Abstract
Apoptosis has been shown to contribute to loss of cardiomyocytes in cardiomyopathy, progressive decline in left ventricular function, and congestive heart failure. Because the molecular mechanisms involved in apoptosis of cardiocytes are not completely understood, we studied the biochemical and ultrastructural characteristics of upstream regulators of apoptosis in hearts explanted from patients undergoing transplantation. Sixteen explanted hearts from patients undergoing heart transplantation were studied by electron microscopy or immunoblotting to detect release of mitochondrial cytochrome c and activation of caspase-3. The hearts explanted from five victims of motor vehicle accidents or myocardial ventricular tissues from three donor hearts were used as controls. Evidence of apoptosis was observed only in endstage cardiomyopathy. There was significant accumulation of cytochrome c in the cytosol, over myofibrils, and near intercalated discs of cardiomyocytes in failing hearts. The release of mitochondrial cytochrome c was associated with activation of caspase-3 and cleavage of its substrate protein kinase C δ but not poly(ADP-ribose) polymerase. By contrast, there was no apparent accumulation of cytosolic cytochrome c or caspase-3 activation in the hearts used as controls. The present study provides in vivo evidence of cytochrome c-dependent activation of cysteine proteases in human cardiomyopathy. Activation of proteases supports the phenomenon of apoptosis in myopathic process. Because loss of myocytes contributes to myocardial dysfunction and is a predictor of adverse outcomes in the patients with congestive heart failure, the present demonstration of an activated apoptotic cascade in cardiomyopathy could provide the basis for novel interventional strategies.
Loss of myocytes is a feature of the cardiomyopathic process that contributes to progressive decline in left ventricular function and congestive heart failure (1, 2). Recent studies have proposed that myocyte loss in cardiomyopathy can occur by apoptosis without an attendant inflammatory response (3). Apoptosis may be the consequence of prolonged growth stimulation of adult myocytes (4), which are terminally differentiated and are unable to divide (5). Growth stimulation initially occurs as a compensatory effort to meet chronically altered hemodynamic demands on the failing myocardium and is mediated by systemic and/or local up-regulation of mediators of adrenergic (6, 7) or renin-angiotensin (8, 9) axes and by various cytokines (10). Local up-regulation of angiotensin II induces immediate-early genes (9, 11), which may lead to increased protein synthesis (12) and myocardial hypertrophy (9, 13, 14) or, alternatively, may up-regulate expression of apoptotic proteins (such as p53) in myocytes (15). Similarly, certain cytokines (such as tumor necrosis factor α) can induce growth (16, 17) as well as apoptosis (18).
The induction of apoptosis as a form of cell death distinct from necrosis (19–23) is associated with activation of aspartate-specific cysteine proteases such as caspase-3 (CPP32/Yama) (24, 25) and cleavage of poly(ADP-ribose) polymerase (PARP) (26), protein kinase C-δ (PKCδ) (27), and certain other proteins (28). Caspase-3 protein is detectable by immunostaining in various human tissues, including cardiomyocytes (29). Direct evidence for involvement of caspase-3 in apoptosis is derived from studies with the baculovirus protein p35, which directly inhibits this protease and blocks induction of apoptosis (30).
Recent studies have indicated that mitochondria may play a crucial role in apoptosis by releasing cytochrome c (31). Addition of purified cytochrome c and dATP to cytosolic extracts from proliferating cells activates caspase-3 responsible for cleavage of PARP (31). The finding that intact cells undergo apoptosis after release of cytochrome c into the cytosol has provided further support for an apoptotic function of this mitochondrial protein (31). Furthermore, recent studies have demonstrated that, in addition to cytochrome c, activation of caspase-3 also requires another protein, Apaf-1, a human homologue of the Caenorhabditis elegans ced-4 protein (32).
Although sequential activation of the apoptotic cascade has been well characterized in nematodes, in cell culture, and in cell free systems, little information is available in human diseases. To investigate the potential involvement of cytochrome c release from mitochondria and activation of caspases in the end-stage cardiomyopathy, a pathological state wherein apoptosis is known to play a role (3), we performed ultrastructural and biochemical analysis of explanted hearts from cardiac allograft recipients. The results support the constitutive activation of a cytochrome c-mediated apoptotic cascade in human cardiomyopathy.
MATERIALS AND METHODS
Myocardial Specimens from Cardiomyopathic and Normal Hearts.
