Abstract
Pups are a highly rewarding stimulus for early postpartum rats. Our previous work supports this notion by showing that suckling activates the mesocorticolimbic system in mothers. In the present study, we tested whether development of behavioral sensitization to cocaine before pregnancy affects the neural response to pups during the early postpartum days (PD). Virgin rats were repeatedly administered cocaine for 14 days (15 mg kg-1) and withdrawn from treatment during breeding and pregnancy. The neural response to suckling was measured at PD 4 to 8 using blood-oxygen-level-dependent (BOLD) MRI or microdialysis. Our results show that BOLD activation in the medial prefrontal cortex (PFC), septum and auditory cortex was curtailed in cocaine-sensitized dams. No differences between cocaine sensitized and saline control dams were observed in the nucleus accumbens, olfactory structures, or in 48 additional major brain regions that were analyzed. Baseline, but not pup-stimulated, dopamine (DA) levels in the medial PFC was lower in cocaine-sensitized dams than in controls. When tested for maternal behaviors, cocaine sensitized dams showed significantly faster retrieval of pups without changes in other maternal behaviors such as grouping, crouching and defending the nest. Taken together, the present findings suggest that maternal motivation to retrieve pups was enhanced by repeated cocaine exposure and withdrawal, a result reminiscent of ‘cross sensitization’ between the drug and a natural reward. Changes in retrieval behavior in cocaine-sensitized mothers might be associated with a hypo-responsive medial PFC.
INTRODUCTION
Mothers recovering from cocaine addiction experience difficulties in their maternal roles as care givers 9, 10. Evidence for this comes from studies reporting reduced mother-child play interactions, low-self esteem, emotional neglect, lack of maternal identity, increased hostility towards own child and inability to cope with stressful situations 1, 9, 20, 26, 40. Although socioeconomic factors affect mothers recovering from cocaine addiction, neurobiological changes resulting from chronic cocaine abuse may also have lasting effects on the maternal brain.
Studies in rats have examined the effects of acute and chronic cocaine administration in gestation and after parturition on the expression of maternal behaviors. Acute cocaine administration during early postpartum causes a dose-dependent disruption in maternal behaviors 29, that returns to normal once plasma cocaine levels drop, suggesting that the acute effects of cocaine are reversible 59. Virgin rats trained to self-administer cocaine were reported to escalate their intake dramatically during pregnancy, but showed a decline during early postpartum days that may have been associated with the attendance of their newborn pups 21. Interestingly, Hecht et al. 21 reported that mothers attend pups by placing them near the active cocaine lever, suggesting that the reinforcing properties of pups compete with those of cocaine. This is supported by Morell and colleagues 38 who reported data suggesting that the motivation for pups during this reproductive stage is greater than the motivation to seek cocaine. Newborn pups are so reinforcing that lactating rats will incessantly attempt to make snout contact with pups, even if prevented to do so by muzzling 56, and will also lever-press to gain access to them 30. Will the intense motivation to seek, attend and protect pups be modified by chronic cocaine exposure early in life, before pregnancy? The present study tested whether development of behavioral sensitization to cocaine in virgin rats affects subsequent maternal behaviors. We hypothesized that repeated cocaine administration before pregnancy would have long-term behavioral and neurobiological outcomes that might contribute to changes in maternal motivation.
Maternal retrieval, nursing and aggression is under the control of several areas of the brain. A core neural circuitry includes the medial preoptic area, paraventricular nucleus of the hypothalamus, ventral pallidum, ventral tegmental area, bed nucleus of the stria terminalis, nucleus accumbens, periaqueductal grey, portions of the amygdala, and the olfactory system 4, 5, 16, 31, 35, 42, 44, 45. Although several of these areas share connectivity with midbrain dopamine neurons, the release of this neurotransmitter in the nucleus accumbens plays an important part in modulating retrieval behavior and nursing of newborn pups 27. The recent use of BOLD fMRI in lactating dams has allowed a detailed analysis of neural processing of an important sensory stimulus for the maternal brain, the suckling stimulus 14. Increasing amounts of data show that the BOLD signal originates from underlying hemodynamic alterations in relation to increases in neuronal activity 11, 52, specifically with changes in local field potentials 33. We previously reported that suckling stimulation robustly activates brain areas of the mesocorticolimbic dopamine system 14. In the present work we observed that females that were sensitized to cocaine before pregnancy showed lower BOLD activation in the PFC, with no changes in the nucleus accumbens (NAC) or ventral tegmental area (VTA) during the postpartum period. Diminished basal levels of extracellular dopamine in the medial PFC were also observed with cocaine pre-exposure. Interestingly, cocaine-sensitized females showed quicker retrieval of pups than cocaine-naive dams, a result reminiscent of behavioral ‘cross-sensitization’ between pup retrieval and cocaine. The enhanced maternal retrieval was not accompanied by differences in other measures of maternal behavior between control and cocaine pretreated dams, a result that does not support the notion that full maternal responding is affected with cocaine pretreatment and withdrawal. Instead, the overall findings support the idea that maternal motivation to retrieve her pups is increased despite the low neural response during mother-pup interactions.
MATERIALS AND METHODS
Animals
Adult Sprague-Dawley females were purchased from Charles River Laboratories (Charles River, MA). Virgin rats were bred in the animal resource facilities of the University of Massachusetts Medical School. Dams were housed with their litters in a temperature and humidity controlled room, under a 12L:12D light-dark cycle with lights off at 1800 hr. Water and Purina rat chow were provided ad libitum. Animals were acquired and cared for in accordance with the guidelines published in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publications No. 85-23, Revised 1985) and adhere to the National Institutes of Health and the American Association for Laboratory Animal Science guidelines. The protocols used in this study were in compliance with the regulations of the Institutional Animal Care and Use Committee at the University of Massachusetts Medical School.
Cocaine administration and behavioral testing
Cocaine administration and behavioral assessments were conducted during the light phase of the light:dark cycle (900 to 1100 hr). Female rats were given a daily injection of cocaine (15 mg kg-1, intraperitoneal) for 14 consecutive days (Figure 1A). Control animals received an injection of 0.9 % sterile saline vehicle (0.1 cc 100 g-1, intraperitoneal). The initial number of animals included in the study was 24 cocaine-pretreated females and 21 controls, which were later divided between microdialysis and fMRI experiments. The behavioral response to cocaine was assessed using a 9-point behavioral rating scale developed by Ellinwood and Balster 13. Briefly, scores were assigned as follows: 1- lying down, eyes closed, 2- lying down, eyes open, 3- normal grooming or chewing cage litter, 4- moving about cage, sniffing and rearing, 5- running movements characterized by rapid changes in position, 6- repetitive exploration of the cage at normal level of activity, 7- repetitive exploration of the cage with hyperactivity, 8- remaining in the same place in cage with fast repetitive head and/or foreleg movement (includes licking, chewing, and gnawing stereotypies) and 9- backing up, jumping, seizures, abnormally maintained postures, dyskinetic movements.
Figure 1.
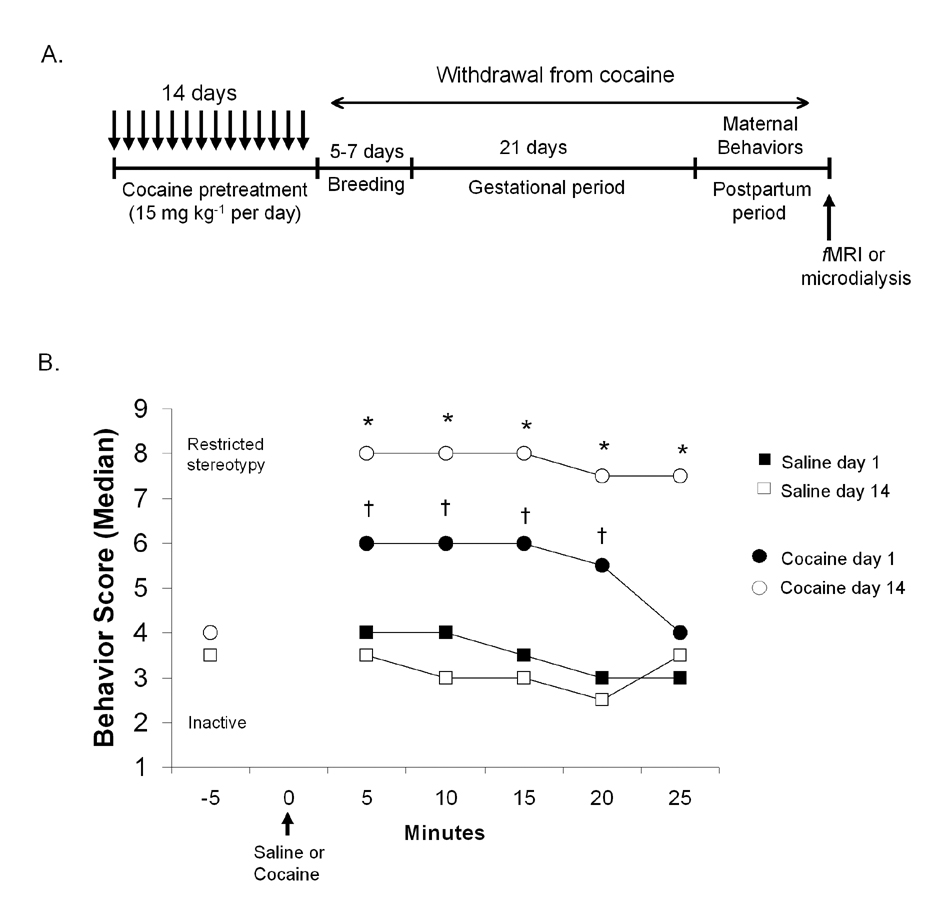
Experimental design and behavioral sensitization to cocaine in virgin rats. A: Experimental design and timeframe for cocaine administration, gestation, maternal behavioral measurements, and functional imaging or microdialysis studies. B: Results from scoring the behavioral response to cocaine. Data are expressed as the median score assigned at each 5 min interval on days 1 and 14 of cocaine treatment. Symbols denote significant differences at p < 0.05 (*cocaine on day 14 versus day 1; † cocaine versus saline injection). Arrow indicates time of cocaine or saline injection.
