the permeability of VDAC to Ca2+ in the open and closed states. The states with higher Ca2+ permeability are different from the state traditionally considered to be the open state.
Materials and Methods
Planar phospholipid membrane studies
Planar phospholipid membranes were generated according to standard methods [20–21]. The membrane was formed from phospholipid monolayers consisting of diphytanoyl phosphatidylcholine, polar extract of soybean phospholipids (both from Avanti Polar Lipids, Alabaster, AL) and cholesterol (Sigma, St Louis, MO) in a 1:1:0.1 mass ratio.
VDAC was purified from mitochondria isolated from rat liver [22–23]. In the ion selectivity measurements, a 0.1 µL aliquot of the VDAC-containing solution (2.5% Triton X100, 50 mM KCl, 10 mM Tris, 1 mM EDTA, 15% DMSO, pH 7.0) was stirred into 4–6 mL of aqueous solution containing 80 mM CaCl2 aqueous (unless stated otherwise) solution on the cis side of the chamber. The trans side, containing 20 mM CaCl2 aqueous solution (unless stated otherwise), was held at virtual ground by the voltage clamp. The solutions were unbuffered and no other ions were deliberately added. After a single channel insertion, a triangular voltage wave at a frequency of 3 mHz from −52 mV to +52 mV was applied to the cis side. The current was recorded at a rate of 1 ms per point with 2 kHz filtering. All experiments were performed at approximately 23°C.
Results
The voltage gating of VDAC channels is not affected by Ca2+
Detergent solubilized VDAC was added to a solution containing no calcium ions (0.4mM EGTA was added to chelate any possible calcium contaminations) bathing a planar phospholipid membrane (Fig. 1). VDAC channels inserted into the membrane and these events were recorded as increases in current. In the absence of calcium ions, VDAC can remain in the open state and closes in response to a 50 mV applied potential. The kinetics of the closure are indistinguishable whether in the absence or presence of 1.6 mM Ca2+ (Fig. 1). Moreover, the presence or absence of Ca2+ does not change the conductance of VDAC. In 1.0 M NaCl the conductance is 3.3 ± 0.3 nS (mean ± S.D.) in the presence of 0.1 mM EGTA and 3.4 ± 0.1 nS (mean ± S.D.) in the presence of 1 mM CaCl2.
Fig. 1.
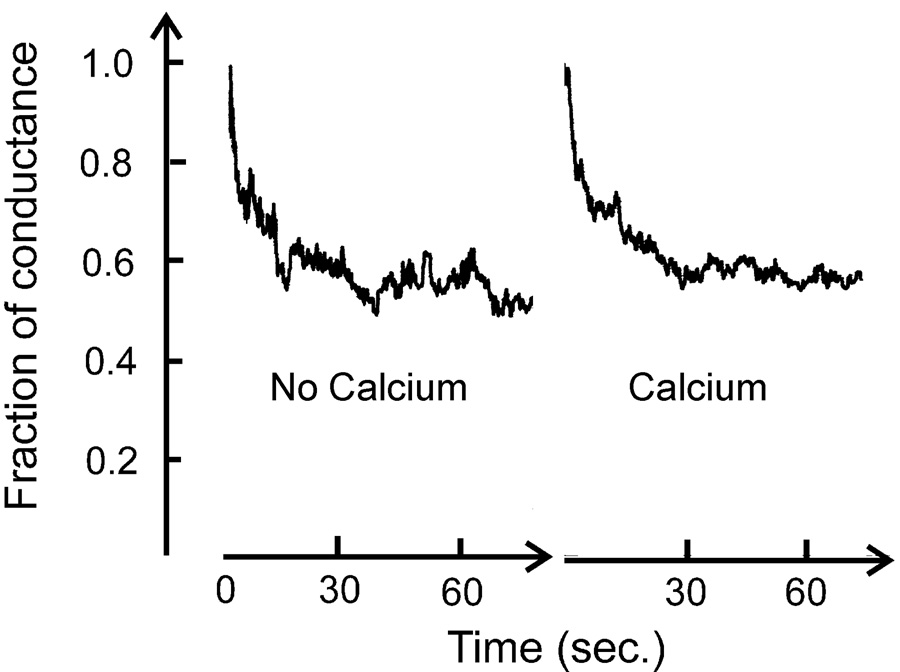
VDAC was reconstituted into a planar phospholipid membrane composed of 0.5% PC, 0.5% asolectin and 0.05% cholesterol in the presence of 1.0 M KCl, 20mM HEPES, pH=7.2. 0.4mM EGTA was then added to each side of the chamber to chelate any possible trace amount of Ca2+. 2mM Ca2+ was added later to compare VDAC’s gating in the presence and absence of Ca2+. (Note: there is enough buffer to control the pH even after acid is released following chelation of some Ca2+) VDAC’s gating was recorded by applying 50 mV (cis side is ground). The total conductance in the absence and presence of Ca2+ is 115 nS and 179 nS, respectively. There is an 18-minute gap between these records and the conductance increased because of VDAC insertion. The percent conductance drop and the rate of decay are essentially the same with and without free Ca2+.