Sixteen explanted hearts were obtained from patients undergoing heart transplantation; 10 patients had idiopathic dilated cardiomyopathy (IDCM), and the remaining 6 had ischemic cardiomyopathy (ISCM). Normal myocardial tissues also were obtained from five victims of road accidents and from three donor hearts before transplantation. The control tissues from accident victims were only accepted if obtained within 6 hours of death. Similarly, donor-heart normal myocardial tissues were accepted only when ischemic times were <60 min.
Of these 16, explanted hearts from 7 patients either were placed in 4% buffered formaldehyde fixative or were snap frozen in liquid nitrogen. Four of the seven patients had IDCM, and three suffered from ISCM. The formalin-fixed tissue was processed by paraffin embedding and was used for histopathologic characterization and for demonstration of apoptosis by nick-end labeling. Histopathologic and end-labeling studies from these seven patients have been reported previously (3). The myocardial tissue from these hearts, which was snap frozen in liquid nitrogen, was stored at −70°C for biochemical characterization of cytochrome c release and caspase activation. The results of the biochemical analysis were compared with the myocardial tissues obtained from five victims of motor vehicle accidents.
The myocardial tissue specimens from the remaining nine hearts were obtained in 0.5% Karnovsky’s fixative for immunoelectron microscopic examination and were compared with the small ventricular tissue samples obtained from three donor hearts. Ultrastructural examination was undertaken for precise localization of cytochrome c in mitochondrial and cytoplasmic compartments.
Cell Culture Studies.
Human U-937 myeloid leukemia cells and neonatal murine cardiomyocytes were used for positive control studies. U-937 cells were grown in RPMI medium 1640 supplemented with 10% heat-inactivated fetal bovine serum, 100 units/ml penicillin, 100 mg/ml streptomycin, and 2 mM l-glutamine. Irradiation was performed at room temperature with a Gamma Cell 1000 (Atomic Energy, Ottawa) under aerobic conditions with a 137Cs source emitting at a fixed-dose rate of 13 Gy/min as determined by dosimetry and described previously (33).
Neonatal rat myocytes were prepared from ventricular muscle (34). Cardiocytes were plated in 25-ml Falcon flasks for each experiment in high-glucose DMEM medium with 10% fetal calf serum. The cells initially were incubated for 24 hours at 37°C in 5% C02. For hypoxic stress, the culture medium in flasks was flushed with sterile nitrogen for 4 min, and flasks were tightly capped and transferred to incubators as described (35). The hypoxic conditions were maintained for 6 and 12 hours. After the indicated time periods, the cells were harvested from flasks by gentle scraping.
Isolation of the Cytosolic Fraction.
Myocardial tissue specimens from seven cardiomyopathic or five normal hearts, treated and untreated cultured neonatal cardiomyocytes, and treated and untreated cultured U-937 cells were homogenized in buffer A (20 mM Hepes, pH 7.5/1.5 mM MgCl2/10 mM KCl/1 mM EDTA/1 mM EGTA/1 mM DTT/0.1 mM PMSF/10 μg/ml leupeptin/aprotinin/pepstatin A) (33). A uniform cell population was washed twice with PBS, and the pellet was suspended in 5 ml of ice-cold buffer A containing 250 mM sucrose. The cells were homogenized by douncing three times in a Dounce homogenizer with a sandpaper-polished pestle. After centrifugation for 5 min at 4°C, the supernatants then were centrifuged at 105,000 × g for 30 min at 4°C. The resulting supernatant was used as the soluble cytosolic fraction.
Immunoblot Analysis.
Proteins from cytosolic fractions or total cell lysates were separated by SDS/PAGE, were transferred to nitrocellulose, and were analyzed by immunoblotting with anti-cytochrome c (provided by L. Procheska, Wright State Univ.) (36), anti-CPP32 (Santa Cruz Biotechnology), anti-PKCδ (Upstate Biotechnology, Lake Placid, NY), anti-PARP (Upstate Biotechnology), or anti-actin (Sigma) antibodies. The blots were developed by ECL chemiluminescence (Amersham Pharmacia). In mammalian cells, CPP32 normally exists as a 32-kDa inactive precursor that is converted proteolytically to active p17/12 subunits when cells are induced to undergo apoptosis (24). Anti-CPP32 antibody recognizes both the cleaved and uncleaved CPP32 proteins. PKCδ exists as a 70-kDa inactive protein and undergoes CPP32-mediated cleavage into a 40-kDa fragment in response to apoptotic inducer (27). On the other hand, PARP is a 116-kDa protein that also is cleaved by CPP32 during apoptosis. Signal intensities of various proteins and their fragments in Western blots were determined by densitometric analysis (UltraScan, LKB).