Following 60 minutes of habituation to the testing cages, a behavioral score was assigned to each rat every 5 minutes for 30 minutes. Testing began 5 minutes prior to cocaine administration and lasted up to 25 min following injections. During testing, one rat was observed for 10 seconds and a score assigned within 10 seconds before passing to the next 13. Behavioral scoring was done for 14 consecutive days. The day after the final saline or cocaine injection, nulliparous control and drug treated rats were housed for 5 days with sexually experienced males. Pregnancy was verified by the presence of a sperm plug in the cage bedding or vaginal canal. In many cases, vaginal smears were observed under a light microscope to confirm the presence of sperm. Females remained undisturbed throughout breeding, pregnancy, and postpartum days 1 to 2, until maternal behavior testing, functional imaging and in vivo microdialysis (Figure 1A).
Maternal behavior testing
The average litter sizes did not vary between the control and cocaine pretreated rats (control litter size was 11.6 ± 1.5 and cocaine sensitized litter size was 12.7 ± 2.4). Group sizes were not standardized and mothers were allowed to freely interact with their pups before the day of testing. Maternal behaviors were assessed on postpartum days 3–4 according to work published by Bridges 7, with minor modifications. Behavioral measurements were done during the light phase of the light-dark cycle (800 to 1100 hr). Dams and pups were removed from their homecage and placed into two separate cages. The nest area was located within the homecage and six pups were placed opposite to the nest, three pups in each corner. After a short 15-minute separation from the pups, dams were returned to their cages for testing. The time taken to retrieve six pups, group and crouch over them was registered during a 15 minute testing session. Afterwards, the number of pups in nest was verified at 30, 45 and 60 minutes after the start of maternal testing. At the end of the study, all pups were returned to their respective mothers. Once all pups were grouped in the nest, a male intruder equal in size to the dam was placed inside the homecage and the latency to attack was also noted.
Functional MRI of pup suckling stimulation
Studies were performed using a multi-concentric dual-coil small animal restrainer (Insight NeuroImaging Systems, Worcester, MA). Prior to breeding, females were acclimated to the restrainer and the imaging protocol as described previously28. To reduce discomfort from ear and nose bars during acclimation and experiments, a topical anesthetic (2.5 % lidocaine/2.5 % prilocaine cream) was applied to skin and soft tissue in the ear canal and over the bridge of the nose. Details of the set-up for suckling stimulation are provided in Ferris et al. 14. Briefly, four pups (4 to 8 days of age) were placed in a weigh boat cradle padded with a double-layer of gauze sheets. The pup cradle was positioned under a ventral opening in the body tube that allowed immediate access to two pairs of teats on each side of the dams’ abdomen (for a total of 4 teats accessible to pups). Gliding a thin plastic sheet over the ventral opening of the body tube controlled access to teats. The end of the plastic sheet was easily accessible from outside of the bore of the magnet. The hindlimbs of dams were loosely tethered and raised just above the floor of the body tube. This provided a visual inspection of the ventrum from outside the magnet and prevented the dam from kicking and injuring pups during suckling. Pups used for the imaging session were separated from the dams for about 45–60 minutes before the start of suckling stimulation. Our previous results indicated that pups attached to teats within seconds after a separation time 14. Suckling was confirmed after the completion of each functional scan.
Functional MRI parameters
Functional imaging was conducted in a Bruker Biospec 4.7-T/40-cm horizontal magnet (Oxford Instrument, Oxford, U.K.) equipped with a Biospec Bruker console (Bruker, Billerica, MA U.S.A) and a 20-G/cm magnetic field gradient insert (ID = 12 cm, 120-µs rise time). Functional imaging was performed with a T2-weighted multi-slice fast spin echo pulse sequence with the following parameters: 14 slices, 1.2 mm thick, field of view of 30 mm, 64 × 64 data matrix, echo train length (ETL) of 16, echo spacing of 7 milliseconds (msec), repetition time (TR) of 2108 msec, effective echo time (TEeff) of 53.2 msec. A single acquisition of all 14 slices took 8.4 sec. For suckling stimulation, 110 repetitions were acquired for a total scan time of 15 minutes. After a 5-minute baseline, pups were allowed access to teats for the remaining 10-minutes. Suckling was terminated by replacement of the plastic partition. A high-resolution anatomical scan was then collected for 6 minutes using a fast spin echo pulse sequence (TEeff = 48 msec, TR = 2500 msec, FOV = 30 mm, 1.2 mm slice thickness, 256 × 256 data matrix, ETL = 16, 12 averages).
Dopamine microdialysis
On postpartum day 4–6, surgical implantation of cannulae (Bioanalytical Systems, Baltimore, MD) into the medial PFC was carried out under 3.5 % isoflurane anesthesia using the following the Bregma coordinates: anterior-posterior 3.2 mm, mediolateral 0.6 mm, dorsoventral 3 mm. Experiments were done on postpartum days 7–9, following 2–3 days of recovery. Pups were removed from home cages and microdialysis probes (Bioanalytical Systems, Baltimore, MD) were inserted into guide cannulas 1.5–3 hours prior to sample collection. A metallic arm swivel (CMA, Chelmsford, MA) extended 40 cm above the homecage walls allowing dams to move freely about the cage during experiments. The semi-permeable dialysis membrane of the probe was positioned 1 mm outside the guide cannula tip (4 mm below dura). The probe was perfused with artificial cerebrospinal fluid continuously before and during sample collection at a flow rate of 1.8 µl/min (aCSF contained, in mM: 145 NaCl, 2.7 KCl, 10 MgCl2, 12 CaCl2, 20 Na2HPO4•7H20, pH 7.4). Samples were collected at 15 min intervals for 2 hours and 15 minutes (45 min baseline and 90 minutes after pup presentation) into 10 µl of 20 % perchloric acid. Thus, dams were separated from pups for 2.5–3.5 hours, after which pups were scattered throughout the cage and samples collected for an additional 90 minutes.
Samples were immediately analyzed for dopamine and dihydrophenylacetic acid (DOPAC) content using high performance liquid chromatography (HPLC) with coulometric detection (ESA, Chelmsford, MA). Mobile phase (ESA, Chelmsford, MA) flowed through the system at a flow rate of 0.5 ml/min. Coulometric electrodes were set at −175 mV (pre-oxidation), +150 mV (oxidation) and the guard cell at +300 mV. A 10 % retention window was used, with a retention time for dopamine of 4.7 min and DOPAC 3.6 min (Figure 7C). An external standard calibration curve for catecholamines and DOPAC was generated (7 different concentrations ranging from 0.001 pmol/10µl to 5.3 pmol/10µL) to quantitatively analyze dialysates. Using the above conditions, we estimated a signal-to-noise ratio for catecholamines of over 2:1, with a detection limit for dopamine over 0.005 pmol/10µL. Data are presented as pmol/10µl sample uncorrected for probe recovery. After experiments, rats were sacrificed and brains excised for gross verification of cannula placement using the forceps minor of the corpus callosum as an anatomical landmark (see Figure 5A–B).
