Abstract
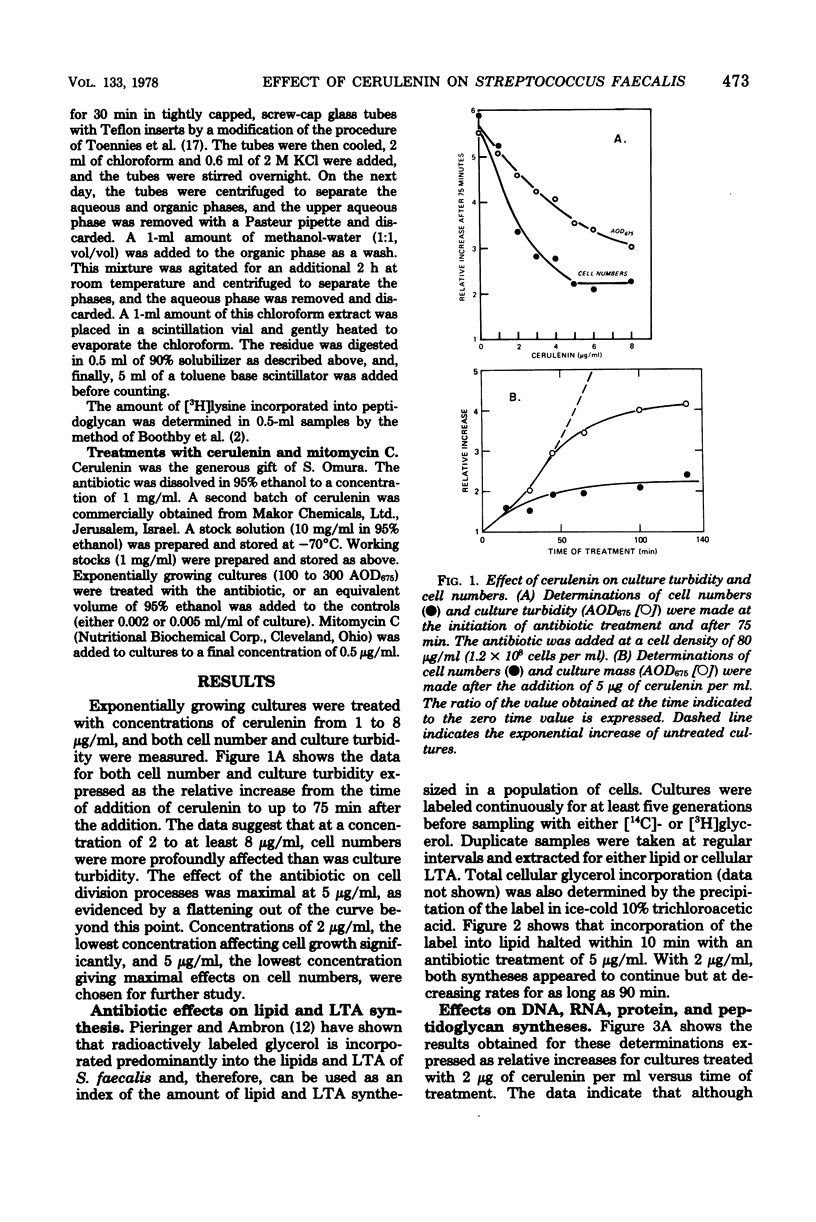
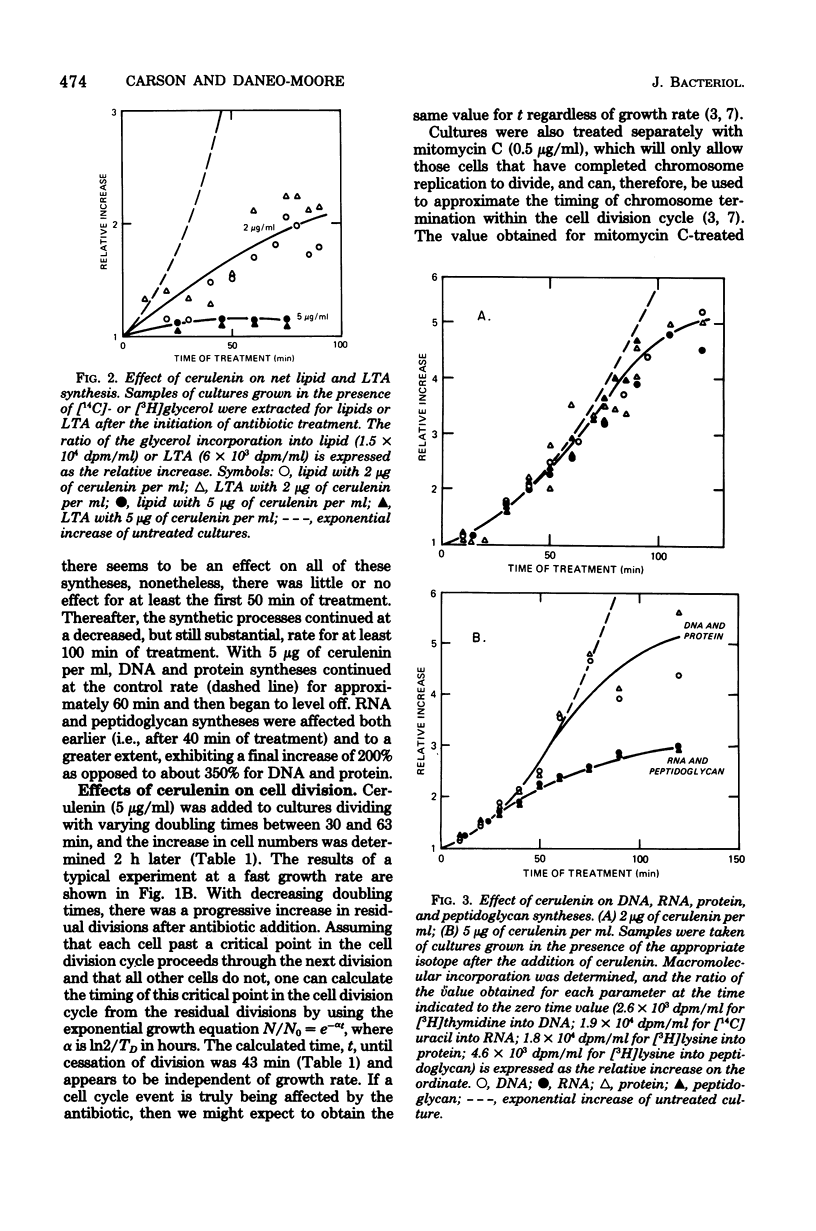
The antibiotic cerulenin has been used to study macromolecular synthesis and cell division in Streptococcus faecalis. The data suggest that lipid and lipoteichoic acid synthesis as well as cell number increase are affected prior to any observable effects on overall mass increase or DNA, RNA, protein, or peptidoglycan synthesis. Treatment with cerulenin of cultures growing at various rates and analysis of the subsequent cell divisions indicate that the antibiotic may block a cell cycle event that precedes the completion of chromosome replication by about 10 min.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbogast L. Y., Henderson T. O. Effect of inhibition of protein synthesis on lipid metabolism in Lactobacillus plantarum. J Bacteriol. 1975 Sep;123(3):962–971. doi: 10.1128/jb.123.3.962-971.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothby D., Daneo-Moore L., Shockman G. D. A rapid, guantitative, and selective estimation of radioactively labeled peptidoglycan in gram-positive bacteria. Anal Biochem. 1971 Dec;44(2):645–653. doi: 10.1016/0003-2697(71)90255-7. [DOI] [PubMed] [Google Scholar]
- Clark D. J. The regulation of DNA replication and cell division in E. coli B-r. Cold Spring Harb Symp Quant Biol. 1968;33:823–838. doi: 10.1101/sqb.1968.033.01.094. [DOI] [PubMed] [Google Scholar]
