Abstract
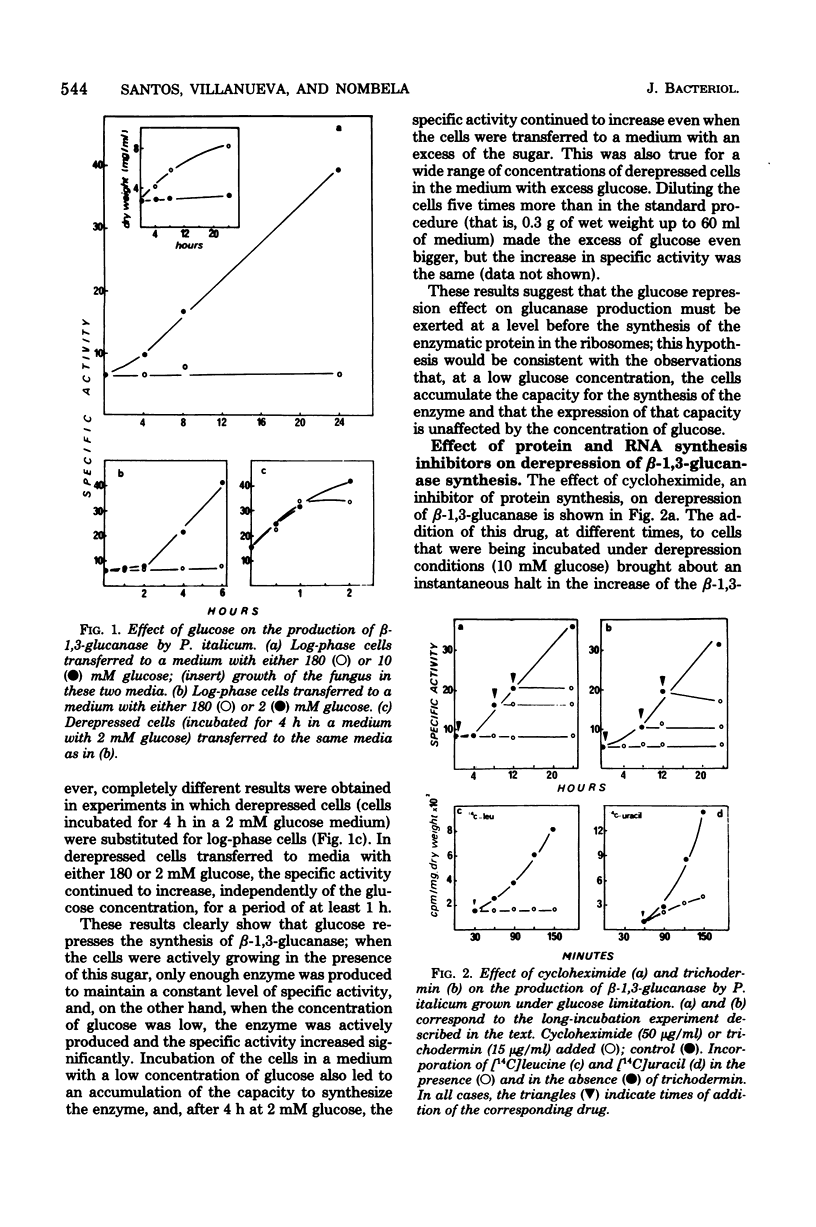
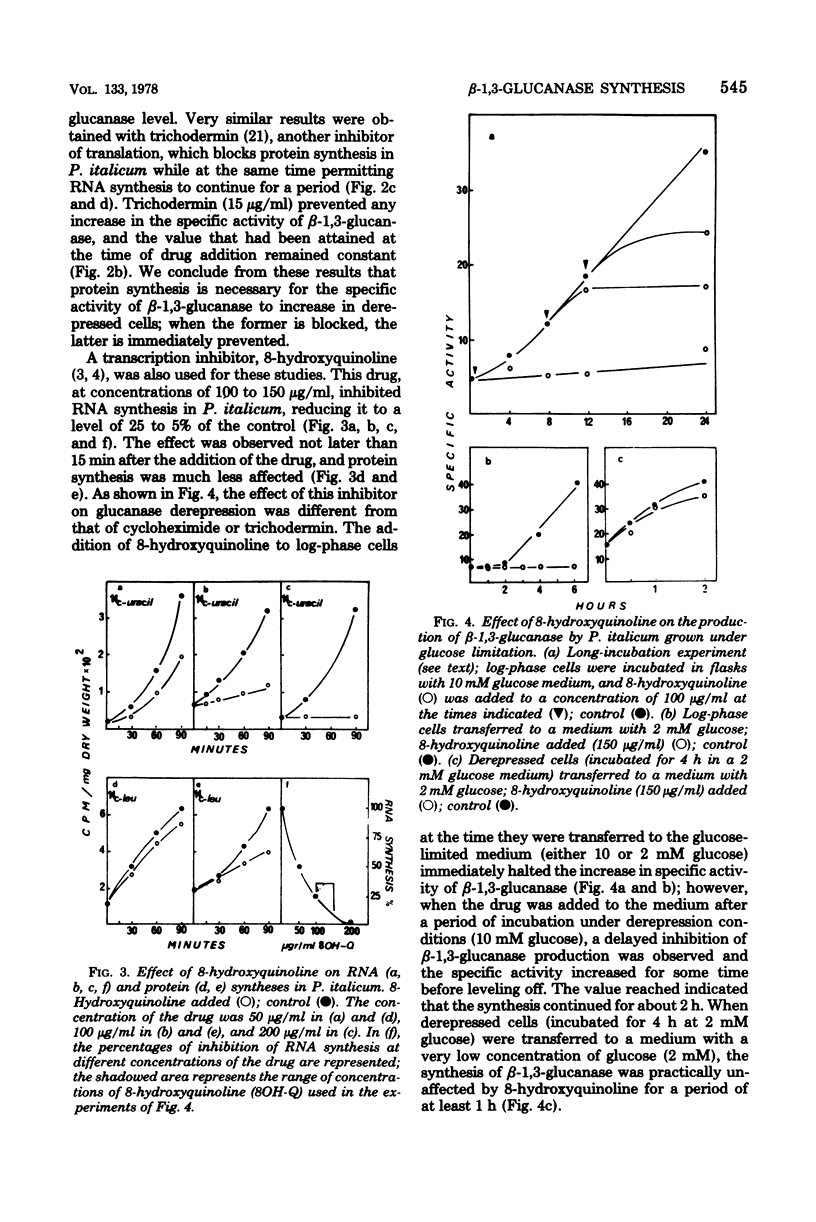
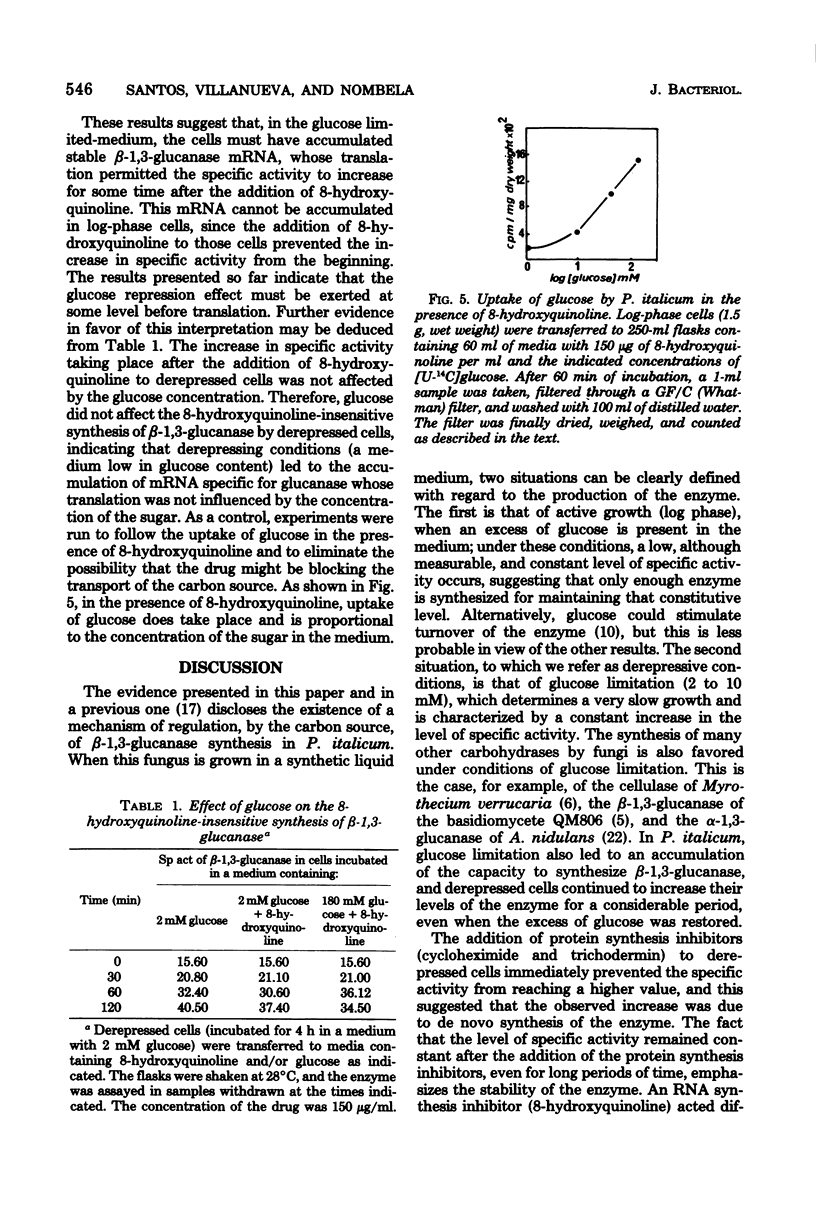
The filamentous fungus Penicillium italicum produced a certain level of beta-1,3-glucanase during active growth in a glucose-supplemented medium; however, at a low glucose concentration (2 to 10 mM), derepression took place and the specific activity of the enzyme increased significantly. Derepressed cells (incubated in a glucose-limited medium) accumulated a capacity for the synthesis of beta-1,3-glucanase, which led to a subsequent increase in the specific activity even when the cells were transferred to a medium with an excess of glucose (180 mM). Two protein synthesis inhibitors, cycloheximide and trichodermin, immediately stopped the increase in specific activity when added to derepressed cells. On the other hand, 8-hydroxyquinoline, an RNA a synthesis inhibitor, acted differently, since it permitted the specific activity to increase for some time after being added to depressed cells. Moreover, the concentration of glucose did not affect the 8-hydroxyquinoline-insensitive synthesis of beta-1,3-glucanase. It is concluded that the glucose repression effect on beta-1,3-glucanase production must be exerted at a pretranslational level that could be either mRNA synthesis or some stage of the process involved in its maturation or stabilization.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Creanor J., May J. W., Mitchison J. M. The effect of 8-hydroxyquinoline on enzyme synthesis in the fission yeast Schizosaccharomyces pombe. Eur J Biochem. 1975 Dec 15;60(2):487–493. doi: 10.1111/j.1432-1033.1975.tb21027.x. [DOI] [PubMed] [Google Scholar]
