Abstract
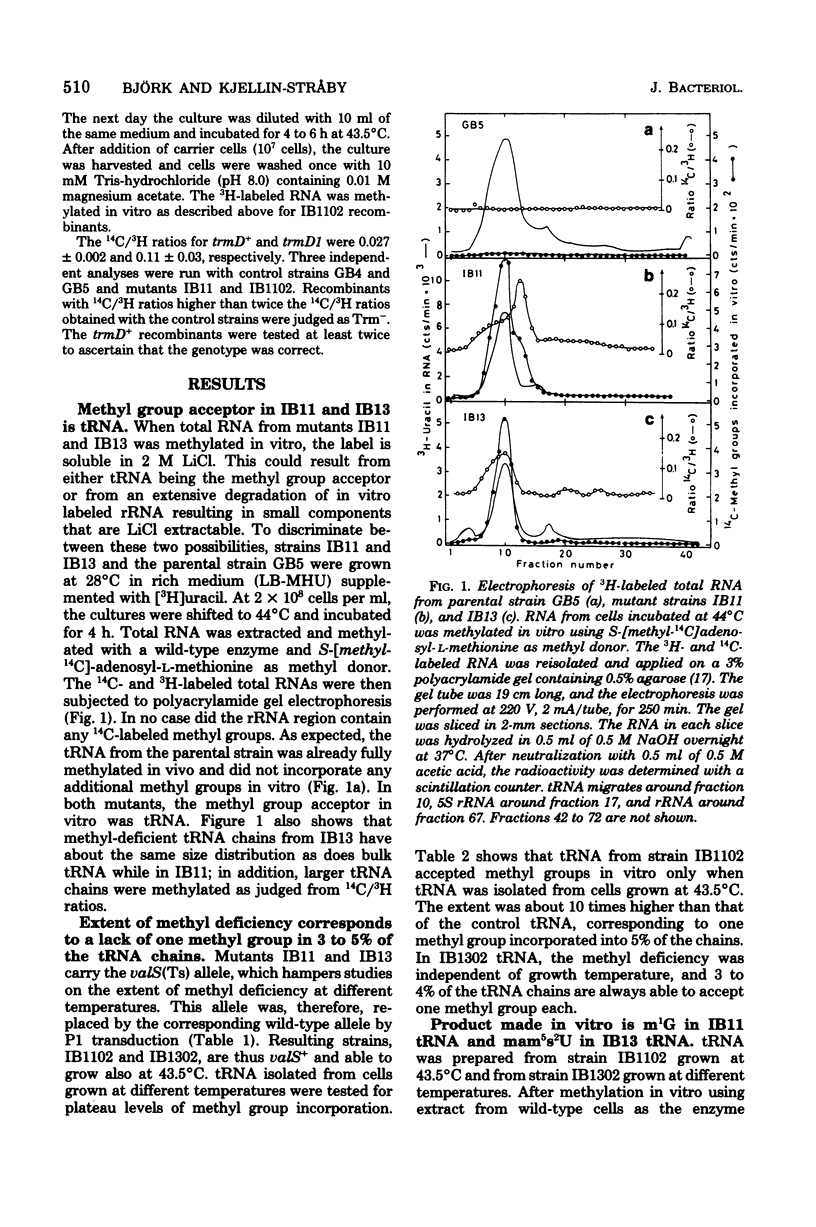
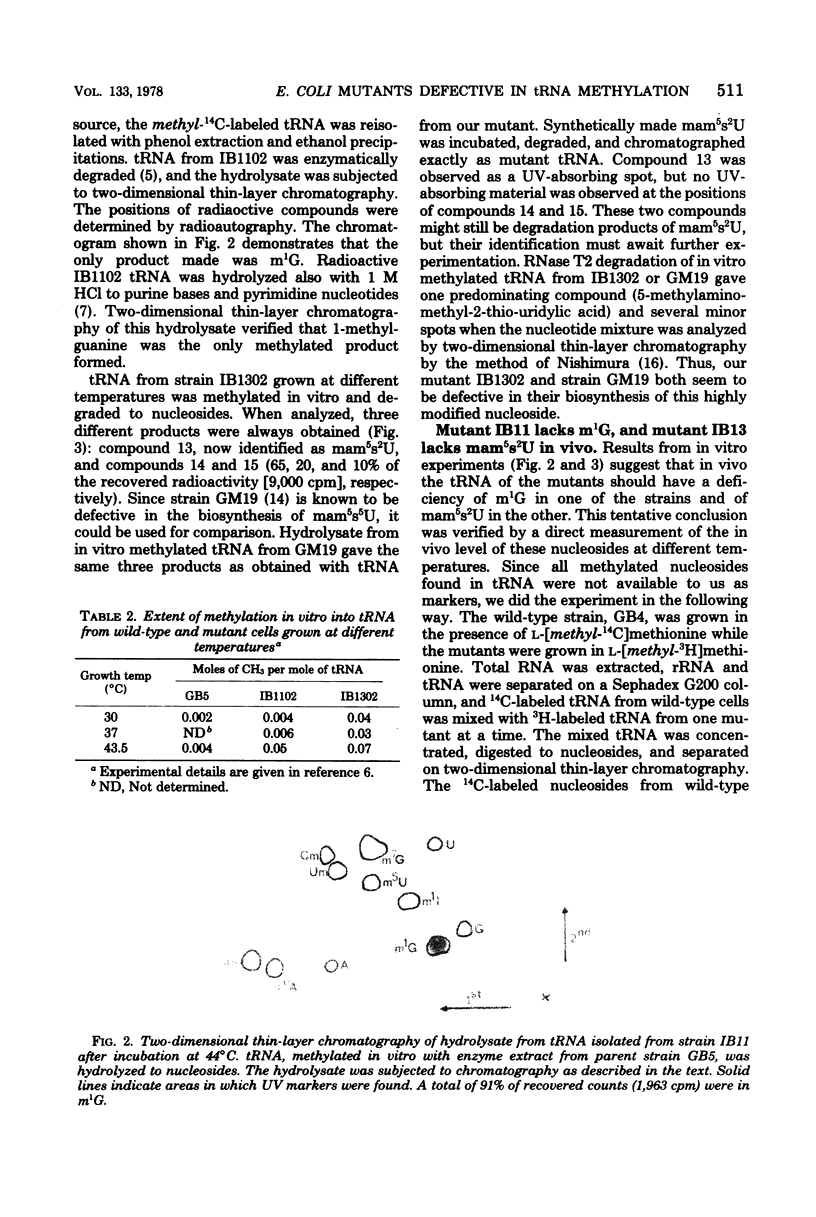
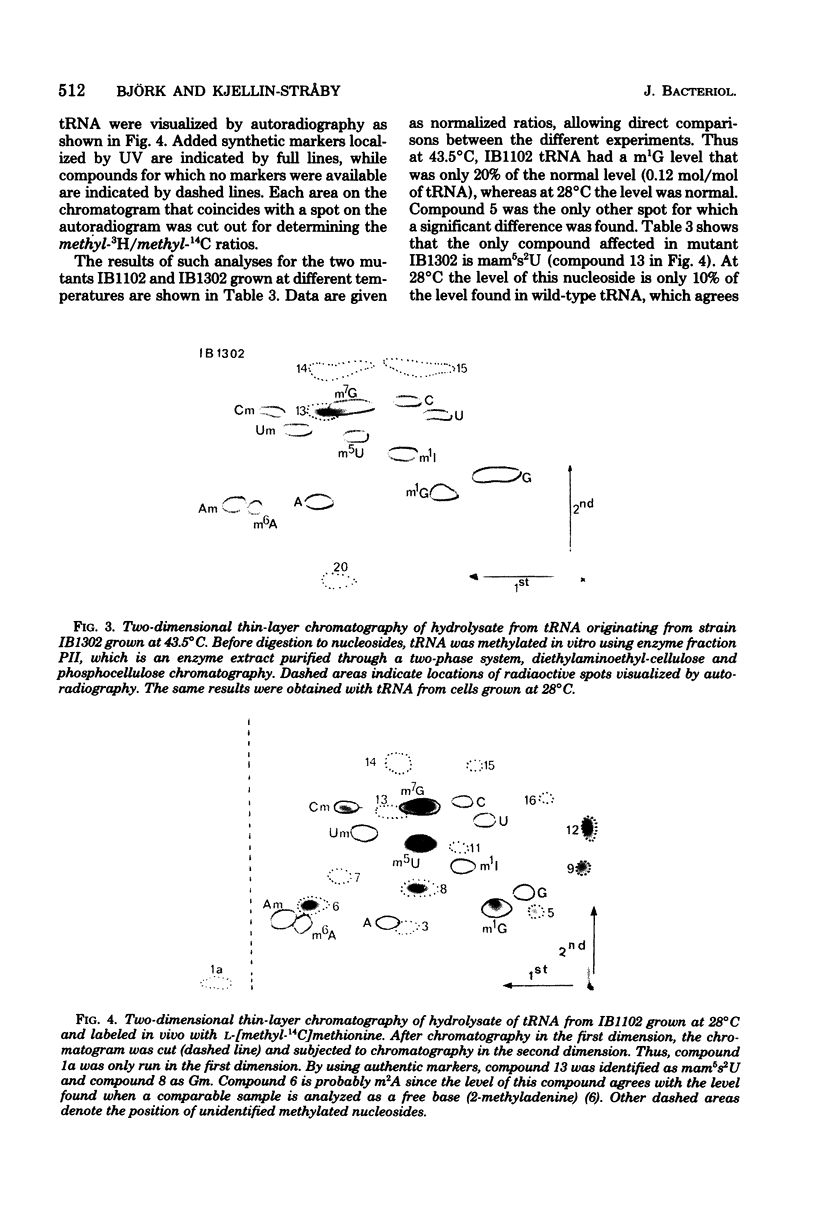
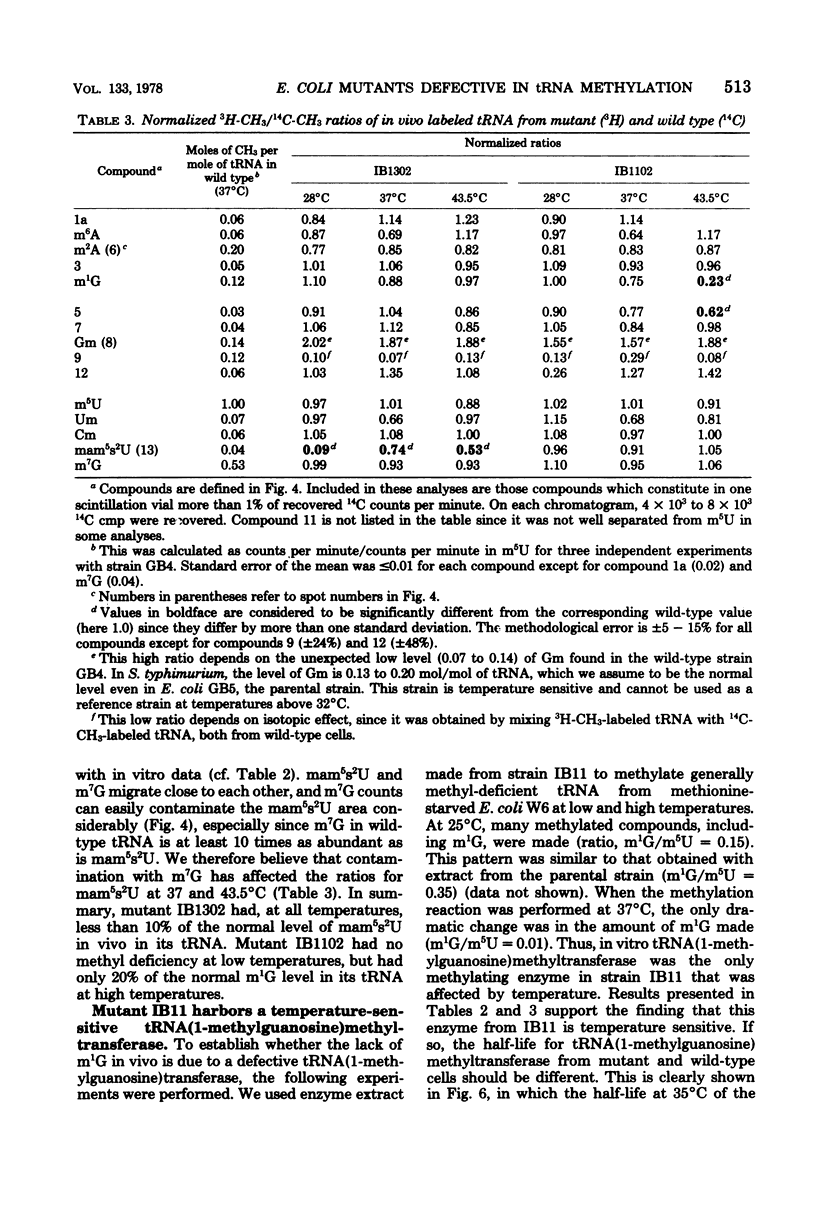
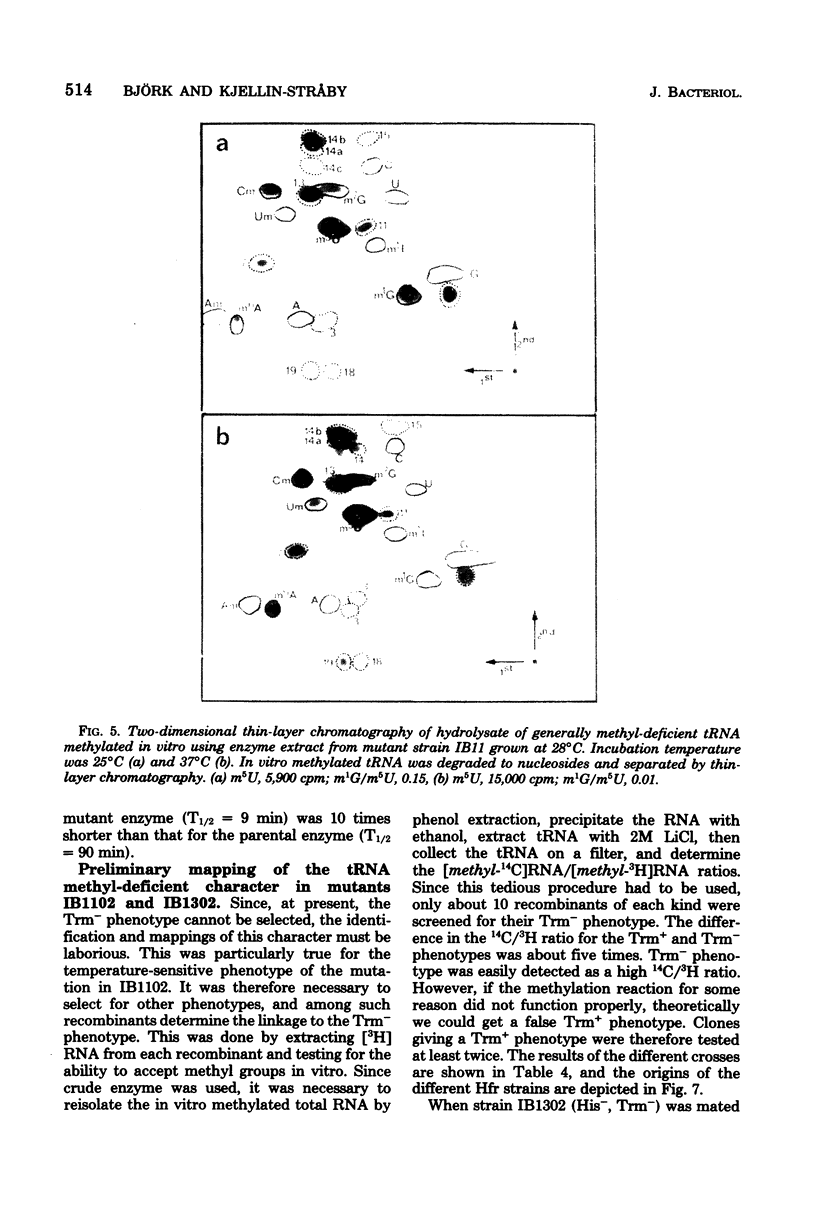
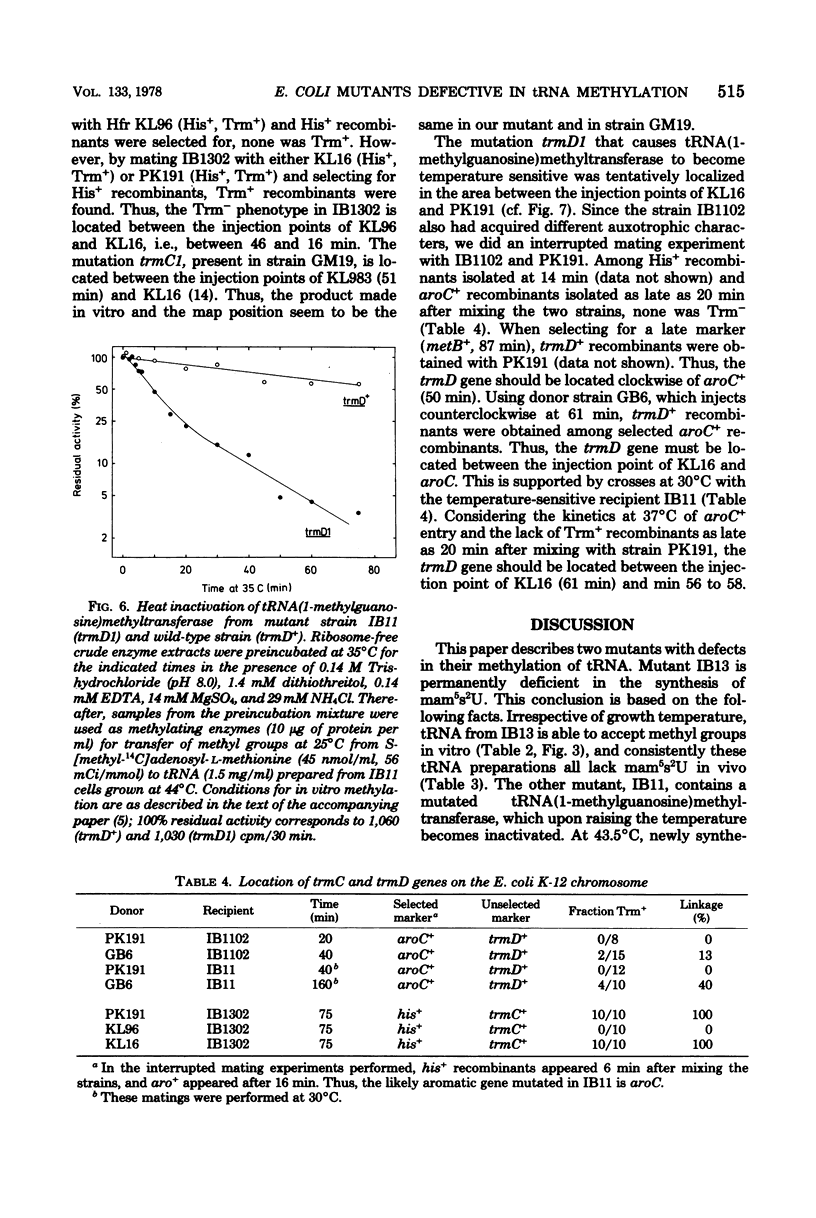
Two tRNA methyltransferase mutants, isolated as described in the accompanying paper (G.R. Björk and K. Kjellin-Stråby, J. Bacteriol. 133:499-207, 1978), are biochemicaaly and genetically characterized. tRNA from mutant IB13 lacks 5-methylaminomethyl-2-thio-uridine in vivo due to a permanently nonfunctional methyltransferase. Thus tRNA from this mutant is a specific substrate for the corresponding tRNA methyltransferase in vitro. In spite of this defect in tRNA, such a mutant is viable. Mutant IB11 is conditionally defective in the biosynthesis of 1-methylguanosine in