Measurement of the permeability of VDAC to Ca2+ and Cl−
The permeability of VDAC to small ions includes Ca2+, which was reported to permeate both the open [4] and closed states [24] (Rostovtseva, T.K., unpublished data) of VDAC. VDAC is the most abundant channel in the MOM (mitochondrial outer membrane). Thus its permeability to Ca2+ is very important as this may affect calcium homeostasis and cell death signaling. However, the value of the ion flux of Ca2+ through VDAC has never been directly and accurately determined. Here, CaCl2 was used to measure the ion flow through VDAC.
The current carried by cations and anions are given by the GHK current equation (constant field equation):
| (1) |
Where IS is current carried by ion S, zS is the charge of s, PS is the permeability of VDAC to S, βS is the activity of S (i denotes cis side, o denotes trans side), V is the potential difference across the membrane, F is the Faraday constant, R is the gas constant and T is the temperature. The single-ion activities were obtained from the salt activity coefficients. For CaCl2, the activity coefficient is 0.5355 for 80 mM solution (cis side) and 0.6644 for 20 mM solution (trans side) [25].
At any given voltage, all the parameters except IS and PS are known in equation 1. Thus we can define:
| (2) |
which is a known parameter, thus
| (3) |
where I is the recorded current. If each XS is different, equation 2 and equation 3 can be combined to fit the current voltage curve to generate the permeabilities to each ion. A four-fold gradient of CaCl2 (with ionic strength in the physiological range) was used as mentioned in the Materials and Methods.
A single VDAC channel was used for the current-voltage measurements. The parameters were calculated by fitting to the current voltage curve. The slope at zero voltage is defined as the conductance of the VDAC channel, the zero-current intercept is Vrev (reversal potential) and the zero-pontential intercept is I0 (zero potential current).
Fig. 2 shows an example of the fitting of equation 2 and 3 to a segment of a current-voltage record. The recorded current was divided into two currents: calcium current and chloride current. The permeabilities were directly obtained from the fitting. Vrev (reversal potential) and I0 (zero potential current) for each state were determined by extrapolating the current-voltage curve to intercept the appropriate axis.
Fig. 2.
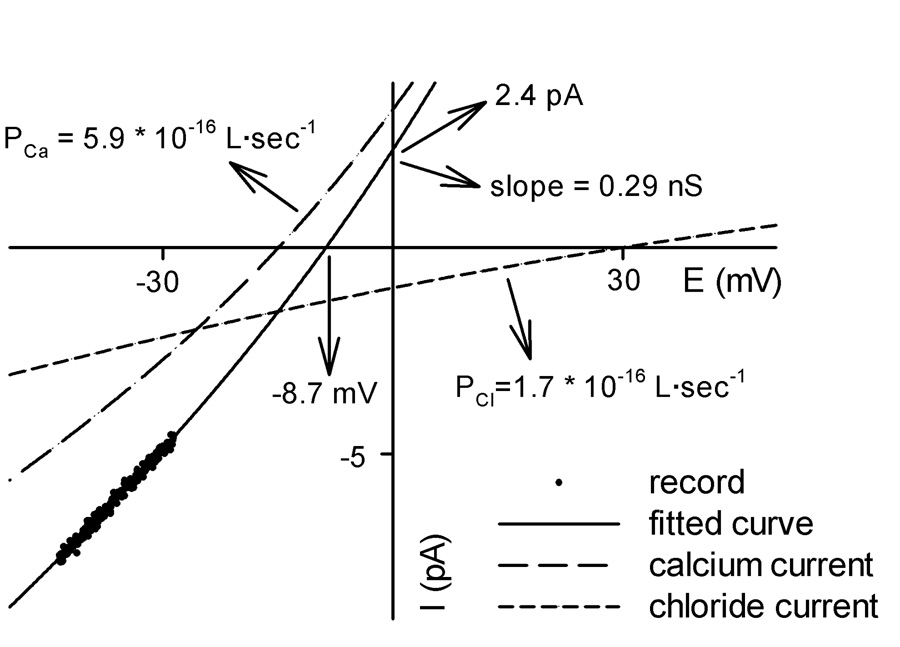
An example of fitting equation 2 and equation 3 to a segment of a single-channel current-voltage record representing one state of a VDAC channel (a closed state here). A four-fold gradient of CaCl2 was present (see Materials and Methods). The arrows and associated numbers indicate the parameters obtained from the fitting.