- Fraser R. S., Creanor J. Rapid and selective inhibition of RNA synthesis in yeast by 8-hydroxyquinoline. Eur J Biochem. 1974 Jul 1;46(1):67–73. doi: 10.1111/j.1432-1033.1974.tb03597.x. [DOI] [PubMed] [Google Scholar]
- Friebe B., Holldorf A. W. Control of Extracellular beta-1,3-glucanase activity in a basidiomycete species. J Bacteriol. 1975 Jun;122(3):818–825. doi: 10.1128/jb.122.3.818-825.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme M. A., Stranks D. W. Regulation of cellulase production by Myrothecium verrucaria grown on non-cellulosic substrates. J Gen Microbiol. 1971 Dec;69(2):145–155. doi: 10.1099/00221287-69-2-145. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis C. M., Fincham J. R. Regulation of nitrate reductase in the basidiomycete Ustilago maydis. J Bacteriol. 1970 Jul;103(1):55–61. doi: 10.1128/jb.103.1.55-61.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- Ortiz J. M., Berkeley R. C., Brewer S. J. Production of exo-beta-N-acetylglucosaminidase by Bacillus subtilis B. J Gen Microbiol. 1973 Aug;77(2):331–337. doi: 10.1099/00221287-77-2-331. [DOI] [PubMed] [Google Scholar]