tRNA due to a temperature-sensitive tRNA (1-methyl-guanosine) methyltransferase. In mutant cells grown at a high temperature, the level of 1-methylguanosine in bulk tRNA is 20% of that of the wild type, demonstrating that in this mutant an 80% deficiency of 1-methylguanosine in tRNA is not lethal. Genetically these two distinct lesions, trmC2, causing 5=methylaminomethyl-2-thio-uridine deficiency, and trmD1, giving a temperature-sensitive tRNA (1-methylguanosine)methyltransferase, are both located between 50 and 61 min on the Escherichia coli chromosome.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avital S., Elson D. A convenient procedure for preparing transfer ribonucleic acid from Escherichia coli. Biochim Biophys Acta. 1969 Apr 22;179(2):297–307. doi: 10.1016/0005-2787(69)90038-0. [DOI] [PubMed] [Google Scholar]
- BOMAN H. G., HJERTEN S. "Molecular sieving" of bacterial RNA. Arch Biochem Biophys. 1962 Sep;Suppl 1:276–282. [PubMed] [Google Scholar]
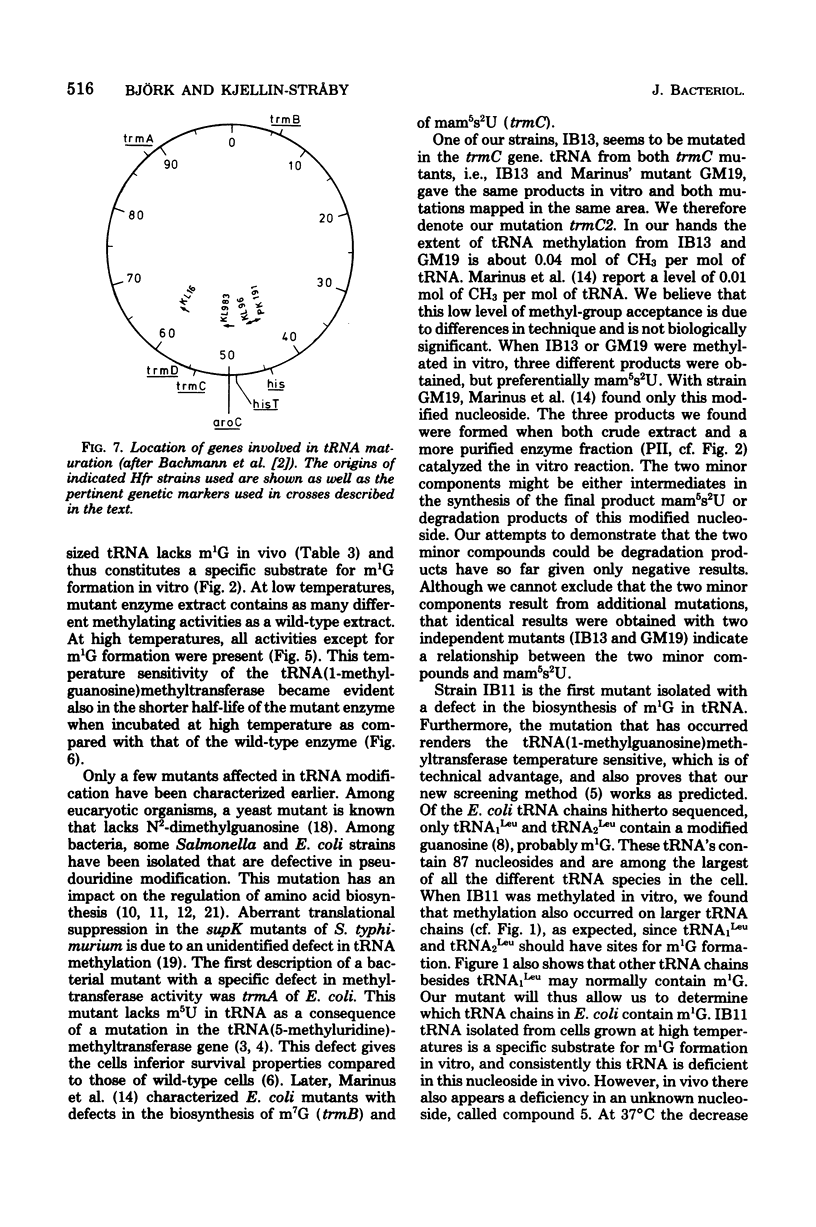
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk G. R., Isaksson L. A. Isolation of mutants of Escherichia coli lac king 5-methyluracil in transfer ribonucleic acid or 1-methylguanine in ribosomal RNA. J Mol Biol. 1970 Jul 14;51(1):83–100. doi: 10.1016/0022-2836(70)90272-x. [DOI] [PubMed] [Google Scholar]
- Björk G. R., Kjellin-Stråby K. General screening procedure for RNA modificationless mutants: isolation of Escherichia coli strains with specific defects in RNA methylation. J Bacteriol. 1978 Feb;133(2):499–507. doi: 10.1128/jb.133.2.499-507.