This method is more accurate than the calculation of ion permeabilities from a linear extrapolation of the current-voltage curve to the axes. The linear extrapolation is not justified because the current shows rectification.
In the open state, the conductance, G = 0.520 ± 0.005 nS, Vrev = 25.5 mV ± 0.2 mV, I0 = −12.4 ± 0.1 pA (mean ± S.E., n = 15). In the positively closed states, G = 0.20 ± 0.02 nS, Vrev = 15 ± 2 mV, I0 = ±2.8 ± 0.5 pA (mean ± S.E., n = 19). In the negatively closed states, G = 0.29 ± 0.05 nS, Vrev = 2 ± 3 mV, I0 = 0 ± 1 pA (mean ± S.E., n = 15).
The distributions of conductance, Vrev, I0 are very narrow for the open state and very broad for the closed states. This is consistent with previous observations [26–27], indicating the presence of multiple closed states. More interestingly, there is a clear difference (P < 0.001) between the states observed at negative and positive voltages. The anion preference drops in both cases as compared to that of the open state but states achieved at negative potentials are more favorable to Ca2+ flux.
Fig. 3 shows the fitted values of the permeability of VDAC to Ca2+ and Cl− in the open state (the units are always 10−16 L·s−1), PCa = 0.94 ± 0.05, PCl = 22.6 ± 0.2 (mean ± S.E., n = 15); in the positively closed states, PCa = 1.4 ± 0.2, PCl = 6.3 ± 0.9 (mean ± S.E., n = 19); And in the negatively closed states, PCa = 4 ± 1, PCl = 5 ± 1 (mean ± S.E., n = 15). Clearly, the VDAC channel is highly selective for chloride ions over calcium ions in the open state. When it is closed by high potentials, the permeability to chloride ions and calcium ions becomes comparable, especially in those states observed in the negative potential region.
Fig. 3.
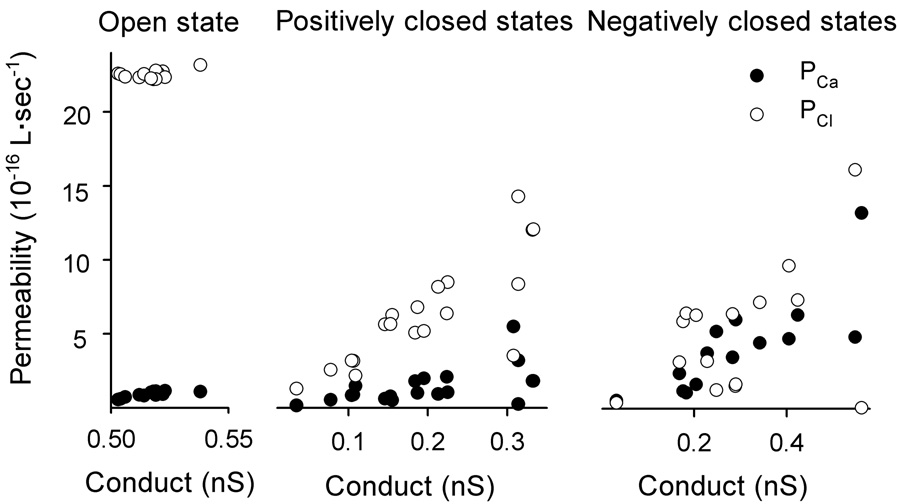
Permeability of VDAC to calcium and chloride ions as a function of the total conductance of the channel. Each value is obtained directly as in Fig. 2.
There is a significant increase in Ca2+ permeability in both sets of closed states but in the negatively closed states the permeability is more that 4 times that of the open state (Fig. 4). In some states, the increase can be more than 10 times (Fig. 3).
Fig. 4.