Immunoelectron Microscopy and Ultrastructural Examination.
Immunoelectron microscopic studies were performed in the remaining 9 of the 16 hearts and in 3 myocardial tissue specimens obtained from donor hearts. The samples were fixed for 2 hours with 0.5% Karnovsky’s fixative, were postfixed with 1.5% OsO4 in 0.2 M cacodylate buffer (pH 7.3), and were dehydrated and embedded in Epon-Araldite. Ultrathin sections were stained with lead citrate and uranyl acetate and were examined with Zeiss 902 electron microscope.
For the electron microscopic immunocytochemical study, ultrathin sections were collected on collodion-coated nickel grids, were etched with 3% H2O2 for 10 min, and were treated with 5% sodium metaperyodate for 15 min. Thin sections then were rinsed in 0.05 M Tris⋅HCl buffer (pH 7.4), were incubated for 30 min with 0.05 M normal goat serum in Tris buffer with 0.09% NaCl (pH 7.4), and were treated with anti-cytochrome c antibody overnight at 4°C. After rinsing in Tris buffer and Tris buffer containing 1% BSA, the sections were incubated for 1 hour at room temperature with the secondary antibody [gold-conjugated goat anti-mouse/anti rabbit IgG, particle size 10 nm (Dako)]. Negative controls were performed by substitution of the primary antibody with Tris buffer. The sections were counterstained with uranyl acetate and lead citrate.
RESULTS
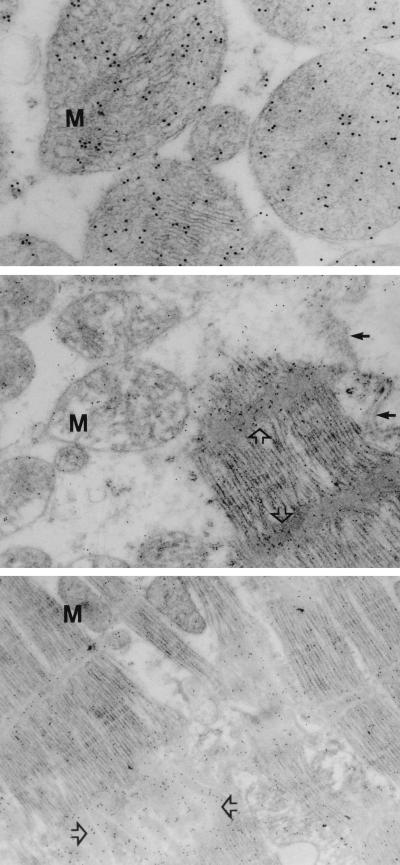
Evidence of apoptosis in myocytes by nick end-labeling studies was only observed in the cardiomyopathic hearts and none of the normal myocardial tissue, as described (3) (Fig. 1). To assess the accumulation of cytochrome c in cardiomyopathic hearts, electron microscopic studies were performed by using immunogold labeling with anti-cytochrome c antibodies in nine hearts (six IDCM hears; three ISCM hearts). The results demonstrate that cytochrome c was localized in mitochondria in donor heart biopsies (Fig. 2 Top). On the other hand, cytochrome c immunoreactivity was observed both in mitochondrial and extramitochondrial compartments in cardiomyopathic hearts. The cytochrome c in these specimens was predominantly distributed in cytoplasm (Table 1), over contractile proteins and Z-bands (Fig. 2 Middle) as well as in the vicinity of intercalated discs (Fig. 2 Bottom). In addition, some anti-cytochrome c reactivity also was noted in the extracellular compartment in ISCM specimens. Of interest, ultrastructural changes of apoptosis had yet not occurred in the nuclei in a majority of cells with prominent cytochrome c accumulation in cytosol.
Figure 1.