Figure 5.
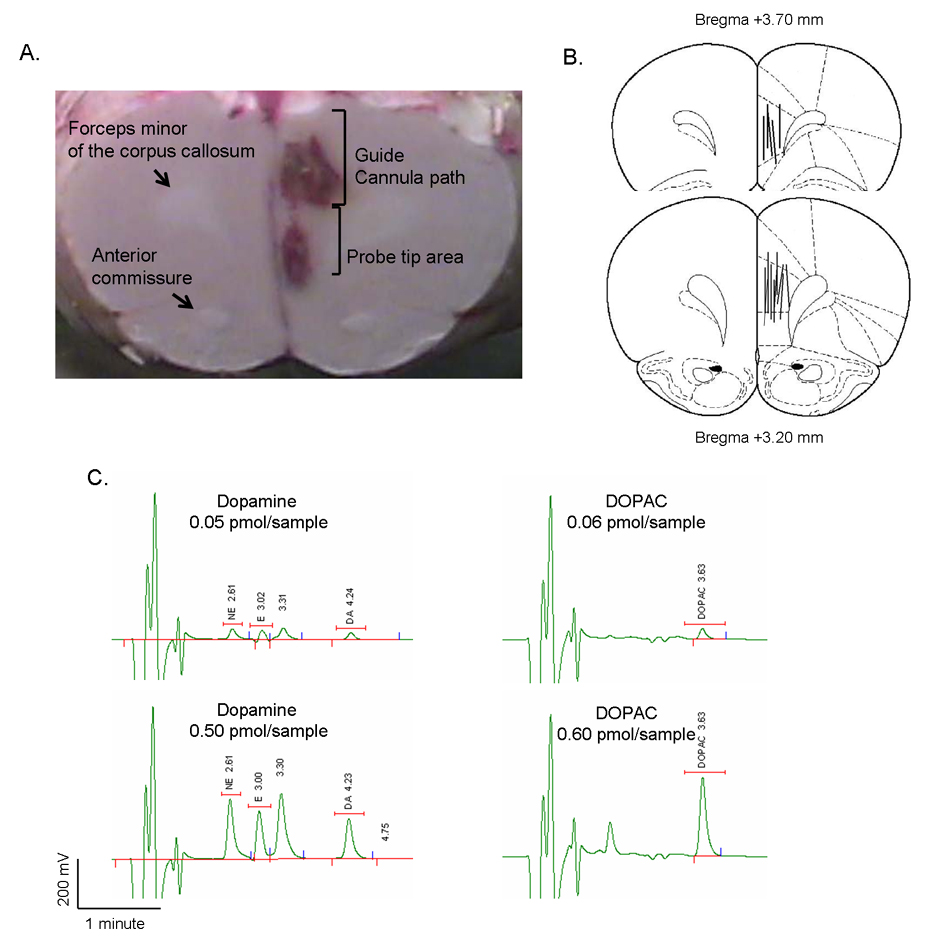
Microdialysis experiments. A: Histological identification of the guide cannula placement into the medial prefrontal cortex. Representative fresh rat brain sectioned after microdialysis experiments. Gross anatomical localization was used to assess correct cannula placement, using the forceps minor of the corpus callosum as a reference landmark. Guide cannula trace and probe tip area are discernable. B: Paxinos and Watson atlas maps indicating site of cannula placement across all animals. C: Chromatograms showing retention times for two concentrations of dopamine and DOPAC.
Statistical analysis
Behavior and dopamine microdialysis
Maternal behaviors were tested in 13 control and 15 cocaine sensitized dams. Microdialysis was done in 10 control and 13 cocaine sensitized. Data were evaluated statistically using analysis of variance to test if saline or cocaine treatments (independent variables) affected maternal behaviors (retrieval, grouping, crouching, and attacks) and dopamine/DOPAC levels. Cocaine-induced behavioral activation was analyzed using a repeated measures analysis of variance.
fMRI
Each functional MRI scan was first inspected for excess motion by estimating the standard deviation from the image center-of-mass using Stimulate software 57. Animals showing an average displacement exceeding 25% of the total inplane (X–Y) voxel resolution (>120 µm out of 468 µm) or more than 25% displacement in the slice (Z) direction (>300 µm out of 1200 µm slice thickness) were excluded (see supplemental Figure 1). Timeseries movies were also generated in order to qualitatively confirm the presence of motion. Scans with prestimulus baseline drifts over 2% were excluded. After applying exclusion criteria, the final n was 9 control and 6 cocaine-sensitized dams. Scans that passed the exclusion criteria and inspected for artifacts were aligned to a 3D atlas of the rat brain.
ROI-based statistical analysis was done using Medical Image Visualization and Analysis (MIVA) software (http://ccni.wpi.edu/cwbench/cwbench-tiles.jsp). Details of the alignment of scans to the rat brain atlas have been published elsewhere 14, 63. Each subject was registered to a fully segmented electronic rat brain atlas based on 2D textbook images 46, 58. The alignment process began by outlining the brain perimeters for each slice of the anatomy image sets. A marching cubes algorithm with automated linearization creates accurate 3D surface shells for each subject 63. This enhanced surface generation strategy eliminates the characteristic stair-stepped behavior of the marching cubes algorithms while simultaneously increasing the accuracy of the geometry representation. These anatomy shells are aligned to the atlas shell. The affined registration involved translation, rotation, and scaling in all 3 dimensions, independently. The matrices that transformed the subject’s anatomy shells to the atlas space were used to embed each slice within the atlas. All transformed pixel locations of the anatomy images were tagged with the segment atlas major and minor regions creating a fully segmented representation of each subject. The inverse transformation matrix [Ti]-1 for each subject (i) was also calculated.
Statistical t tests were performed on each subject within their original coordinate system. The baseline period was 32 repetitions immediately preceding the stimulus and the stimulation window was the 32 repetitions that immediately followed (4.5 minutes each period). Statistical t tests used a 95 % confidence level, two-tailed distribution, and heteroscedastic variance assumptions. In order to provide a conservative estimate of significance, a false-positive detection-controlling algorithm was introduced into the analysis 17. This ensured that the false-positive detection rate was below out cutoff of 5 % 14. Statistically significant pixels were assigned their percentage change values (stimulus mean minus control mean) and all other pixels were set to zero. Statistical composite maps were created.
The segmented atlas was cropped and rendered onto 14 slices of 2562 resolution corresponding to the field of view of the subjects. The cropped atlas served as a segmented composite with coordinates for row, column and slice. A statistical composite was created for each group. A Bonferroni correction factor was used to maintain an overall uncertainty level of 5% for comparisons between groups. The individual analyses were summed within groups. The composite statistics were built using the inverse transformation matrices. Each composite pixel location (ie, row, column, slice), premultiplied by [Ti]-1, mapped it within a subject voxel (i). A trilinear interpolation of the subject’s voxel values (percent change) determined the statistical contribution of the subject (i) to the composite location. The use of [Ti]-1 ensured that the full volume set of the composite was populated with subject contributions. The average value from all subjects within the group determined the composite value. Due to the inability to align each subject at a pixel-level resolution, the composite BOLD response maps may result somewhat broader in their spatial coverage than in the individual maps. However, the subjects did align very well at a ROI-level resolution. Statistical comparisons were performed using analysis of variance and t-test analysis for BOLD data (p < 0.05, corrected for multiple-groups comparisons).
RESULTS
Cocaine-induced behavioral sensitization
Prior to cocaine administration on days 1 through 14, all animals displayed in place activities or were in a normal, alert state (categories 3 and 4 of the Ellinwood and Balster rating scale). As shown in Figure 1B, repeated cocaine administration across 14 days resulted in behavioral sensitization. On day 1, cocaine administration resulted in normal, alert activity, hyperactivity and slow patterned exploration of the testing cages (median category 6, ranging from 4 to 7), while saline injected animals largely displayed in place activities (median category 3.5, ranging from 2 to 4) (Kruskall-Wallis ANOVA H1, 16 > 8.6, p < 0.03 for all time points in Figure 1B). By day 14 of cocaine administration, animals mainly expressed exacerbation of repetitive movements, or stereotypies, that restricted their movement about the cage (median category 8, ranging from 7 to 8), while saline treated rats showed in place activities (median category 3, ranging from 2 to 4). The behavioral score assigned to cocaine treated animals on day 14 was significantly greater than their response to cocaine on day 1 (repeated measures ANOVA F6, 23 = 16.27, p < 0.001) or the behavioral score assigned to saline treated animals (Kruskall-Wallis ANOVA H1, 16 > 9.1, p < 0.002 for all time points in Figure 1B).