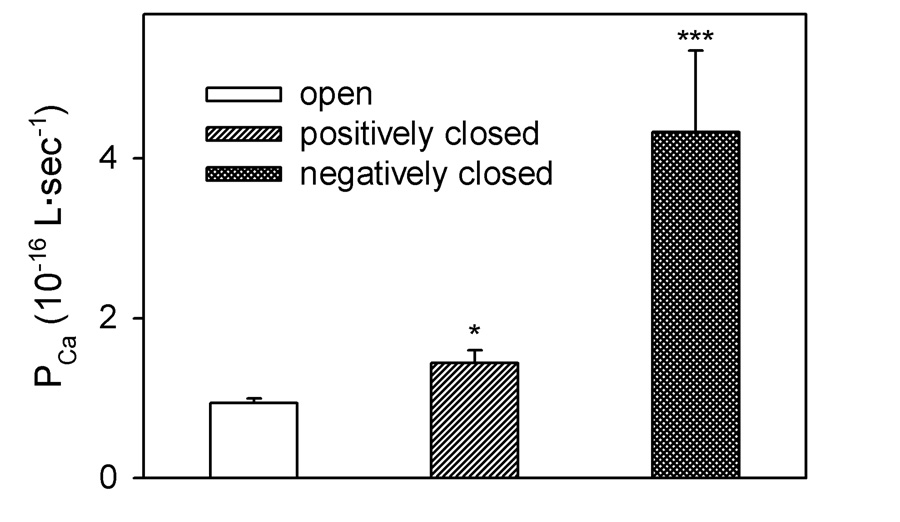
Comparison of the permeability of Ca2+ through VDAC in different states. The values are mean ± S.E. of 15 measurements in the open state and the negatively closed states and 19 measurements in the positively closed states. (*P < 0.05, *** P < 0.001, compared to the open state)
Discussions
The gating of VDAC channels is not influenced by the presence of Ca2+ in the medium, but the flow of Ca2+ through VDAC is affected by VDAC gating. In its open state, VDAC is poorly permeable to Ca2+. Extrapolating our results down to a more physiological Ca2+ concentration of 1 µM, the flux would be 20 ions per sec. This increases 4 fold when VDAC closes but in some states to as much as 10 fold (Fig 3,Fig 4). It is possible that some signals may lock VDAC in the more Ca2+ conducting states and expedite the Ca2+ flux.
In order to obtain accurate estimates for permeability and flux, one needs to have accurate values for activity coefficients. Unfortunately, only salt activity coefficients are available and thus we used these to convert concentrations into activities. However, one can estimate single-ion activity ratios by measuring the reversal potential for salt gradients using ion exchange membranes. Using the salt activity ratios, the calculated permeability ratio of chloride over calcium for the open state of VDAC is more than 25 fold, which is very close to ideal anion selectivity. The permeability ratio doubled if one uses the single ion activity ratios. Thus the calcium flux through VDAC in the open state is even lower, and therefore VDAC closure could enhance calcium flux even more than the value we report.
High-conducting states can be permeable to Ca2+
We observed VDAC functional states that have a similar conductance as that of the open state, but with higher permeability to Ca2+ (Fig. 3). This is consistent with previous publications (e.g. [26]) and the “cationic state” of VDAC previously reported [28]. In our experiments, those states only occur rarely at high negative voltages. They are considered closed states because they are expected to have reduced permeability to metabolites, most of which are anionic. In the Ca2+ experiments, these states occurred exclusively at negative potentials.
Ca2+ and VDAC gating
We observed no significant Ca2+ influence on the voltage gating property of VDAC. A possible reason for the difference between the results presented here and those of Báthori et al. [29] is the different methods used to make planar membranes. We use the monolayer method of Montal and Mueller [20] to generate solvent-free membranes. The hole in the partition that supports the planar membrane is coated with petrolatum (a hydrocarbon mixture ranging from C20 to C90), then phospholipids in hexane are layered on the top of the buffer solution. Once the hexane has evaporated, the planar membranes are made by apposing monolayers from the two sides of the membrane. In contrast, Báthori et al. [29] made the planar membrane by directly painting a decane solution of phospholipids across the hole on the partition separating two sides of the chamber.
The major difference here is the solvent and its propensity to partition into the planar membrane. At room temperature, hexane, which has a vapor pressure of 0.172 atm [30], evaporates very quickly from the monolayers and thus does not contaminate the membrane. On the other hand, decane, with the vapor pressure of 0.00136 atm, two orders of magnitudes lower than that of hexane [30], evaporates much more slowly than hexane. In addition, the painting method for making planar membranes directly applies the decane solution to the hole, which has already been submerged into the buffer solution. Considering the virtual insolubility of decane in water, all the decane will remain with the lipids. Indeed, the decane annulus is necessary to allow the bilayer to interface with the much thicker plastic partition. Unlike the petrolatum coating used in the monolayer method, decane molecules are 5 times smaller and diffuse easily into phospholipid membranes. This solvent increases the membrane thickness by 1 nm, and may change such properties as the surface tension and fluidity of the membrane and affect the stability, conductance and voltage gating of VDAC. In addition, Ca2+ can interact with charged lipids and spontaneously thin the membrane, favoring channel formation, which may account for the regulation of Ca2+ on observed channel conductance. For gramicidin channels, an increase in membrane thickness (even due to the presence of decane) reduces the channel lifetime by orders of magnitude [31–33].
Báthori et al. [29] also reported that Ca2+ increases the rate of proton flow across the mitochondria outer membrane in permeabilized cells, consistent with their reported Ca2+ effect on VDAC channel formation. However, Ca2+ may act indirectly in permeabilized cells. In fact numerous publications report flux studies through VDAC reconstituted in liposomes in the presence of as much as 1 mM EDTA. In these studies, sugars and short polymers were shown to flow through VDAC [e.g. 34–35]. Also, the presence of Ca2+ does not increase the flux [fig. 5C of Ref. 8].