Apoptosis in end-stage heart failure. Analysis of apoptosis was performed by end-labeling of DNA fragments in rat mammary tissue (A; used as a positive control) and myocardial specimens from normal (B) and cardiomyopathic (C and D) hearts. The end-labeling of the DNA fragments was obtained by addition of biotinylated dUTP in the presence of terminal deoxynucleotidyltransferase (TdT) (Trevigen, Gaithersburg, MD). The nucleosomal DNA fragments were visualized with the help of avidin–biotin complex followed by chromogen substrate, which stained the apoptotic nuclei blue (A, C, and D, arrowheads, which appear black here). The apoptotic nuclei (arrowheads) can be seen in acinar cells in the mammary lobules (A). In contrast, apoptotic cells are not observed in the normal myocardial tissue (B) obtained from a victim of motor vehicle accident. Eosin counterstaining demonstrates myocardial silhouette, and the unstained nuclear regions appear clear (open arrows). In a myocardial specimen from a cardiomyopathic heart (C), two myocytes with apoptosis are shown by blue-stained nuclei (arrowheads, which appear black here). The occurrence of apoptosis in myocytes (arrowheads) is further confirmed by double staining for α-muscle actin with antibody HHF-35 (brown product, which appears gray here) (D).
Figure 2.
Ultrastructural localization of cytochrome c. High magnifications of normal myocardial specimen from a donor heart (Top) and ischemic cardiomyopathic hearts (Middle and Bottom) demonstrate striking difference in cytochrome c immunoreactivity. Localization of cytochrome c is represented by (black) gold particles. The cytochrome c is predominantly localized in mitochondria (M) in normal hearts (Top). On the other hand, cytochrome c is substantially reduced in mitochondria (Middle, M) in cardiomyopathic heart and is concentrated either over Z-bands (Middle, open arrows) or near intercalated disc (Bottom, open arrows). Small arrows indicate the presence of normal sarcolemma (Middle).
Table 1.
Ultrastructural immunolocalization of cytochrome c in cardiomyopathic and control myocardial tissues
| No. | Case | Mitochondria | Sarcoplasm | Z-bands | Intercalated discs | Extracellular |
|---|---|---|---|---|---|---|
| 1. | Donor 1 | ++++ | + | ± | ± | − |
| 2. | Donor 2 | ++++ | ± | ± | − | − |
| 3. | Donor 3 | ++++ | ± | − | ± | ± |
| 1. | ISCM1 | + | + | ++ | + | ± |
| 2. | ISCM2 | ± | ++ | + | + | + |
| 3. | ISCM3 | + | ± | + | ± | ± |
| 4. | IDCM1 | + | + | ++ | ++ | ± |
| 5. | IDCM2 | + | ± | + | + | − |
| 6. | IDCM3 | + | ++ | ++ | + | − |
| 7. | IDCM4 | + | + | + | + | ± |
| 8. | IDCM5 | + | + | ++ | + | − |
| 9. | IDCM6 | ± | ± | + | + | − |
±, sparse, very few granules; +, few granules; ++, moderate amount; ++++, abundant granules.
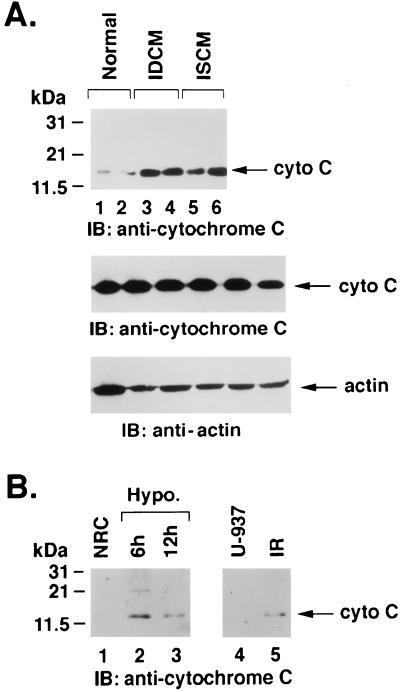
Electrophoretic resolution and immunoblot analyses were performed in multiple frozen myocardial specimens from seven cardiomyopathic hearts to confirm mitochondrial release of cytochrome c in the cytoplasmic S-100 cell lysates. The results demonstrate that cytochrome c was substantially increased in cytosolic fraction of myopathic hearts (Fig. 3A Top). Total levels of cytochrome c in normal and cardiomyopathic hearts were similar (Fig. 3A Middle). As a control, cytoplasmic S-100 cell extracts also were analyzed by anti-actin. The results confirm similar levels of actin in all specimens tested (Fig. 3A Bottom). No cytoplasmic accumulation of cytochrome c was evident in control hearts from accident victims (Fig. 3A Top). The cytosolic localization of cytochrome c was similar to that seen in irradiated U-937 cells (Fig. 3B). Similarly, mitochondrial release of cytochrome c also was observed in neonatal murine cardiomyocytes exposed to hypoxia (Fig. 3B).