Maternal retrieval affected by cocaine pretreatment
Litter size ranged from 8 to 15 pups and was not affected by prior cocaine treatment. Figure 2 summarizes data for maternal behaviors. Under the same testing conditions, cocaine treated dams showed a shorter latency to retrieve all pups to the nesting area (Kruskall-Wallis ANOVA H1, 28 > 4.4, p < 0.03 for all pups). No differences in the latency to group pups into the nest, crouch over them and attack an intruder were observed (Figure 2). Pup grouping and maternal crouching over pups commenced at a similar time as controls (Figure 2). As a result, cocaine sensitized dams showed a significant delay between retrieval of the last pup and the commencement of crouching (Figure 3) (t26=2.05, p = 0.002). Control mothers immediately attended pups after retrieval, whereas cocaine pre-exposed dams took longer to begin attending pups after retrieving them.
Figure 2.
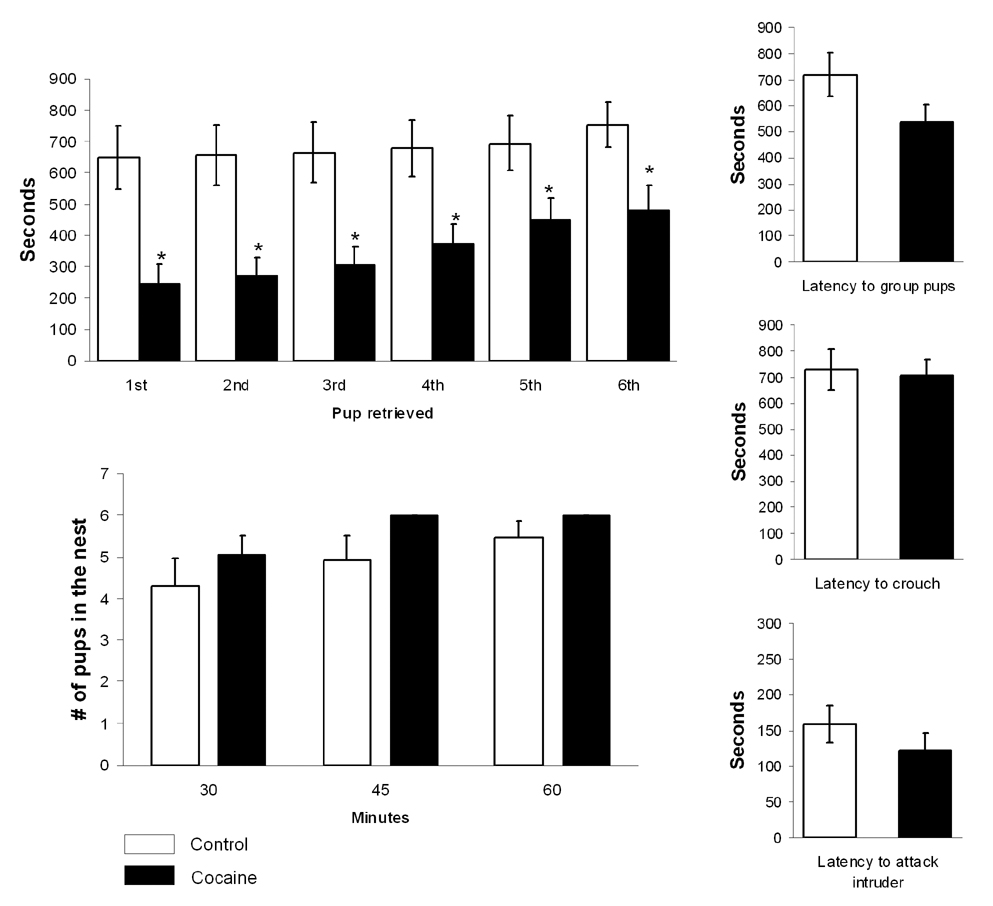
Maternal behaviors in control and cocaine sensitized dams on postpartum days 3 to 4. Shown above are the latency to retrieve, group and crouch over 6 pups during a 15 minute test, number of pups in the nest at various time intervals and latency to attack a nest intruder. *Significantly different (p < 0.01). Latencies are presented in seconds (mean ± SEM).
Figure 3.
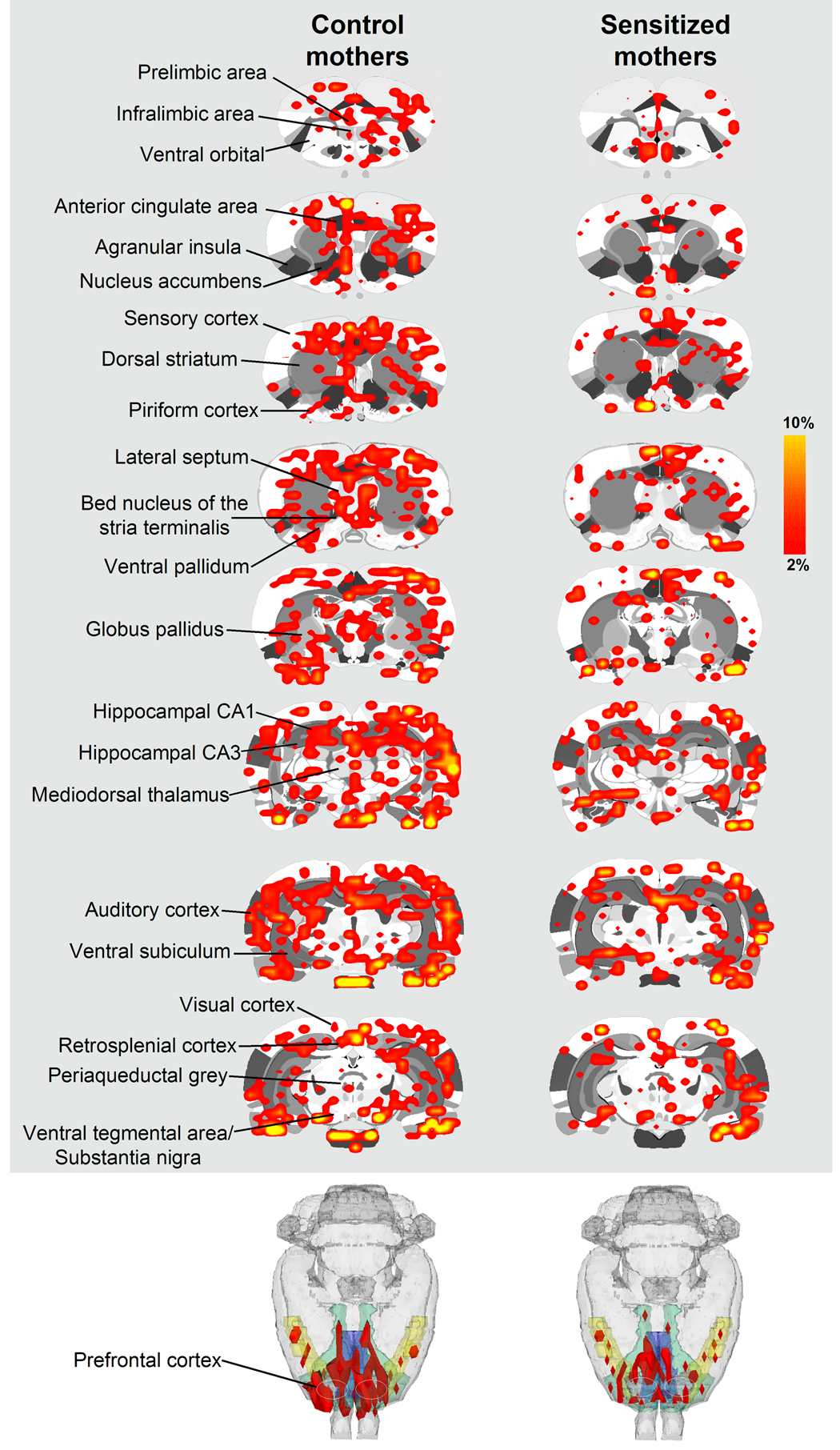
BOLD signal changes in response to suckling stimulation in control and cocaine sensitized dams. Top columns show 2-dimensional rat atlas maps segmented into various regions of interest (some of them indicated on the left). Colored areas correspond to regions of the brain that showed statistically significantly increases in BOLD signal intensity with suckling. Scale bar hue to the right indicates percent increase in BOLD. Shown in the bottom are 3-dimensional renditions highlighting the volume of frontal cortex (in red) activated by suckling in drug naïve and cocaine sensitized rats. The segment of frontal cortex includes rostral limbic cortical areas and the strip of motor cortex caudally.