Fig. 5.
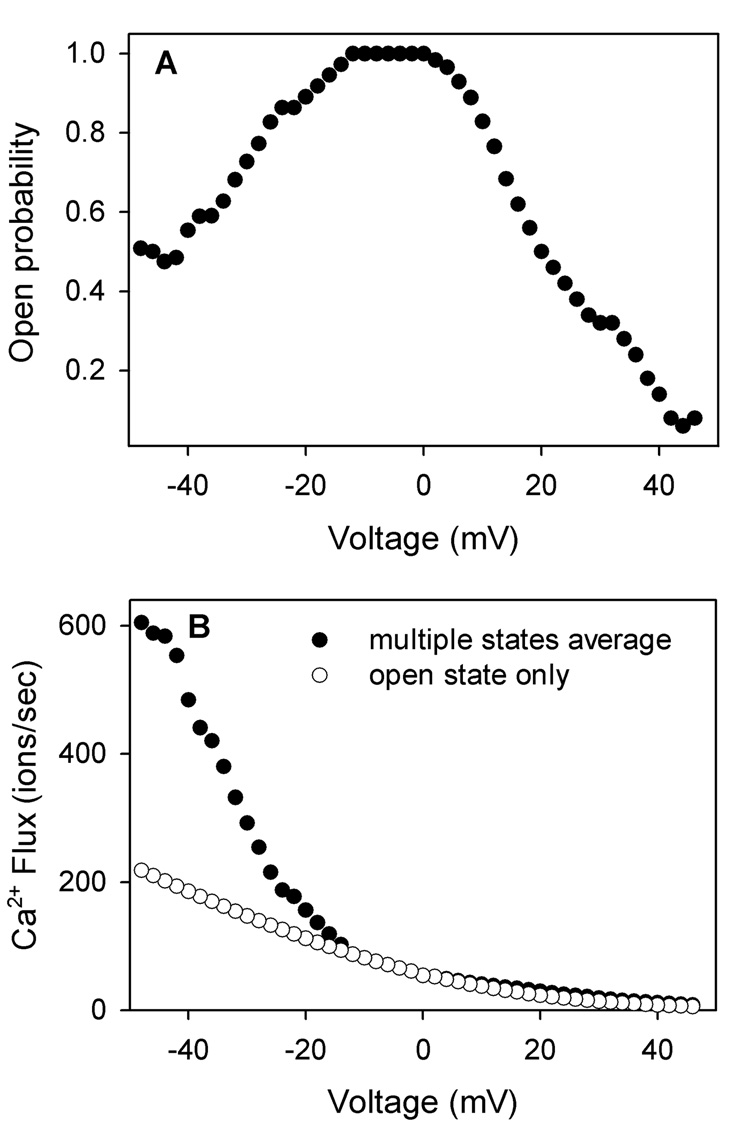
(A) The open probability of VDAC in response to voltage. (B) A comparison of the average calcium flux through a single VDAC channel in response to voltage calculated for what it would be in the presence of 1 µM cytosolic Ca2+ (0 µM in the intermembrane space). The filled circles are the weighted average flux of Ca2+ through the open and closed states, assuming the same voltage gating property as in panel A. The open circles are assuming that VDAC is always in the open state.
Ionic strength and VDAC permeability to Ca2+
The permeability to Ca2+ measured here is very different from that reported by Gincel et al. [6]. They measured a PCa/PCl of 0.38 and claim a high flux of calcium through the open VDAC channel. This conclusion supports their hypothesis that the open state of VDAC favors Ca2+ flux, mitochondrial swelling and cell death. The main observed difference between their experiments and results shown here is the measured reversal potential in CaCl2 gradients. Their observed reversal potential of 10 mV is less than half of what is measured here. This is most likely due to the difference in salt concentration (Table 1).
Table 1.
Comparison of the conditions and results
| This paper | Gincel et al. [6] | |
|---|---|---|
| CaCl2 gradient | 80 mM: 20 mM | 250 mM: 150 mM |
| Ionic strength | 150 mM | 600 mM |
| Debye length | 0.78 nm | 0.39 nm |
| PCa/PCl | 0.04 | 0.35 |
Assuming a linear concentration gradient inside the channel, the difference in CaCl2 concentration between the two sets of experiments is 50 mM vs. 200 mM. The ionic strength is 150mM vs. 600mM. Thus our ionic strength is at a physiological level. The difference in ionic strength translates into a difference in screening of charges within the channel. The calculated Debye Length at room temperature is:
| (4) |
Thus the Debye length is 0.78 nm for our experiments vs. 0.39 nm. Hence, in the higher ionic strength there are fewer electrostatic interactions between the positive charges in the inner wall of VDAC and Ca2+ ions permeating through the VDAC.