Figure 3.
Accumulation of cytochrome c in the cytosol. (A) Proteins from the cytosolic S-100 fractions were separated by 12.5% SDS/PAGE and were analyzed by immunoblotting (IB) with anti-cytochrome c antibody. (Top) Normal (Lanes 1 and 2), IDCM (Lanes 3 and 4), and ISCM (Lanes 5 and 6) hearts. Total lysates also were analyzed by immunoblotting with anti-cytochrome c (Middle) and anti-actin (Bottom) antibodies. (B) Neonatal rat cardiomyocytes (NRC) cultured in normoxic (Lane 1) or hypoxic conditions for 6 (Lane 2) and 12 (Lane 3) hours (Left), U-937 control cells (Lane 4), and cells treated with 20 Gy ionizing radiation and harvested at 6 hours (Lane 5). S-100 cytosolic extracts were prepared and analyzed by immunoblotting with cytochrome c antibody. Cytosolic accumulation of cytochrome c was only observed in cardiomyopathic myocardial specimens, irradiated U-937 cells, and hypoxic cardiomyocytes. Normal myocardial tissues and control cells show no evidence of cytosolic cytochrome c.
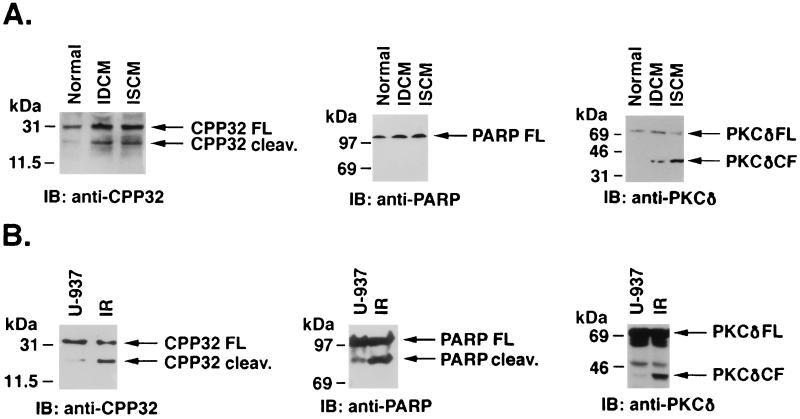
Recent studies have demonstrated that release of cytochrome c from mitochondria leads to activation of caspase-3 (32). To assess activation of caspase-3 in cardiomyopathic hearts, cell lysates were subjected to immunoblotting with anti-caspase-3 antibody. The results demonstrate that, in contrast to normal hearts, there was a significant increase in activation of caspase-3 in cardiomyopathic hearts (Fig. 4A). The signal intensities of cleaved and uncleaved CPP32 fragments were determined by densitometric scanning. The results demonstrate that the cleavage of CPP32 in normal hearts was negligible compared with 45–55% in cardiomyopathic hearts (Fig. 4A). The activation of caspase-3 usually leads to cleavage of cytoplasmic (such as PKCδ) and nuclear (such as PARP or lamin) substrates for manifestation of apoptosis (26–28). Therefore, to confirm the functional significance of caspase-3 in cardiomyopathic hearts, cell lysates also were subjected to immunoblotting with anti-PKCδ or anti-PARP antibodies. The results demonstrate cleavage of PKCδ in IDCM and ISCM hearts. The activation of caspase-3 and cleavage of PKCδ was comparable to that seen in U-937 cells (Fig. 4B). However, unlike U-937 cells, PARP cleavage was not seen in cardiomyopathic hearts (Fig. 4A). Cleavage of both PKCδ and PARP was not observed in control myocardial specimens (Fig. 4A). These results support a mechanism that links mitochondrial release of cytochrome c to activation of caspase-3 in cardiomyopathic hearts.
Figure 4.
