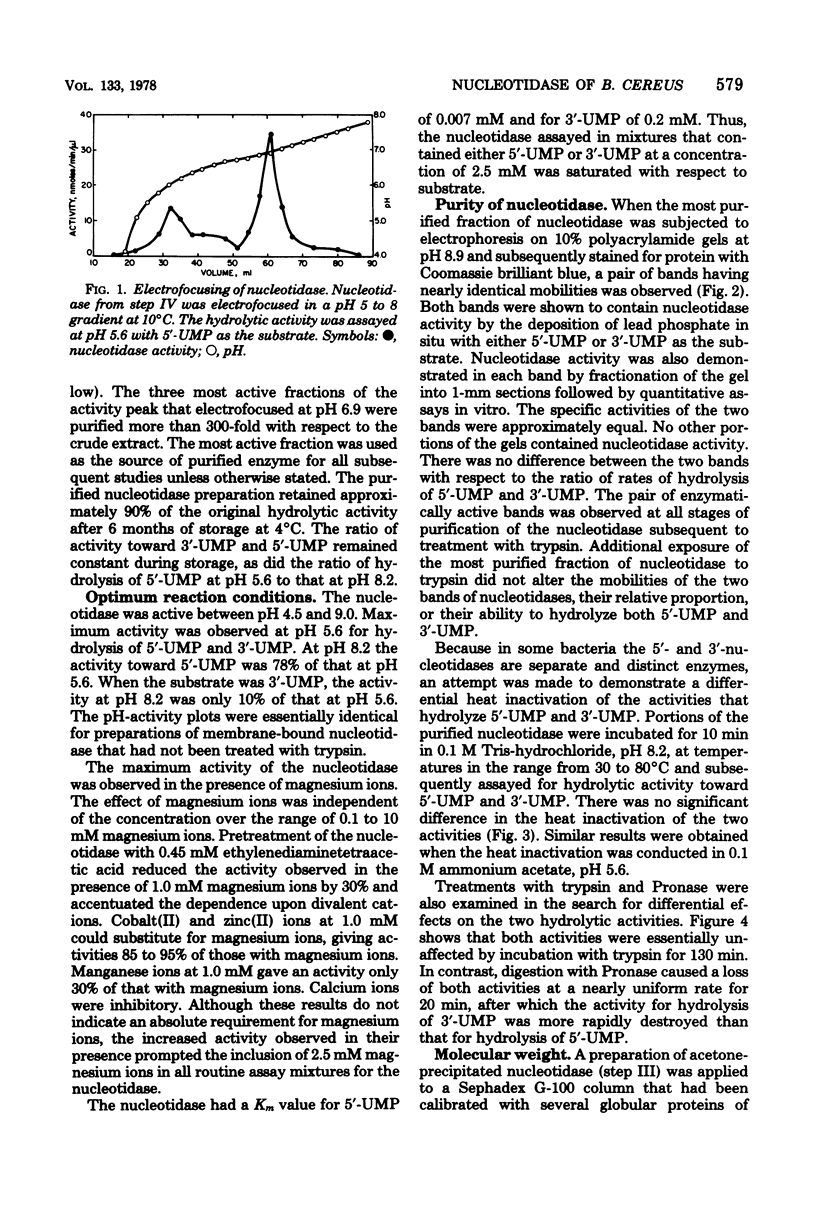
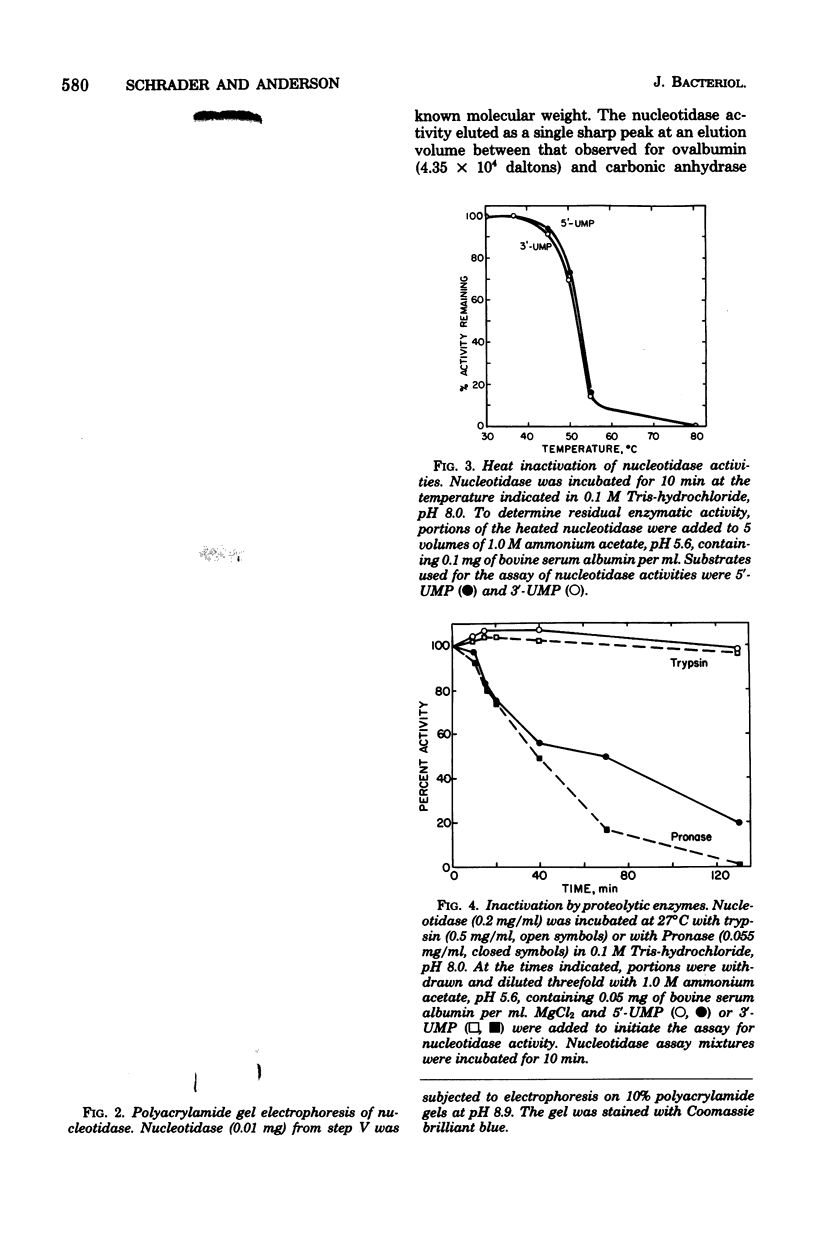
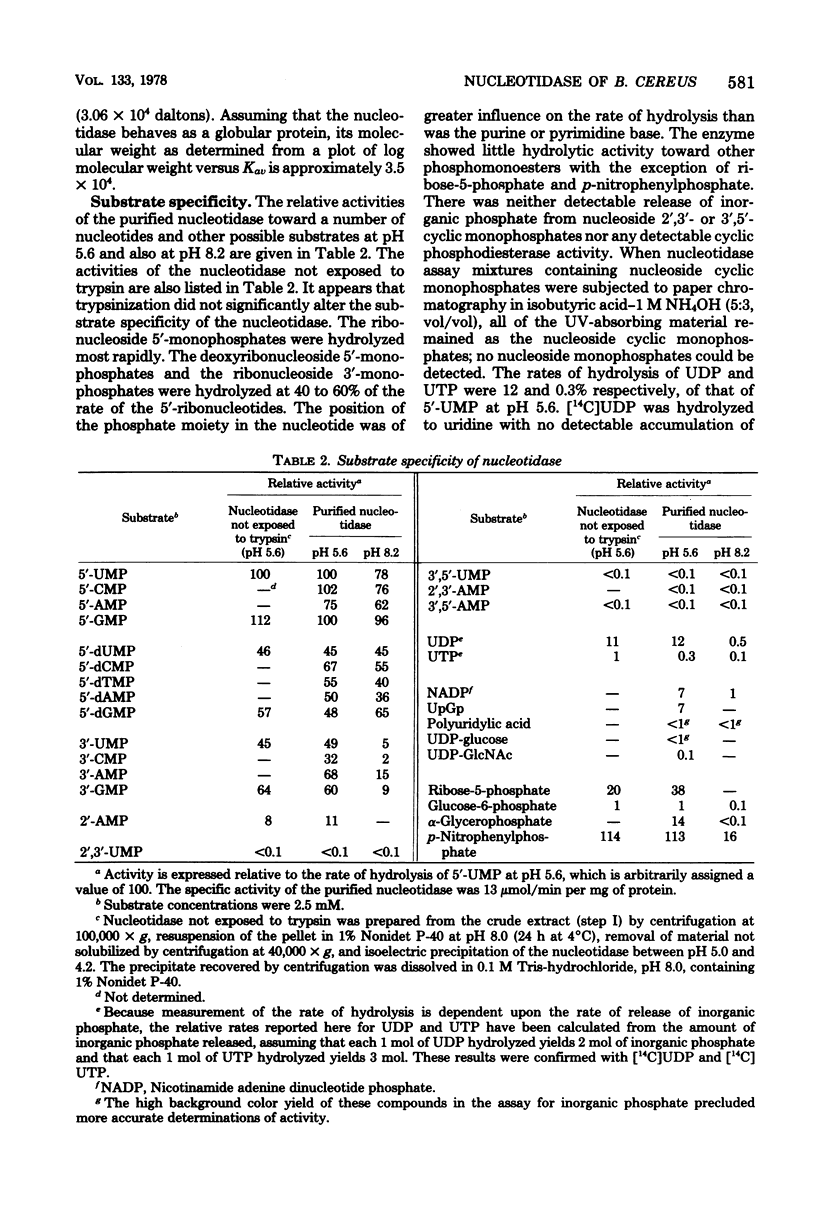
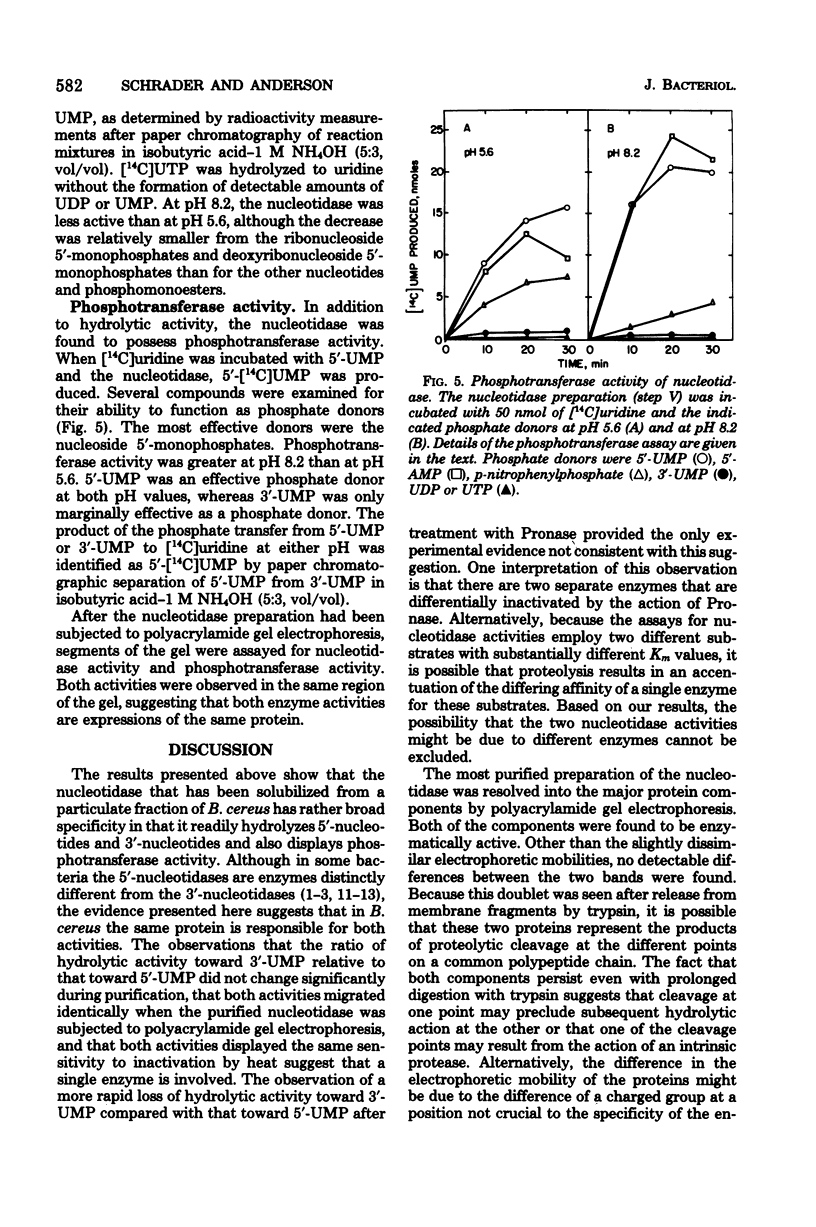
Abstract
A membrane-bound nucleotidase of Bacillus cereus T was solubilized by digestion with trypsin and subsequently purified more than 300-fold. The purified nucleotidase was most active on ribonucleoside 5'-monophosphates and was slightly less active (40 to 60%) on deoxyribonucleoside 5'-monophosphates and ribonucleoside 3'-monophosphates. In addition to hydrolytic activity, the nucleotidase preparation possessed phosphotransferase activity by which phosphate is transferred from a phosphate donor to the 5' position of nucleosides.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANRAKU Y. A NEW CYCLIC PHOSPHODIESTERASE HAVING A 3'-NUCLEOTIDASE ACTIVITY FROM ESCHERICHIA COLI B. I. PURIFICATION AND SOME PROPERTIES OF THE ENZYME. J Biol Chem. 1964 Oct;239:3412–3419. [PubMed] [Google Scholar]