In addition to the difference in ionic solutions, Gincel et al. [6] did not compare the Ca2+ flow through VDAC in open and closed states. Perhaps, even under their conditions, the closed states of VDAC could be more permeable to Ca2+.
The physiological impact of VDAC gating
In our observations, the permeability of Ca2+ through VDAC is different in the states observed at positive versus negative voltages (Fig 3,Fig 4). This clearly shows an oriented insertion of mammalian VDAC into planar membranes and an asymmetrical VDAC function. The impact of VDAC gating on the regulation of Ca2+ flux will depend on the orientation of VDAC in mitochondria and the sign of the potential across the outer membrane.
At present, there is no way of relating the orientation of VDAC in planar membranes and that in the mitochondrial outer membrane. However, it is still interesting to consider the impact of VDAC closure and selectivity change on the calcium flux. Fig. 5A shows the open probability of VDAC in response to voltages. By combining this information with the permeability of VDAC to Ca2+ in the different states, one can calculate the time-averaged permeability of VDAC to calcium in response to voltages. If we assume that VDAC in mitochondria is in the orientation, whose closure greatly favors calcium flux from the cytosol into mitochondria, the calcium flux in response to 1 µM cytosolic Ca2+ can be calculated (Fig. 5B).
Porcelli and coworkers [36] measured the pH difference between the mitochondrial intermembrane space and the cytosol. Considering the highly-buffered nature of these compartments, it is reasonable to conclude that protons will be at equilibrium between these two compartments and thus calculate the potential difference across the outer membrane. This turns out to be 40 mV negative potential in the intermembrane space compared to the cytosol [36]. Thus, from Fig. 5 there is a 3 fold increase of calcium flux if VDAC closes with a 40 mV potential. Considering that there are the VDAC modulator [37–38] and other proteins which may favor VDAC closure and may favor particular closed states, the actual effect could be even larger. Thus the closure of VDAC actually favors Ca2+ flux, even though the closed states has a smaller pore diameter (1.8 nm in the closed states compared to 2.5 nm in the open state) [39–40].
Accumulating evidence links VDAC gating with apoptosis [2, 13]. It is clear that the open state of VDAC facilitates metabolite flow and thus maintains mitochondria in a healthy state, preventing cytochrome c release. In addition, in the open state, Ca2+ permeability is low. In the closed state, VDAC reduces metabolite flux, increases Ca2+ permeation, and thus sensitizes mitochondria to apoptotic signals. The gating process of VDAC is not only an interesting biophysical phenomenon, it also changes the properties of VDAC to initiate or be in harmony with changes in the physiological state of the cell.
Footnotes
VDAC gating has evolved not to regulate the flow of K+ and Cl− ions, but to regulate the flow of metabolites. We use small ions because it is convenient to do so to follow the different conformational states of VDAC. However, one must think about VDAC from its physiological function, to allow metabolites, especially ATP and ADP to cross the outer membrane. Thus VDAC closure refers to closure to the flow of metabolites. The normal closed states of VDAC allow a substantial flow of small ions. The conductance to KCl is generally 40–50% of the open state conductance.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Vander Heiden MG, Chandel NS, Li XX, Schumacker PT, Colombini M, Thompson CB. Outer mitochondrial membrane permeability can regulate coupled respiration and cell survival. Proc. Natl. Acad. Sci. U. S. A. 2000;97:4666–4671. doi: 10.1073/pnas.090082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan W, Lai JC, Miller P, Stein CA, Colombini M. Phosphorothioate oligonucleotides reduce mitochondrial outer membrane permeability to ADP. Am. J. Physiol. Cell Physiol. 2006;292:C1388–C1397. doi: 10.1152/ajpcell.00490.2006. [DOI] [PubMed] [Google Scholar]
- 3.Krauskopf A, Eriksson O, Craigen WJ, Forte MA, Bernardi P. Properties of the permeability transition in VDAC1(−/−) mitochondria. Biochim. Biophys. Acta. 2006;1757:590–595. doi: 10.1016/j.bbabio.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Colombini M. Structure and mode of action of a voltage-dependent anion-selective channel (VDAC) located in the outer mitochondrial membrane. Ann. New York Acad. Sci. 1980;341:552–563. doi: 10.1111/j.1749-6632.1980.tb47198.x. [DOI] [PubMed] [Google Scholar]