Activation of caspase-3 in cardiomyopathic hearts. (A) Proteins from total cell lysates of normal, IDCM, and ISCM were separated by SDS/PAGE, were transferred to nitrocellulose, and were analyzed by immunoblotting (IB) with anti-CPP32, anti-PARP, and anti-PKCδ antibodies. (B) Similarly, both control and irradiated U-937 cell lysates were analyzed by immunoblotting with anti-CPP32, anti-PARP, and anti-PKCδ antibodies. Cleavage of CPP32 and PKCδ is seen in cardiomyopathic hearts similar to U937 cells. PARP cleavage is not observed in cardiomyopathic hearts, unlike U-937 cells. Control myocardial lysates and untreated U-937 cells show only uncleaved intact precursors (FL or full-length).
DISCUSSION
Explanted hearts with end-stage cardiomyopathy exhibit release of cytochrome c from mitochondria into the cytoplasm. Cytochrome c is predominantly localized in cytoplasm around myofibrils and intercalated discs. The cytosolic accumulation of cytochrome c is associated with cleavage of caspase-3 and PKCδ. Cytochrome c release and cleavage of caspase-3 and PKCδ occur in both ischemic and idiopathic dilated cardiomyopathic hearts alike. Although evidence of cytochrome c depletion from mitochondria and caspase activation are almost universally seen, ultrastructural alterations of apoptosis in nuclei is not observed in these cells. The activation of nuclear substrate of caspase-3, PARP, also is not observed. The cytochrome c is confined to the mitochondria in normal myocardium, and there is no activation of caspase-3 in normal specimens.
The mammalian cysteine proteases are structurally similar to ced-3 and ced-4, which control programmed cell death in the nematode C. elegans. Similar to ced-3, caspase-3 exists in the cytosolic fraction as an inactive 32-kDa precursor that is proteolytically cleaved in apoptotic cells to active subunits (27). Caspase-3 inhibitors effectively block the ability of cytoplasm from the apoptotic cells to induce apoptotic changes in the normal nuclei in vitro (27). Once activated, the caspase-3 production is self-sustained by autocatalysis (27). However, the initial activation has recently been proposed to require release of mitochondrial respiratory chain protein, cytochrome c (31). Cytochrome c is localized in the intermembrane space loosely attached to the surface of the inner membrane and, as expected, was observed only in the mitochondria (both biochemically and ultrastructurally) of the normal heart. On the other hand, an ≈15- to 20-fold higher concentration was present in the cytoplasm of all myopathic tissues. Involvement of mitochondria in execution of apoptosis has been suggested by the existence of apoptotic process even in anucleate cells (37, 38) and the ability of mitochondria-rich fraction to induce apoptosis in nuclei in Xenopus egg extracts (39). Substantial quantitative release of cytochrome c in cytoplasm has been demonstrated in cultured U-937 cells undergoing apoptosis on exposure to DNA-damaging agents (33). During apoptosis, permeability transition pores are formed in the mitochondrial membrane (40) mediated by an inner mitochondrial membrane protein, adenine nucleotide translocator (41). Ligands of adenine nucleotide translocator, such as atractyloside and bongkrekic acid, alter the probability of permeability transition pore formation and modulate ability of mitochondria in induction of apoptosis in cell-free systems (40). It is interesting to note that adenine nucleotide translocator antibody has been frequently isolated from the sera in cardiomyopathy patients and may be involved in the pathogenesis of cardiomyopathy (42, 43).
Although protease cleavage and cytochrome c in the myocardial cytoplasmic extracts support the phenomenon of apoptosis in end-stage heart failure, presence of significant expression of upstream mediators of apoptosis is intriguing, especially when nuclear alterations of apoptosis is not seen in the same cells. This may suggest that the myocytes may be in the preapoptotic stage long before morphologic changes become manifest. One also can surmise that the presence of precursors of apoptosis indicates the commitment of these cells to programmed self-destruction and not the actual occurrence or execution of the process. It is also possible that externally induced apoptosis, especially in a differentiated cell, may follow an unconventional course, unlike classical, spontaneous, single cell-related physiological apoptosis. Furthermore, cleavage of cytoplasmic substrate of caspase-3 but absence of ultrastructural nuclear fragmentation also may suggest a possible dissociation of cytoplasmic and nuclear processes of apoptosis.
CONCLUSIONS
Continued loss of myocytes leads to myocardial dysfunction. Because decreased systolic function is a powerful predictor of adverse outcome in congestive heart failure, strategies that reduce the biological signals responsible for myocyte loss and chamber remodeling should improve clinical outcome. The intervention aimed at prevention of apoptosis may be a step in this direction, and documentation of the apoptotic cascade, therefore, becomes necessary for the development of novel therapeutic regimens.