Description of gross motion during scanning
Acclimating animals to the imaging restraint minimizes many of the sources of motor artifact in awake animals (physiological, gross motion) 28. Here we observed that most motion during scanning occurs in the Y plane (up-down head movements; see Supplementary Figure 1). Animals moving beyond 25% of the inplane single voxel resolution were excluded.
Cocaine pretreatment attenuates suckling stimulated BOLD signal changes
Functional MRI data were analyzed as percent BOLD signal changes following pup presentation and also as the number of activated voxels across 51 major regions of interest. Control dams showed BOLD signal increases in response to suckling in areas of the reward system (i.e., nucleus accumbens, prefrontal cortex, ventral tegmental area, caudate-putamen, substantia nigra) (Figure 3). In comparison to controls, cocaine sensitized dams showed curtailment in the number of activated BOLD voxels in the medial PFC (infralimbic/ ventral orbital area of the PFC, F1, 13 = 9.0, p = 0.01), septum (F1, 13 = 5.8, p = 0.03) and the primary auditory cortex (F1, 13 = 12.9, p = 0.003) (Figure 3 and Figure 4A). The lateral prefrontal cortex (corresponding to the primary motor cortex) also showed a reduced BOLD response to suckling stimulation, however it did not hold out significantly different (p = 0.06). In addition to the number of voxels, percent change in BOLD was greater in the infralimbic/orbital region of the PFC of control versus sensitized dams (Figure 4B) (two-tailed Student’s t-test t11=2.6 p = 0.04). No differences in percent changes in BOLD were observed in all other regions of interest analyzed [including the septum and auditory cortex, which showed reduced number of activated voxels in cocaine sensitized rats]. Little negative BOLD signal changes were observed in both groups, with no significant effects for cocaine pretreatment.
Figure 4.
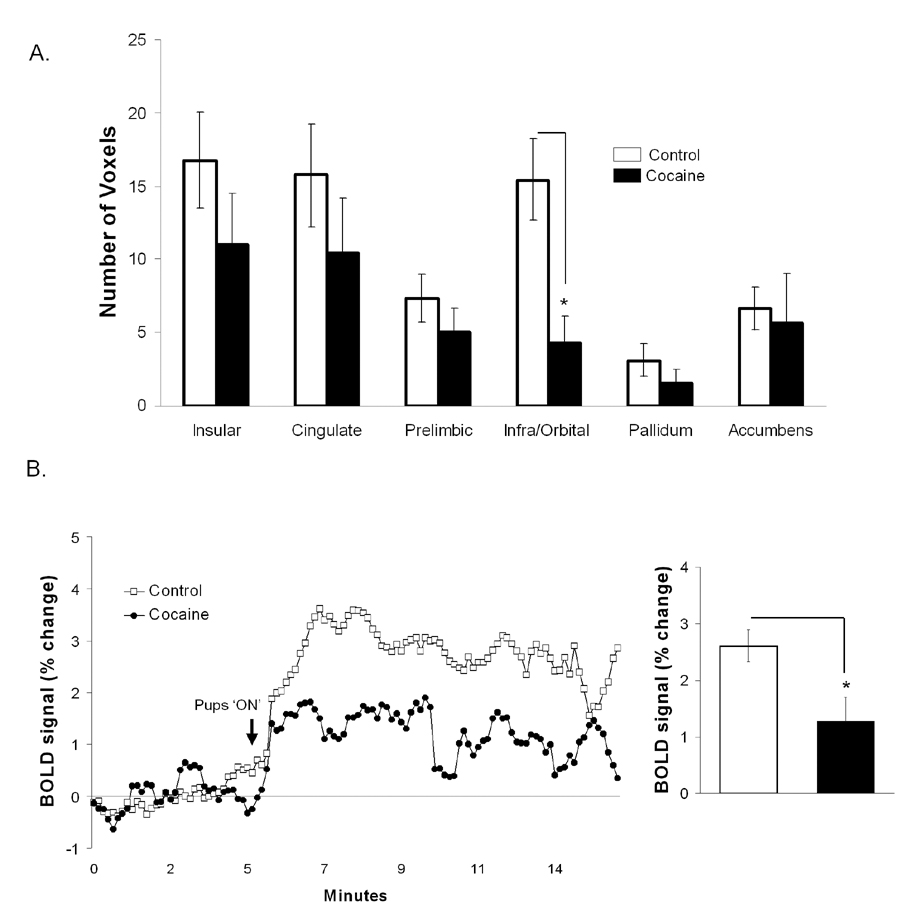
BOLD signal response to suckling in control and cocaine sensitized dams. A: Number of brain voxels showing changes in the BOLD signal. B: Percent change in BOLD signal in drug naïve and cocaine pretreated rats. Arrow indicates the time pups are given access to the mother’s ventrum. Asterisks indicate significant differences in comparison to control dams (p < 0.05). All data are presented as mean ± SEM.
Baseline and pup-stimulated dopamine and DOPAC levels in the medial PFC
Levels of dopamine and DOPAC were estimated for the entire period of sampling (2 hrs 15 min). The overall concentrations of dopamine were significantly different between drug naïve and cocaine sensitized animals, even after 3 hrs of acclimation to dialysis probe placement and experimental setup (Figure 6). Average extracellular dopamine levels (uncorrected for probe recovery) during the baseline period were estimated to be 0.21 ± 0.06 picomol per 10 µl sample in drug naïve dams and 0.07 ± 0.02 picomol per 10 µl sample in cocaine sensitized mothers (two-tailed Student’s t-test t21 = 2.07, p = 0.02). The control baseline value for dopamine is similar to previously reported levels in males 51. Average extracellular DOPAC levels were estimated to be 0.17 ± 0.05 pg per sample in drug naïve animals and 0.09 ± 0.01 pg per sample in cocaine sensitized animals (two-tailed Student’s t-test t21 = 2.09, p = 0.08). Pups significantly elevated extracellular dopamine concentrations in the medial PFC of control and cocaine-sensitized mothers, however this increment lasted for 15–30 minutes for both dopamine and DOPAC. When analyzed as percent change from pre-stimulus dopamine or DOPAC concentrations, no differences were observed between the two treatment conditions (Figure 6).
Figure 6.
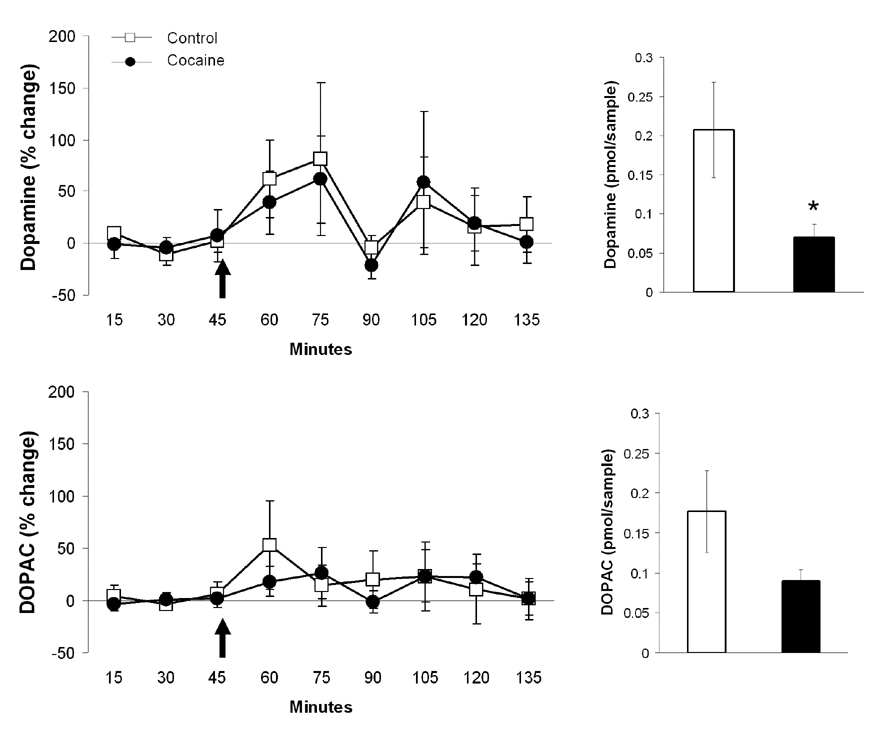
Extracellular dopamine and DOPAC levels over time in the medial prefrontal cortex of control and cocaine sensitized dams. Arrows indicate the time pups were presented. Bar graphs on the right summarize baseline dopamine and DOPAC levels. Data are expressed in picomol per 10 µl samples (mean ± SEM). Asterisk denote statistical differences at p <0.05.