- 5.Hodge T, Colombini M. Regulation of metabolite flux through voltage-gating of VDAC channels. J. Membr. Biol. 1987;157:271–279. doi: 10.1007/s002329900235. [DOI] [PubMed] [Google Scholar]
- 6.Gincel D, Zaid H, Shoshan-Barmatz V. Calcium binding and translocation by the voltage-dependent anion channel: A possible regulatory mechanism in mitochondrial function. Biochem. J. 2001;358:147–155. doi: 10.1042/0264-6021:3580147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimizu S S, Konishi A, Kodama T, Tsujimoto Y Y. BH4 domain of antiapoptotic Bcl-2 family members closes voltage-dependent anion channel and inhibits apoptotic mitochondrial changes and cell death. Proc. Natl. Acad. Sci. U.S.A. 2000;97:3100–3105. doi: 10.1073/pnas.97.7.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kusano H, Shimizu S, Koya RC, Fujita H H, Kamada S, Kuzumaki N N, Tsujimoto Y. Human gelsolin prevents apoptosis by inhibiting apoptotic mitochondrial changes via closing VDAC. Oncogene. 2000;19:4807–4814. doi: 10.1038/sj.onc.1203868. [DOI] [PubMed] [Google Scholar]
- 9.Azoulay- Zohar H, Israelson A, Abu-Hamada S, Shoshan-Barmatz V. In self-defense: Hexokinase promotes VDAC closure and prevents mitochondria-mediated apoptotic cell death. Biochemical J. 2004;377:347–355. doi: 10.1042/BJ20031465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pastorino JG, Shulga N, Hoek JB. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J. Biol. Chem. 2002;277:7610–7618. doi: 10.1074/jbc.M109950200. [DOI] [PubMed] [Google Scholar]
- 11.Madesh M, Hajnóczky G. VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. J. Cell Biol. 2001;155:1003–1015. doi: 10.1083/jcb.200105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vander Heiden MG, Chandel NS, Schumacker PT, Thompson CB. Bcl-xL prevents cell death following growth factor withdrawal by facilitating mitochondrial ATP/ADP exchange. Mol. Cell. 1999;3:159–167. doi: 10.1016/s1097-2765(00)80307-x. [DOI] [PubMed] [Google Scholar]
- 13.Vander Heiden MG, Li XX, Gottleib E, Hill RB, Thompson CB, Colombini M. Bcl-xL promotes the open configuration of the voltage-dependent anion channel and metabolite passage through the outer mitochondrial membrane. J. Biol. Chem. 2001;276:19414–19419. doi: 10.1074/jbc.M101590200. [DOI] [PubMed] [Google Scholar]
- 14.Lai JC, Tan W, Benimetskaya L, Miller P, Colombini M, Stein CA. A pharmacologic target of G3139 in melonoma cells may be the mitochondrial VDAC. Proc. Natl. Acad. Sci. U. S. A. 2006;103:7494–7499. doi: 10.1073/pnas.0602217103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan W, Loke YH, Stein CA CA, Miller P, Colombini M. Phosphorothioate Oligonucleotides Block the VDAC Channel. Biophys. J. 2007 doi: 10.1529/biophysj.107.105379. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCormack JG, Bromidge ES, Dawes NJ. Characterization of the effects of Ca2+ on the intramitochondrial Ca2+-sensitive dehydrogenases within intact rat-kidney mitochondria. Biochim. Biophys. Acta. 1988;934:282–292. doi: 10.1016/0005-2728(88)90088-6. [DOI] [PubMed] [Google Scholar]
- 17.Panov AV, Scaduto RC. Influence of calcium on NADH and succinate oxidation by rat heart submitochondrial particles. Arch. Biochem. Biophys. 1995;316:815–820. doi: 10.1006/abbi.1995.1109. [DOI] [PubMed] [Google Scholar]
- 18.Gronblad M, Akerman KE. Electron-dense endoplasmic reticulum-like profiles closely associated with mitochondria in glomus cells of the carotid body after fixation with oxalate. Exp. Cell Res. 1984;152:161–168. doi: 10.1016/0014-4827(84)90240-4. [DOI] [PubMed] [Google Scholar]
- 19.Hajnóczky G, Csordás G, Madesh M, Pacher P. The machinery of local Ca2+ signalling between sarco-endoplasmic reticulum and mitochondria. J. Physiol. 2000;529:69–81. doi: 10.1111/j.1469-7793.2000.00069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montal M, Mueller P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc. Natl. Acad. Sci. U. S. A. 1972;69:3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colombini M. Characterization of channels isolated from plant mitochondria. Methods Enzymol. 1987;148:465–475. doi: 10.1016/0076-6879(87)48045-2. [DOI] [PubMed] [Google Scholar]
- 22.Freitag H, Benz R, Neupert W. Isolation and properties of the porin of the outer mitochondrial membrane from Neurospora crassa. Methods Enzymol. 1983;97:286–294. doi: 10.1016/0076-6879(83)97140-9. [DOI] [PubMed] [Google Scholar]