Acknowledgments
We thank Lawrence Procheska (Wright State Univ., Dayton, Ohio) for providing antibody to cytochrome c. This investigation was supported by Public Health Service Grant CA 75216 (to S.K.) awarded by the National Cancer Institute, Department of Health and Human Services and by Medical Research Council of Canada Grant MT-14361 (to S.S.).
ABBREVIATIONS
- PARP
poly(ADP-ribose) polymerase
- PKCδ
protein kinase C-δ
- IDCM
idiopathic dilated cardiomyopathy
- ISCM
ischemic cardiomyopathy
Footnotes
A Commentary on this article begins on page 7614.
References
- 1.Eichhorn E J, Bristow M R. Circulation. 1996;94:2285–2296. doi: 10.1161/01.cir.94.9.2285. [DOI] [PubMed] [Google Scholar]
- 2.Beltrami C A, Finato N, Rocco M, Feruglio G A, Puricelli S. J Mol Cell Cardiol. 1995;27:291–305. doi: 10.1016/s0022-2828(08)80028-4. [DOI] [PubMed] [Google Scholar]
- 3.Narula J, Haider N, Virmani R, DiSalvo T G, Kolodgie F D, Hajjar R J, Schmidt U, Semigran M J, Dec G W, Khaw B A. N Engl J Med. 1996;335:1182–1189. doi: 10.1056/NEJM199610173351603. [DOI] [PubMed] [Google Scholar]
- 4.Katz A M. Ann Intern Med. 1994;121:363–371. doi: 10.7326/0003-4819-121-5-199409010-00009. [DOI] [PubMed] [Google Scholar]
- 5.Nadal-Ginard B. Cell. 1978;15:855–864. doi: 10.1016/0092-8674(78)90270-2. [DOI] [PubMed] [Google Scholar]
- 6.Francis G S, Benedict C, Johnstone D E, Kirlin P C, Nicklas J, Liang C S, Kubo S H, Rudin-Toretski E, Yusuf S. Circulation. 1990;82:1724–1729. doi: 10.1161/01.cir.82.5.1724. [DOI] [PubMed] [Google Scholar]
- 7.Bristow M R, Minobe W, Rasmussen R, Larrabee P, Skerl L, Klein J W, Anderson F L, Murray J, Mestroni L, Karvande S V, et al. J Clin Invest. 1992;89:803–815. doi: 10.1172/JCI115659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CONSENSUS Trial Study Group. N Engl J Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 9.Sadoshima J, Xu Y, Slayter H S, Izumo S. Cell. 1993;75:977–984. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- 10.Levine B, Kalman J, Mayer L, Fillit H M, Packer M. N Engl J Med. 1990;323:236–241. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 11.Komuro I, Katoh Y, Kaida T, Shibazaki Y, Kurabayashi M, Hoh E, Takaku F, Yazaki Y. J Biol Chem. 1991;266:1265–1268. [PubMed] [Google Scholar]
- 12.Aceto J F, Baker K M. Am J Physiol. 1990;258:H806–H813. doi: 10.1152/ajpheart.1990.258.3.H806. [DOI] [PubMed] [Google Scholar]
- 13.Weber K T, Brilla C G. Circulation. 1991;83:1849–1865. doi: 10.1161/01.cir.83.6.1849. [DOI] [PubMed] [Google Scholar]
- 14.Baker K M, Chernin M I, Wixson S K, Aceto J F. Am J Physiol. 1990;259:H324–H332. doi: 10.1152/ajpheart.1990.259.2.H324. [DOI] [PubMed] [Google Scholar]
- 15.Raynolds M, Peacock S J, Roden R L. FASEB Abstr. 1995;9:1281. [Google Scholar]
- 16.Brenner D A, O’Hara M, Angel P, Chojkier M, Karin M. Nature (London) 1989;337:661–663. doi: 10.1038/337661a0. [DOI] [PubMed] [Google Scholar]
- 17.Westwick J K, Weitzel C, Minden A, Karin M, Brenner D. J Biol Chem. 1994;269:26396–26401. [PubMed] [Google Scholar]
- 18.Tartaglia L A, Ayres T M, Wong G H, Goeddel D V. Cell. 1993;74:845–853. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- 19.Kerr J F R, Wyllie A H, Currie A R. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wyllie A H, Kerr J F R, Currie A R. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 21.Searle J, Kerr J F, Bishop C J. Pathol Annu. 1982;17:229–259. [PubMed] [Google Scholar]