DISCUSSION
The present study provides evidence that development of behavioral sensitization to cocaine before pregnancy alters maternal retrieval during the early postpartum period. Under similar testing conditions, cocaine pre-exposed females displayed shorter latency to obtain pups than control mothers, in a manner suggestive of increased sensitization to pups. Interestingly, the faster retrieval behavior was not accompanied by alterations in other maternal behaviors, such as grouping, hovering over pups and aggression towards a male intruder. The sensitized response to pups was associated with a curtailed prefrontal cortical BOLD response to suckling, with no changes in mesolimbic or olfactory pathways. The fact that the observed behavioral and physiological measures were altered even after such an extended period of withdrawal from cocaine (ca. >30 days) suggests that enduring changes in prefrontal cortical dopamine may have occurred after repeated cocaine administration. To test this, in vivo microdialysis was used to measure dopamine and DOPAC in the medial PFC. Basal extracellular levels of dopamine were significantly lower in cocaine-sensitized dams than in controls. Presentation of pups resulted in a significant rise in dopamine or DOPAC in these groups; however, they did not differ in their response to pups. This suggests that additional neurotransmitter systems or biochemical steps in dopamine metabolism other than dopamine release may underlie the observed hypo-responsiveness in the medial PFC to pups.
The results of the present work complements a significant body of literature reporting the effects of repeated cocaine across gestation and early in the postpartum period. Chronic gestational and postpartum cocaine has been shown to disrupt maternal retrieval and crouching 24, 29. Vernotica et al. 59 showed that cocaine dose-dependently interferes with the expression of maternal retrieval, nest building, crouching and aggressiveness towards nest intruders for up to 4 hours after treatment but not at 24 hours after the last injection. Thus, maternal responsiveness was affected only when cocaine was present at high levels in the bloodstream of dams and was restored following the elimination of the drug from plasma. In fact, work by Johns et al. 25 indicated that after repeated administration of cocaine through gestation and the postpartum period there was a disruption in the onset and maintenance of retrieval, licking, crouching that was not present following cessation of chronic cocaine treatment (ie, cocaine withdrawal). In the aforementioned work, cocaine was administered during pregnancy or at some point after parturition. Here we focused primarily on the long-term effects of repeated cocaine given before pregnancy on subsequent maternal behaviors.
We hypothesized that alterations brought forth by repeated psychostimulant exposure would have long-term behavioral and neurobiological consequences resulting in changes in maternal motivational state. A study by Fiorino and Phillips 15, using a different behavioral measure, support this notion. They showed that amphetamine sensitization facilitated several measures of sexual motivation in male rats. In their study, amphetamine treatment was given 21 days prior to behavioral testing. Amphetamine treated males showed shorter latencies to mount and intromit than controls, despite no differences in ejaculation latency and post-ejaculatory interval 15. Elevated levels of dopamine in the nucleus accumbens were associated with the observed behavioral changes in response to this natural incentive (sex) 15. Here, maternal motivation seems to have been affected by previous cocaine administration. Although maternal retrieval was faster, other behavioral patterns reflecting maternal care and protection were unaffected. A broader interpretation of these findings may be that the biological adaptations occurring as a consequence of cocaine sensitization impel animals to seek rewarding stimuli with a greater intensity.
The lower baseline levels of extracellular dopamine in the medial PFC could represent a biological event influencing maternal retrieval. Several studies have reported on the role of nucleus accumbens dopamine in regulating maternal behavior. Interaction with pups has been reported to enhance the release of dopamine in the accumbens of dams 19. Destruction of dopamine terminals in this area reduces retrieval 18. Blocking accumbens dopamine receptors with a non-selective antagonist (Cis-flupenthixol) reduced maternal retrieval but also increased timespent in a nursing posture 27. Work by Numan et al. 43 has further demonstrated that retrieval behavior is under selective control of D1 receptors in the accumbens shell. The role of dopamine and its receptors in subregions of the medial prefrontal cortex has been suggested across several studies 8, 22, 37, but has not been studied in sufficient detail. Our present findings are to some extent in accordance with previous work showing changes in medial PFC dopaminergic activity in rats 2, 54, 61, 62. Across studies, the release of dopamine was reduced in the medial PFC of male rats after withdrawal from repeated cocaine or amphetamine administration. Cocaine-induced changes in dopamine release appear up to 2 weeks of withdrawal but recover by 30 days 62; however, as suggested by our present results employing a 30 day withdrawal period in females, there may be a need for further experiments to support or rule out longer lasting effects of repeated cocaine on extracellular dopamine signaling. Our data are not entirely consistent with the above-cited reports, which mainly focus on cocaine-associated changes in dopamine release and not differences in basal levels 2, 54, 61, 62. Interestingly, a closer inspection of the reported baseline values for dopamine across these reports reveals that they tend to be lower in cocaine-pretreated animals than saline controls 2, 54, 61.
Rewarding stimuli, or stimuli signalling the proximity of a natural reward, activate the medial PFC 50, 60. The mPFC may also play a role in responding to the incentive properties of pups rather than nonspecifically organizing motoric aspects of maternal behaviors. However, the specific role of medial prefrontal subregions in maternal behaviors in rats has not been studied in detail. Lesioning limbic prefrontal territories, like the anterior cingulate in rodents, dramatically reduce maternal retrieval behavior 55. But ablation of extensive lateral cortical areas has not proven to interfere dramatically with maternal behavioral patterns in hamsters, rats and cats 3, 39, 55. Involvement of the PFC in responding to infant-related cues is supported by several human imaging studies 36, 41, 47. In one study, orbital frontal PFC activation was observed upon presenting first-time mothers with a picture of their own newborn infant. The pattern of neural activation was correlated with self-reported levels of positive moods 41. Work by our group using functional MRI in awake, lactating dams showed that suckling stimulation from pups activates regions of the reward system, nucleus accumbens, dorsal striatum, substantia nigra, VTA and the medial PFC 14. Increases in BOLD activation were significantly greater with suckling than with cocaine administration 14. This has been supported by more recent work showing robust increases in c-Fos expression in the medial PFC of postpartum rats actively engaged in pup seeking in place conditioning tests 37.
Our observation that BOLD activity in response to suckling was reduced in the orbital- and infralimbic of cocaine-sensitized dams might be interpreted in terms of a reduction in reward value or motivational impact of the suckling stimulus. However, this finding from fMRI experiments does not reconcile with the faster retrieval behavior in sensitized dams. To interpret these results it is important to consider that the pattern of neuronal activity involved in the active search for a reward might vary from neuronal activity during the consummation of a reward 48. During imaging sessions dams are passively receiving suckling stimulation from pups, thus prefrontal cortical BOLD activation could reflect neuronal activity during a consummatory phase instead of a preceding appetitive phase. To corroborate this hypothesis, future fMRI studies will be designed to test both the distal response to pup cues (appetitive) versus the passive neural response to suckling (consummatory).
There were several caveats in the present experiments that need to be addressed in future studies. We did not assess whether there are additional changes in other maternal measures that might lead to negative outcomes in the health and development of pups. These may include time spent hovering over pups, time spent nursing, number of nursing and licking and grooming bouts during the day. In addition, measures of maternal anxiety-like behaviors and locomotor activity need to be considered in future experimental designs. With regards to the dopamine microdialysis, the fact that we did not observe any differential responses to pups between cocaine sensitized and control dams may be in part associated with the placement of dialysis cannulas in the pre- versus infralimbic cortex in a majority of the dams. Importantly, we are very hesitant to extrapolate the present findings in rats directly to real-life human circumstances, which by far is more complex in terms of the maternal psychological, emotional state and social organization. We believe that the increased maternal retrieval observed in cocaine sensitized rats, may not correspond to better maternal care but instead might reflect changes in responding to natural incentives; in this case it is the pups. Regarding the fMRI findings, it is important to mention that differences in the BOLD response between control and cocaine sensitized dams may correspond to changes in basal activity in the medial PFC 23, in addition to neural activity once suckling commences. A higher basal activity of neurons within medial PFC most likely would’ve also contributed to the present results.