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk G. R., Neidhardt F. C. Physiological and biochemical studies on the function of 5-methyluridine in the transfer ribonucleic acid of Escherichia coli. J Bacteriol. 1975 Oct;124(1):99–111. doi: 10.1128/jb.124.1.99-111.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk G. R., Svensson I. Analysis of methylated constituents from RNA by thin-layer chromatography. Biochim Biophys Acta. 1967 Apr 18;138(2):430–432. doi: 10.1016/0005-2787(67)90504-7. [DOI] [PubMed] [Google Scholar]
- Björk G. R. Transductional mapping of gene trmA responsible for the production of 5-methyluridine in transfer ribonucleic acid of Escherichia coli. J Bacteriol. 1975 Oct;124(1):92–98. doi: 10.1128/jb.124.1.92-98.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank H. U., Söll D. The nucleotide sequence of two leucine tRNA species from Escherichia coli K12. Biochem Biophys Res Commun. 1971 Jun 4;43(5):1192–1197. doi: 10.1016/0006-291x(71)90589-4. [DOI] [PubMed] [Google Scholar]
- Bruni C. B., Colantuoni V., Sbordone L., Cortese R., Blasi F. Biochemical and regulatory properties of Escherichia coli K-12 hisT mutants. J Bacteriol. 1977 Apr;130(1):4–10. doi: 10.1128/jb.130.1.4-10.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang G. W., Roth J. R., Ames B. N. Histidine regulation in Salmonella typhimurium. 8. Mutations of the hisT gene. J Bacteriol. 1971 Oct;108(1):410–414. doi: 10.1128/jb.108.1.410-414.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther R. P., Hatfield W. Biochemical characterization of an Escherichia coli hisT strain. J Bacteriol. 1977 Apr;130(1):552–557. doi: 10.1128/jb.130.1.552-557.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Rapid mapping of conditional and auxotrophic mutations in Escherichia coli K-12. J Bacteriol. 