- 22.Bursch W, Kliene L, Tenniswood M. Biochem Cell Biol. 1990;68:1071–1074. doi: 10.1139/o90-160. [DOI] [PubMed] [Google Scholar]
- 23.Arends M J, Morris R G, Wyllie A H. Am J Pathol. 1990;136:593–608. [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandes-Alnemri T, Litwack G, Alnemri E S. J Biol Chem. 1994;269:30761–30764. [PubMed] [Google Scholar]
- 25.Nicholson D W, Ali A, Thornberry N A, Vaillancourt J P, Ding C K, Gallant M, Gareau Y, Griffin P R, Labelle M, Lazebnik Y A, et al. Nature (London) 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 26.Tewari M, Quan L T, O’Rourke K, Desnoyers S, Zeng G, Beidler D R, Poirier G G, Salversen G S, Dixit V M. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- 27.Emoto Y, Manome Y, Meinhardt G, Kisaki H, Kharbanda S, Robertson M, Ghayur T, Wong W W, Kamen R, Weichselbaum R, Kufe D. EMBO J. 1995;14:6148–6156. doi: 10.1002/j.1460-2075.1995.tb00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Zelenski N G, Yang J, Sakai J, Brown M S, Goldstein J L. EMBO J. 1996;15:1012–1020. [PMC free article] [PubMed] [Google Scholar]
- 29.Krajewska M, Wang H G, Krajewski S, Zapata J M, Shabaik A, Gascoyne R, Reed J C. Cancer Res. 1997;57:1605–1613. [PubMed] [Google Scholar]
- 30.Bump N J, Hackett M, Hugunin M, Seshagiri S, Brady K, Chen P, Ferenz C, Franklin S, Ghayur T, Li P, et al. Science. 1995;269:1885–1888. doi: 10.1126/science.7569933. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Kim C N, Yang J, Jemmerson R, Wang X. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 32.Zou H, Henzel W, Liu X, Lustehg A, Wang X. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 33.Kharbanda S, Pandey P, Schofield L, Israels S, Roncinske R, Yoshida K, Bharti A, Yuan Z M, Saxena S, Weichselbaum R, et al. Proc Natl Acad Sci USA. 1997;94:6939–6942. doi: 10.1073/pnas.94.13.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simpson P, Savion S. Circ Res. 1982;50:101–116. doi: 10.1161/01.res.50.1.101. [DOI] [PubMed] [Google Scholar]
- 35.Khaw B A, Torchilin V P, Vural I, Narula J. Nat Med. 1995;1:1195–1198. doi: 10.1038/nm1195-1195. [DOI] [PubMed] [Google Scholar]
- 36.Kirken R A, Lincoln A J, Fink P S, Prochaska L J. Protein Expression Purif. 1995;6:707–715. doi: 10.1006/prep.1995.1093. [DOI] [PubMed] [Google Scholar]
- 37.Jacobson M D, Burne J F, Raff M C. EMBO J. 1994;13:1899–1910. doi: 10.1002/j.1460-2075.1994.tb06459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulze-Osthoff K, Walczak H, Droge W, Krammer P H. J Cell Biol. 1994;127:15–20. doi: 10.1083/jcb.127.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newmeyer D D, Farschon D M, Reed J C. Cell. 1994;79:353–364. doi: 10.1016/0092-8674(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 40.Zamzami N, Susin S A, Marchetti P, Hirsch T, Gomez-Monterrey I, Castedo M, Kroemer G. J Exp Med. 1996;183:1533–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernardi P, Broekemeier K M, Pfeiffer D R. J Bioenerg Biomembr. 1994;26:509–517. doi: 10.1007/BF00762735. [DOI] [PubMed] [Google Scholar]
- 42.Schulze K, Schultheiss H P. Eur Heart J. 1995;16:64–67. doi: 10.1093/eurheartj/16.suppl_o.64. [DOI] [PubMed] [Google Scholar]
- 43.Takemoto M, Kusachi S, Urabe N, Inoue K, Tsuji T. Jpn Circ J. 1993;57:1150–1158. doi: 10.1253/jcj.57.1150. [DOI] [PubMed] [Google Scholar]