Conclusions
Repeatedly treating female rats with cocaine prior to pregnancy had lasting effects on medial PFC activation during nursing and altered maternal retrieval behavior. In addition to differential BOLD responses in the medial PFC, we also observed that the auditory cortical and septal neural response followed along the same lines, a reduced activation in cocaine-experienced dams. The findings of the auditory cortex may have an important functional basis since this area has been studied for its role in maternal responding 12. Acoustic features of pup ultrasonic calls are represented differently in the auditory cortex of maternal and virgin rats, with mothers being sensitive to variations in call frequencies 32. In the case of the septum, dopaminergic innervation of this region has been linked to spatial orientation in rats 53. Also, presenting mothers with pups following a 48 hr separation induced Fos-like immunoreactivity in this area 34. Whether or not the present neural changes resulted in cross-sensitization between cocaine and pups remains open to testing. When virgin rats are repeatedly exposed to pups, this results in heightened levels of pup retrieval behavior and other ‘maternal’-like responses 49, termed pup-sensitization 6. Pup sensitization is believed to occur naturally as part of the normal changes in the brain that take place during late gestation and early postpartum days 49. If there is a cross sensitization between cocaine and pups then it might be the case that there exists a converging circuitry, perhaps involving the medial PFC.
Supplementary Material
Motion exclusion criteria (see text). Graph shows average center-of-mass displacement in X (left-right), Y (up-down) and Z (forward-back) for each animal scanned. Inset shows average displacement in all three directions. Arrows (red) show individual scans that were discared due to excess motion.
Acknowledgments
The authors thank Ms. Tara Messenger-Stolberg for her excellent technical assistance through the course of the study. This work was supported by a grant from the National Institute on Drug Abuse (DA13517) to Craig F. Ferris.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Amir LH, Donath SM. Does maternal smoking have a negative physiological effect on breastfeeding? The epidemiological evidence. Birth. 2002;29:112–123. doi: 10.1046/j.1523-536x.2002.00152.x. [DOI] [PubMed] [Google Scholar]
- 2.Beyer CE, Steketee JD. Dopamine depletion in the medial prefrontal cortex induces sensitized-like behavioral and neurochemical responses to cocaine. Brain Res. 1999;833:133–141. doi: 10.1016/s0006-8993(99)01485-7. [DOI] [PubMed] [Google Scholar]
- 3.Bjursten LM, Norrsell K, Norrsell U. Behavioural repertory of cats without cerebral cortex from infancy. Exp Brain Res. 1976;25:115–130. doi: 10.1007/BF00234897. [DOI] [PubMed] [Google Scholar]
- 4.Bosch OJ, Kromer SA, Brunton PJ, Neumann ID. Release of oxytocin in the hypothalamic paraventricular nucleus, but not central amygdala or lateral septum in lactating residents and virgin intruders during maternal defence. Neuroscience. 2004;124:439–448. doi: 10.1016/j.neuroscience.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 5.Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain oxytocin correlates with maternal aggression: link to anxiety. J Neurosci. 2005;25:6807–6815. doi: 10.1523/JNEUROSCI.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bridges R, Zarrow MX, Gandelman R, Denenberg VH. Differences in maternal responsiveness between lactating and sensitized rats. Dev Psychobiol. 1972;5:123–127. doi: 10.1002/dev.420050205. [DOI] [PubMed] [Google Scholar]
- 7.Bridges RS. A quantitative analysis of the roles of dosage, sequence, and duration of estradiol and progesterone exposure in the regulation of maternal behavior in the rat. Endocrinology. 1984;114:930–940. doi: 10.1210/endo-114-3-930. [DOI] [PubMed] [Google Scholar]
- 8.Broad KD, Hinton MR, Keverne EB, Kendrick KM. Involvement of the medial prefrontal cortex in mediating behavioural responses to odour cues rather than olfactory recognition memory. Neuroscience. 2002;114:715–729. doi: 10.1016/s0306-4522(02)00231-2. [DOI] [PubMed] [Google Scholar]
- 9.Coyer SM. Mothers recovering from cocaine addiction: factors affecting parenting skills. J Obstet Gynecol Neonatal Nurs. 2001;30:71–79. [PubMed] [Google Scholar]
- 10.Coyer SM. Women in recovery discuss parenting while addicted to cocaine. MCN Am J Matern Child Nurs. 2003;28:45–49. doi: 10.1097/00005721-200301000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Devor A, Tian P, Nishimura N, Teng IC, Hillman EM, Narayanan SN, Ulbert I, Boas DA, Kleinfeld D, Dale AM. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. J Neurosci. 2007;27:4452–4459. doi: 10.1523/JNEUROSCI.0134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehret G. Left hemisphere advantage in the mouse brain for recognizing ultrasonic communication calls. Nature. 1987;325:249–251. doi: 10.1038/325249a0. [DOI] [PubMed] [Google Scholar]
- 13.Ellinwood EH, Jr, Balster RL. Rating the behavioral effects of amphetamine. Eur J Pharmacol. 1974;28:35–41. doi: 10.1016/0014-2999(74)90109-5. [DOI] [PubMed] [Google Scholar]
- 14.Ferris CF, Kulkarni P, Sullivan JM, Jr, Harder JA, Messenger TL, Febo M. Pup suckling is more rewarding than cocaine: evidence from functional magnetic resonance imaging and three-dimensional computational analysis. J Neurosci. 2005;25:149–156. doi: 10.1523/JNEUROSCI.3156-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiorino DF, Phillips AG. Facilitation of sexual behavior and enhanced dopamine efflux in the nucleus accumbens of male rats after D-amphetamine-induced behavioral sensitization. J Neurosci. 1999;19:456–463. doi: 10.1523/JNEUROSCI.19-01-00456.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleming A, Vaccarino F, Tambosso L, Chee P. Vomeronasal and olfactory system modulation of maternal behavior in the rat. Science. 1979;203:372–374. doi: 10.1126/science.760196. [DOI] [PubMed] [Google Scholar]
- 17.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 18.Hansen S. Maternal behavior of female rats with 6-OHDA lesions in the ventral striatum: characterization of the pup retrieval deficit. Physiol Behav. 1994;55:615–620. doi: 10.1016/0031-9384(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 19.Hansen S, Bergvall AH, Nyiredi S. Interaction with pups enhances dopamine release in the ventral striatum of maternal rats: a microdialysis study. Pharmacol Biochem Behav. 1993;45:673–676. doi: 10.1016/0091-3057(93)90523-v. [DOI] [PubMed] [Google Scholar]
- 20.Harmer AL, Sanderson J, Mertin P. Influence of negative childhood experiences on psychological functioning, social support, and parenting for mothers recovering from addiction. Child Abuse Negl. 1999;23:421–433. doi: 10.1016/s0145-2134(99)00020-4. [DOI] [PubMed] [Google Scholar]
- 21.Hecht GS, Spear NE, Spear LP. Changes in progressive ratio responding for intravenous cocaine throughout the reproductive process in female rats. Dev Psychobiol. 1999;35:136–145. [PubMed] [Google Scholar]
- 22.Hernandez-Gonzalez M, Navarro-Meza M, Prieto-Beracoechea CA, Guevara MA. Electrical activity of prefrontal cortex and ventral tegmental area during rat maternal behavior. Behav Processes. 2005;70:132–143. doi: 10.1016/j.beproc.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Hyder F, Rothman DL, Shulman RG. Total neuroenergetics support localized brain activity: implications for the interpretation of fMRI. Proc Natl Acad Sci U S A. 2002;99:10771–10776. doi: 10.1073/pnas.132272299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johns JM, Noonan LR, Zimmerman LI, Li L, Pedersen CA. Effects of chronic and acute cocaine treatment on the onset of maternal behavior and aggression in Sprague-Dawley rats. Behav Neurosci. 1994;108:107–112. doi: 10.1037//0735-7044.108.1.107. [DOI] [PubMed] [Google Scholar]
- 25.Johns JM, Noonan LR, Zimmerman LI, Li L, Pedersen CA. Effects of short- and long-term withdrawal from gestational cocaine treatment on maternal behavior and aggression in Sprague-Dawley rats. Dev Neurosci. 1997;19:368–374. doi: 10.1159/000111234. [DOI] [PubMed] [Google Scholar]