- Pastan I., Adhya S. Cyclic adenosine 5'-monophosphate in Escherichia coli. Bacteriol Rev. 1976 Sep;40(3):527–551. doi: 10.1128/br.40.3.527-551.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmsdorf H. J., Cailla H. L., Spitz E., Moran M. J., Rickenberg H. V. Effect of sugars on early biochemical events in development of Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3183–3187. doi: 10.1073/pnas.73.9.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos T., Sanchez M., Villanueva J. R., Nombela C. Regulation of the beta-1,3-glucanase system in Penicillium italicum: glucose repression of the various enzymes. J Bacteriol. 1978 Feb;133(2):465–471. doi: 10.1128/jb.133.2.465-471.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos T., Villanueva J. R., Nombela C. Production and catabolite repression of Penicillium italicum beta-glucanases. J Bacteriol. 1977 Jan;129(1):52–58. doi: 10.1128/jb.129.1.52-58.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian K. N., Sorger G. J. Regulation of nitrate reductase in Neurospora crassa: regulation of transcription and translation. J Bacteriol. 1972 May;110(2):547–553. doi: 10.1128/jb.110.2.547-553.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. R., Terry K., Matchett W. H. Temporal separation of transcription and translation in Neurospora. J Bacteriol. 1970 Aug;103(2):370–374. doi: 10.1128/jb.103.2.370-374.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonneveld B. J. Morphogenesis in Aspergillus nidulans. The significance of a alpha-1, 3-glucan of the cell wall and alpha-1, 3-glucanase for cleistothecium development. Biochim Biophys Acta. 1972 Jun 26;273(1):174–187. doi: 10.1016/0304-4165(72)90205-x. [DOI] [PubMed] [Google Scholar]