- 23.Song J, Midson C, Blachly-Dyson E, Forte M, Colombini M. The topology of VDAC as probed by biotin modification. J. Biol. Chem. 1998;273:24406–24413. doi: 10.1074/jbc.273.38.24406. [DOI] [PubMed] [Google Scholar]
- 24.Schein SJ, Colombini M, Finkelstein A. Reconstitution in planar lipid bilayers of a voltage-dependent anion-selective channel obtained from Paramecium mitochondria. J. Membr. Biol. 1976;30:99–120. doi: 10.1007/BF01869662. [DOI] [PubMed] [Google Scholar]
- 25.Staples BR, Nuttall RL. The activity and osmotic coefficients of aqueous calcium chloride at 298.15K. J. Phys. Chem. Ref. Data. 1977;6:385–407. [Google Scholar]
- 26.Zhang DW, Colombini M. Group IIIA-Metal hydroxides indirectly neutralize the voltage sensor of the voltage-dependent mitochondrial channel, VDAC, by interacting with a dynamic binding site. Biochim. Biophys. Acta. 1990;1025:127–134. doi: 10.1016/0005-2736(90)90089-7. [DOI] [PubMed] [Google Scholar]
- 27.Colombini M. Voltage gating in VDAC: toward a molecular mechanism, Section 4, Chapter 10. In: Miller C, editor. Ion Channel Reconstitution. New York: Plenum Press; 1986. pp. 533–552. [Google Scholar]
- 28.Pavlov E, Grigoriev SM, Dejean LM, Zweihorn CL, Mannella CA, Kinnally KW. The mitochondrial channel VDAC has a cation-selective open state. Biochim. Biophys. Acta. 2005;1710:96–102. doi: 10.1016/j.bbabio.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Báthori G, Csordás G, Garcia-Perez C, Davies E, Hajnóczky G. Ca2+-dependent control of the permeability properties of the mitochondrial outer membrane and voltage-dependent anion-selective channel (VDAC) J Biol Chem. 2006;281:17347–17358. doi: 10.1074/jbc.M600906200. [DOI] [PubMed] [Google Scholar]
- 30.Jordan TE. Vapor pressure of organic compounds. New York: Interscience publishers, Inc.; 1954. [Google Scholar]
- 31.Elliott JR, Needham D, Dilger JP, Haydon DA. The effects of bilayer thickness and tension on gramicidin single-channel lifetime. Biochim. Biophys. Acta. 1983;735:95–103. doi: 10.1016/0005-2736(83)90264-x. [DOI] [PubMed] [Google Scholar]
- 32.Huang HW. Deformation free energy of bilayer membrane and its effect on gramicidin channel lifetime. Biophys. J. 1986;50:1061–1070. doi: 10.1016/S0006-3495(86)83550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lundbaek JA, Andersen OS. Lysophospholipids modulate channel function by altering the mechanical properties of lipid bilayers. J. Gen. Physiol. 1994;104:645–673. doi: 10.1085/jgp.104.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colombini M. Structure and mode of action of a voltage dependent anion-selective channel (VDAC) located in the outer mitochondrial membrane, . Ann. N. Y. Acad. Sci. 1980;341:552–563. doi: 10.1111/j.1749-6632.1980.tb47198.x. [DOI] [PubMed] [Google Scholar]
- 34.Komarov AG, Graham BH, Craigen WJ, Colombini M. The physiological properties of a novel family of VDAC-like proteins from Drosophila melanogaster. Biophys. J. 2004;86:152–162. doi: 10.1016/S0006-3495(04)74093-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porcelli AM, Ghelli A, Zanna C, Pinton P, Rizzuto R, Rugolo M. pH difference across the outer mitochondrial membrane measured with a green fluorescent protein mutant. Biochem. Biophys. Res. Commun. 2005;326:799–804. doi: 10.1016/j.bbrc.2004.11.105. [DOI] [PubMed] [Google Scholar]
- 37.Liu MY, Colombini M. A soluble mitochondrial protein increases the voltage dependence of the mitochondrial channel, VDAC. J. Bioenerg. Biomembr. 1992;24:41–46. doi: 10.1007/BF00769529. [DOI] [PubMed] [Google Scholar]
- 38.Holden MJ, Colombini M. The outer mitochondrial membrane channel, VDAC, is modulated by a protein localized in the intermembrane space. Biochim. Biophys. Acta. 1993;1144:396–402. doi: 10.1016/0005-2728(93)90126-z. [DOI] [PubMed] [Google Scholar]
- 39.Peng S, Blachly-Dyson E, Colombini M, Forte M. Large scale rearrangement of protein domains is associated with voltage gating of the VDAC channel. Biophys. J. 1992;62:123–135. doi: 10.1016/S0006-3495(92)81799-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song J, Midson C, Blachly-Dyson E, Forte M, Colombini M. The sensor regions of VDAC are translocated from within the membrane to the surface during the gating processes. Biophys. J. 1998;74:2926–2944. doi: 10.1016/S0006-3495(98)78000-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