- Cleveland R. F., Daneo-Moore L., Wicken A. J., Shockman G. D. Effect of lipoteichoic acid and lipids on lysis of intact cells of Streptococcus faecalis. J Bacteriol. 1976 Sep;127(3):1582–1584. doi: 10.1128/jb.127.3.1582-1584.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland R. F., Wicken A. J., Daneo-Moore L., Shockman G. D. Inhibition of wall autolysis in Streptococcus faecalis by lipoteichoic acid and lipids. J Bacteriol. 1976 Apr;126(1):192–197. doi: 10.1128/jb.126.1.192-197.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels M. J. Lipid synthesis in relation to the cell cycle of Bacillus megaterium KM and Escherichia coli. Biochem J. 1969 Dec;115(4):697–701. doi: 10.1042/bj1150697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Daneo-Moore L., Boothby D., Shockman G. D. Effect of inhibition of deoxyribonucleic acid and protein synthesis on the direction of cell wall growth in Streptococcus faecalis. J Bacteriol. 1974 May;118(2):681–692. doi: 10.1128/jb.118.2.681-692.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. O., Fisher W. D. Stimulation of cell division by membrane-active agents. Biochem Biophys Res Commun. 1973 Jan 23;50(2):200–210. doi: 10.1016/0006-291x(73)90827-9. [DOI] [PubMed] [Google Scholar]
- McCarthy C. Synthesis and release of sulfolipid by Mycobacterium avium during growth andcell division. Infect Immun. 1976 Nov;14(5):1241–1252. doi: 10.1128/iai.14.5.1241-1252.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieringer R. A., Ambron R. T. A method for the specific labeling of the glycerol in glyceride-containing lipids of Streptococcus faecalis ATCC 9790. J Lipid Res. 1973 May;14(3):370–372. [PubMed] [Google Scholar]