1973 Feb;113(2):798–812. doi: 10.1128/jb.113.2.798-812.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinus M. G., Morris N. R., Söll D., Kwong T. C. Isolation and partial characterization of three Escherichia coli mutants with altered transfer ribonucleic acid methylases. J Bacteriol. 1975 Apr;122(1):257–265. doi: 10.1128/jb.122.1.257-265.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S. Minor components in transfer RNA: their characterization, location, and function. Prog Nucleic Acid Res Mol Biol. 1972;12:49–85. [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Phillips J. H., Kjellin-Stråby K. Studies on microbial ribonucleic acid. IV. Two mutants of Saccharomyces cerevisiae lacking N-2-dimethylguanine in soluble ribonucleic acid. J Mol Biol. 1967 Jun 28;26(3):509–518. doi: 10.1016/0022-2836(67)90318-x. [DOI] [PubMed] [Google Scholar]
- Reeves R. H., Roth J. R. Transfer ribonucleic acid methylase deficiency found in UGA supressor strains. J Bacteriol. 1975 Oct;124(1):332–340. doi: 10.1128/jb.124.1.332-340.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogg H., Brambilla R., Keith G., Staehelin M. An improved method for the separation and quantitation of the modified nucleosides of transfer RNA. Nucleic Acids Res. 1976 Jan;3(1):285–295. doi: 10.1093/nar/3.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer C. E., Smith G. R., Cortese R., Ames B. N. [Mutant tRNA His ineffective in repression and lacking two pseudouridine modifications]. Nat New Biol. 1972 Jul 19;238(81):72–74. doi: 10.1038/newbio238072a0. [DOI] [PubMed] [Google Scholar]