- ANRAKU Y. A NEW CYCLIC PHOSPHODIESTERASE HAVING A 3'-NUCLEOTIDASE ACTIVITY FROM ESCHERICHIA COLI B. II. FURTHER STUDIES ON SUBSTRATE SPECIFICITY AND MODE OF ACTION OF THE ENZYME. J Biol Chem. 1964 Oct;239:3420–3424. [PubMed] [Google Scholar]
- Brownlee S. T., Heath E. C. An extracellular 5'-nucleotidase with both monoesterase and diesterase activity from Micrococcus sodonensis. Arch Biochem Biophys. 1975 Jan;166(1):1–7. doi: 10.1016/0003-9861(75)90357-4. [DOI] [PubMed] [Google Scholar]
- Brunngraber E. F., Chargaff E. A nucleotide phosphotransferase from Escherichia coli. Purification and properties. Biochemistry. 1973 Jul 31;12(16):3005–3012. doi: 10.1021/bi00740a009. [DOI] [PubMed] [Google Scholar]
- Brunngraber E. F., Chargaff E. Transferase from Escherichia coli effecting low-energy phosphate transfer to nucleosides and nucleotides. Proc Natl Acad Sci U S A. 1970 Sep;67(1):107–112. doi: 10.1073/pnas.67.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cercignani G., Serra M. C., Fini C., Natalini P., Palmerini C. A., Magni G., Ipata P. L. Properties of 5'-nucleotidase from Bacillus cereus obtained by washing intact cells with water. Biochemistry. 1974 Aug 13;13(17):3628–3634. doi: 10.1021/bi00714a035. [DOI] [PubMed] [Google Scholar]
- Chao H. M. Nucleoside phosphotransferase from Erwinia herbicola, a new membrane-bound enzyme. J Biol Chem. 1976 Apr 25;251(8):2330–2333. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mauck J., Glaser L. Periplasmic nucleoside diphosphate sugar hydrolase from Bacillus subtilis. Biochemistry. 1970 Mar 3;9(5):1140–1147. doi: 10.1021/bi00807a014. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Chou J. Release of surface enzymes in Enterobacteriaceae by osmotic shock. J Bacteriol. 1967 Dec;94(6):1934–1945. doi: 10.1128/jb.94.6.1934-1945.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C. The 5'-nucleotidases (uridine diphosphate sugar hydrolases) of the Enterobacteriaceae. Biochemistry. 1968 Oct;7(10):3766–3773. doi: 10.1021/bi00850a059. [DOI] [PubMed] [Google Scholar]
- Neu H. C. The cyclic phosphodiesterases (3'-nucleotidases) of the enterobacteriaceae. Biochemistry. 1968 Oct;7(10):3774–3780. doi: 10.1021/bi00850a060. [DOI] [PubMed] [Google Scholar]
- Nisonson I., Tannenbaum M., Neu H. C. Surface localization of Escherichia coli 5'-nucleotidase by electron microscopy. J Bacteriol. 1969 Nov;100(2):1083–1090. doi: 10.1128/jb.100.2.1083-1090.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagil E., Beacham I. R. Uptake of adenosine 5'-monophosphate by Escherichia coli. J Bacteriol. 1975 Feb;121(2):401–405. doi: 10.1128/jb.121.2.401-405.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