- Polonovski J., Wald R., Paysant M., Rampini C., Barbu E. Métabolisme du phosphatidylglycérol et du cardiolipide chez Staphylococcus aureus. Ann Inst Pasteur (Paris) 1971 May;120(5):589–598. [PubMed] [Google Scholar]
- Shockman G. D., Daneo-Moore L., Higgins M. L. Problems of cell wall and membrane growth, enlargement, and division. Ann N Y Acad Sci. 1974 May 10;235(0):161–197. doi: 10.1111/j.1749-6632.1974.tb43265.x. [DOI] [PubMed] [Google Scholar]
- Stárka J., Moravová J. Phospholipids and cellular division of Escherichia coli. J Gen Microbiol. 1970 Feb;60(2):251–257. doi: 10.1099/00221287-60-2-251. [DOI] [PubMed] [Google Scholar]
- Toennies G., Das D. N., Feng F. New observations on the determination of bacterial lipid phosphorus. Can J Microbiol. 1968 Apr;14(4):484–485. doi: 10.1139/m68-079. [DOI] [PubMed] [Google Scholar]
- Van Schaik F. W., Veerkamp J. H. Biochemical changes in Bifidobacterium bifidum var. pennsylvanicus after cell wall inhibition. VIII. Composition and metabolism of phospholipids at different stages and conditions of growth. Biochim Biophys Acta. 1975 May 22;388(2):213–225. [PubMed] [Google Scholar]
- Vance D., Goldberg I., Mitsuhashi O., Bloch K. Inhibition of fatty acid synthetases by the antibiotic cerulenin. Biochem Biophys Res Commun. 1972 Aug 7;48(3):649–656. doi: 10.1016/0006-291x(72)90397-x. [DOI] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Studies on the group F antigen of lactobacilli: isolation of a teichoic acid-lipid complex from Lactobacillus fermenti NCTC 6991. J Gen Microbiol. 1970 Mar;60(3):293–301. doi: 10.1099/00221287-60-3-293. [DOI] [PubMed] [Google Scholar]