- 26.Johnson AL, Morrow CE, Accornero VH, Xue L, Anthony JC, Bandstra ES. Maternal cocaine use: estimated effects on mother-child play interactions in the preschool period. J Dev Behav Pediatr. 2002;23:191–202. doi: 10.1097/00004703-200208000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keer SE, Stern JM. Dopamine receptor blockade in the nucleus accumbens inhibits maternal retrieval and licking, but enhances nursing behavior in lactating rats. Physiol Behav. 1999;67:659–669. doi: 10.1016/s0031-9384(99)00116-x. [DOI] [PubMed] [Google Scholar]
- 28.King JA, Garelick TS, Brevard ME, Chen W, Messenger TL, Duong TQ, Ferris CF. Procedure for minimizing stress for fMRI studies in conscious rats. J Neurosci Methods. 2005;148:154–160. doi: 10.1016/j.jneumeth.2005.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinsley CH, Turco D, Bauer A, Beverly M, Wellman J, Graham AL. Cocaine alters the onset and maintenance of maternal behavior in lactating rats. Pharmacol Biochem Behav. 1994;47:857–864. doi: 10.1016/0091-3057(94)90288-7. [DOI] [PubMed] [Google Scholar]
- 30.Lee A, Clancy S, Fleming AS. Mother rats bar-press for pups: effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav Brain Res. 1999;100:15–31. doi: 10.1016/s0166-4328(98)00109-0. [DOI] [PubMed] [Google Scholar]
- 31.Lee A, Li M, Watchus J, Fleming AS. Neuroanatomical basis of maternal memory in postpartum rats: selective role for the nucleus accumbens. Behav Neurosci. 1999;113:523–538. doi: 10.1037//0735-7044.113.3.523. [DOI] [PubMed] [Google Scholar]
- 32.Liu RC, Linden JF, Schreiner CE. Improved cortical entrainment to infant communication calls in mothers compared with virgin mice. Eur J Neurosci. 2006;23:3087–3097. doi: 10.1111/j.1460-9568.2006.04840.x. [DOI] [PubMed] [Google Scholar]
- 33.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 34.Lonstein JS, Simmons DA, Swann JM, Stern JM. Forebrain expression of c-fos due to active maternal behaviour in lactating rats. Neuroscience. 1998;82:267–281. doi: 10.1016/s0306-4522(97)00283-2. [DOI] [PubMed] [Google Scholar]
- 35.Lonstein JS, Stern JM. Role of the midbrain periaqueductal gray in maternal nurturance and aggression: c-fos and electrolytic lesion studies in lactating rats. J Neurosci. 1997;17:3364–3378. doi: 10.1523/JNEUROSCI.17-09-03364.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, Bohning DE, George MS. A potential role for thalamocingulate circuitry in human maternal behavior. Biol Psychiatry. 2002;51:431–445. doi: 10.1016/s0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- 37.Mattson BJ, Morrell JI. Preference for cocaine- versus pup-associated cues differentially activates neurons expressing either Fos or cocaine- and amphetamine-regulated transcript in lactating, maternal rodents. Neuroscience. 2005;135:315–328. doi: 10.1016/j.neuroscience.2005.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mattson BJ, Williams S, Rosenblatt JS, Morrell JI. Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav Neurosci. 2001;115:683–694. doi: 10.1037//0735-7044.115.3.683. [DOI] [PubMed] [Google Scholar]
- 39.Murphy MR, MacLean PD, Hamilton SC. Species-typical behavior of hamsters deprived from birth of the neocortex. Science. 1981;213:459–461. doi: 10.1126/science.7244642. [DOI] [PubMed] [Google Scholar]
- 40.Nair P, Black MM, Schuler M, Keane V, Snow L, Rigney BA, Magder L. Risk factors for disruption in primary caregiving among infants of substance abusing women. Child Abuse Negl. 1997;21:1039–1051. doi: 10.1016/s0145-2134(97)00064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nitschke JB, Nelson EE, Rusch BD, Fox AS, Oakes TR, Davidson RJ. Orbitofrontal cortex tracks positive mood in mothers viewing pictures of their newborn infants. Neuroimage. 2004;21:583–592. doi: 10.1016/j.neuroimage.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 42.Numan M. Motivational systems and the neural circuitry of maternal behavior in the rat. Dev Psychobiol. 2007;49:12–21. doi: 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- 43.Numan M, Numan MJ, Pliakou N, Stolzenberg DS, Mullins OJ, Murphy JM, Smith CD. The effects of D1 or D2 dopamine receptor antagonism in the medial preoptic area, ventral pallidum, or nucleus accumbens on the maternal retrieval response and other aspects of maternal behavior in rats. Behav Neurosci. 2005;119:1588–1604. doi: 10.1037/0735-7044.119.6.1588. [DOI] [PubMed] [Google Scholar]
- 44.Numan M, Numan MJ, Schwarz JM, Neuner CM, Flood TF, Smith CD. Medial preoptic area interactions with the nucleus accumbens-ventral pallidum circuit and maternal behavior in rats. Behav Brain Res. 2005;158:53–68. doi: 10.1016/j.bbr.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 45.Numan M, Smith HG. Maternal behavior in rats: evidence for the involvement of preoptic projections to the ventral tegmental area. Behav Neurosci. 1984;98:712–727. doi: 10.1037//0735-7044.98.4.712. [DOI] [PubMed] [Google Scholar]
- 46.Paxinos G, Watson C. The Rat Brain In Stereotaxic Coordinates. 3 edn. Boston: Academic Press; 1997. [DOI] [PubMed] [Google Scholar]
- 47.Ranote S, Elliott R, Abel KM, Mitchell R, Deakin JF, Appleby L. The neural basis of maternal responsiveness to infants: an fMRI study. Neuroreport. 2004;15:1825–1829. doi: 10.1097/01.wnr.0000137078.64128.6a. [DOI] [PubMed] [Google Scholar]
- 48.Roesch MR, Olson CR. Neuronal activity related to reward value and motivation in primate frontal cortex. Science. 2004;304:307–310. doi: 10.1126/science.1093223. [DOI] [PubMed] [Google Scholar]
- 49.Rosenblatt JS. Nonhormonal basis of maternal behavior in the rat. Science. 1967;156:1512–1514. doi: 10.1126/science.156.3781.1512. [DOI] [PubMed] [Google Scholar]
- 50.Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb Cortex. 2000;10:272–284. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- 51.See RE, Lynch AM, Aravagiri M, Nemeroff CB, Owens MJ. Chronic haloperidol-induced changes in regional dopamine release and metabolism and neurotensin content in rats. Brain Res. 1995;704:202–209. doi: 10.1016/0006-8993(95)01114-5. [DOI] [PubMed] [Google Scholar]
- 52.Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 2006;9:569–577. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- 53.Simon H, Taghzouti K, Le Moal M. Deficits in spatial-memory tasks following lesions of septal dopaminergic terminals in the rat. Behav Brain Res. 1986;19:7–16. doi: 10.1016/0166-4328(86)90042-2. [DOI] [PubMed] [Google Scholar]
- 54.Sorg BA, Davidson DL, Kalivas PW, Prasad BM. Repeated daily cocaine alters subsequent cocaine-induced increase of extracellular dopamine in the medial prefrontal cortex. J Pharmacol Exp Ther. 1997;281:54–61. [PubMed] [Google Scholar]
- 55.Stamm JS. The function of the median cerebral cortex in maternal behavior of rats. J Comp Physiol Psychol. 1955;48:347–356. doi: 10.1037/h0042977. [DOI] [PubMed] [Google Scholar]
- 56.Stern JM, Keer SE. Maternal motivation of lactating rats is disrupted by low dosages of haloperidol. Behav Brain Res. 1999;99:231–239. doi: 10.1016/s0166-4328(98)00108-9. [DOI] [PubMed] [Google Scholar]
- 57.Strupp JP. Stimulate: A GUI based fMRI analysis software package. Neuroimage. 1996;3:S607. [Google Scholar]
- 58.Swanson LW. Brain maps: structure of the rat brain. 2nd edn. Boston: Elsevier Science; 1999. [Google Scholar]
- 59.Vernotica EM, Lisciotto CA, Rosenblatt JS, Morrell JI. Cocaine transiently impairs maternal behavior in the rat. Behav Neurosci. 1996;110:315–323. doi: 10.1037//0735-7044.110.2.315. [DOI] [PubMed] [Google Scholar]
- 60.Watanabe M. Reward expectancy in primate prefrontal neurons. Nature. 1996;382:629–632. doi: 10.1038/382629a0. [DOI] [PubMed] [Google Scholar]
- 61.Williams JM, Steketee JD. Effects of repeated cocaine on the release and clearance of dopamine within the rat medial prefrontal cortex. Synapse. 2005;55:98–109. doi: 10.1002/syn.20093. [DOI] [PubMed] [Google Scholar]
- 62.Williams JM, Steketee JD. Time-dependent effects of repeated cocaine administration on dopamine transmission in the medial prefrontal cortex. Neuropharmacology. 2005;48:51–61. doi: 10.1016/j.neuropharm.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 63.Wu Z, Sullivan JMJ. Multiple material marching cubes algorithm. IJNME. 2003;58:189–207. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Motion exclusion criteria (see text). Graph shows average center-of-mass displacement in X (left-right), Y (up-down) and Z (forward-back) for each animal scanned. Inset shows average displacement in all three directions. Arrows (red) show individual scans that were discared due to excess motion.


