Abstract
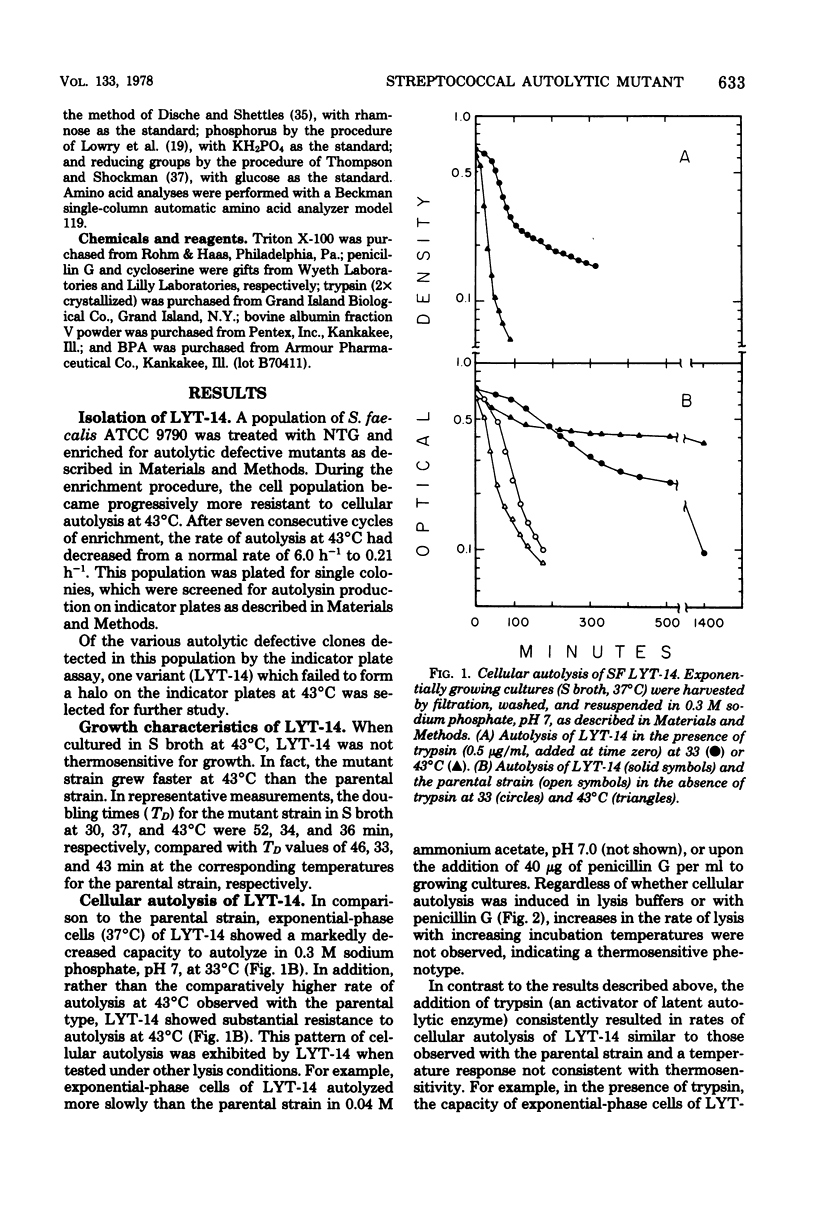
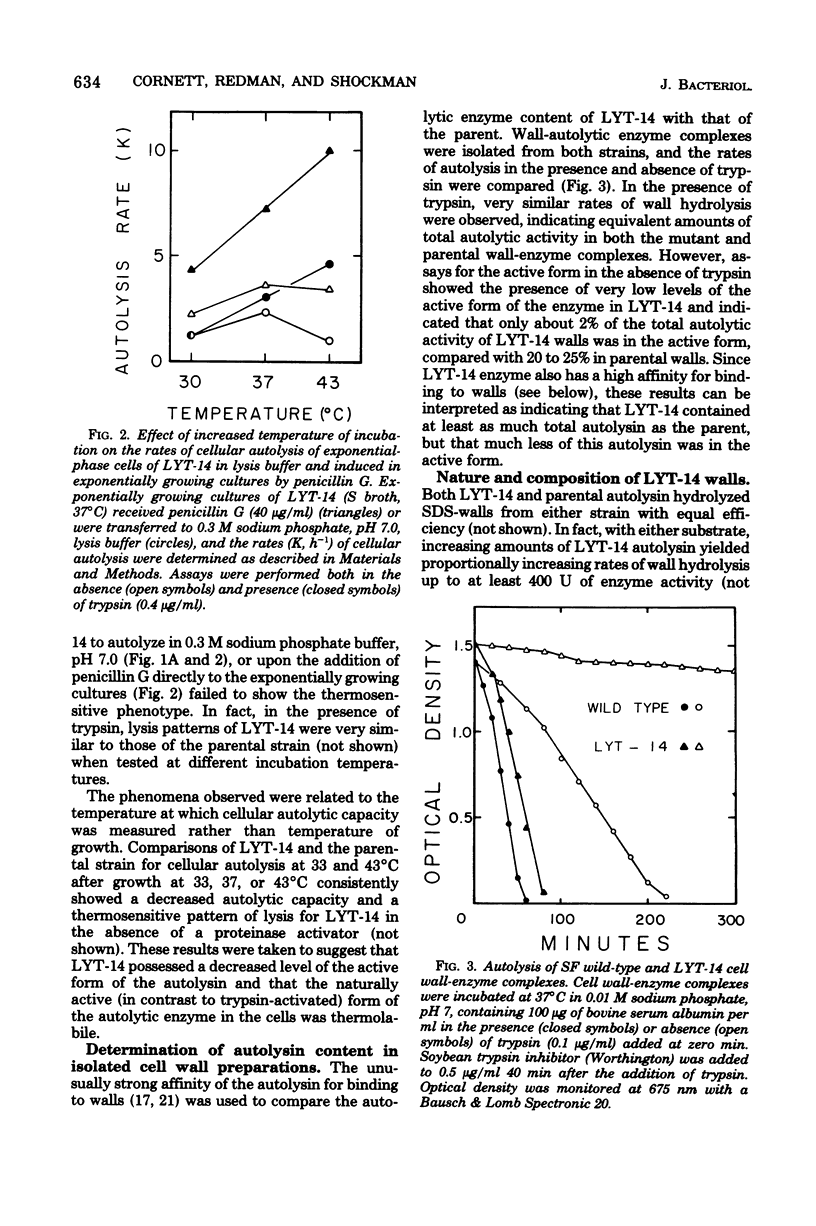
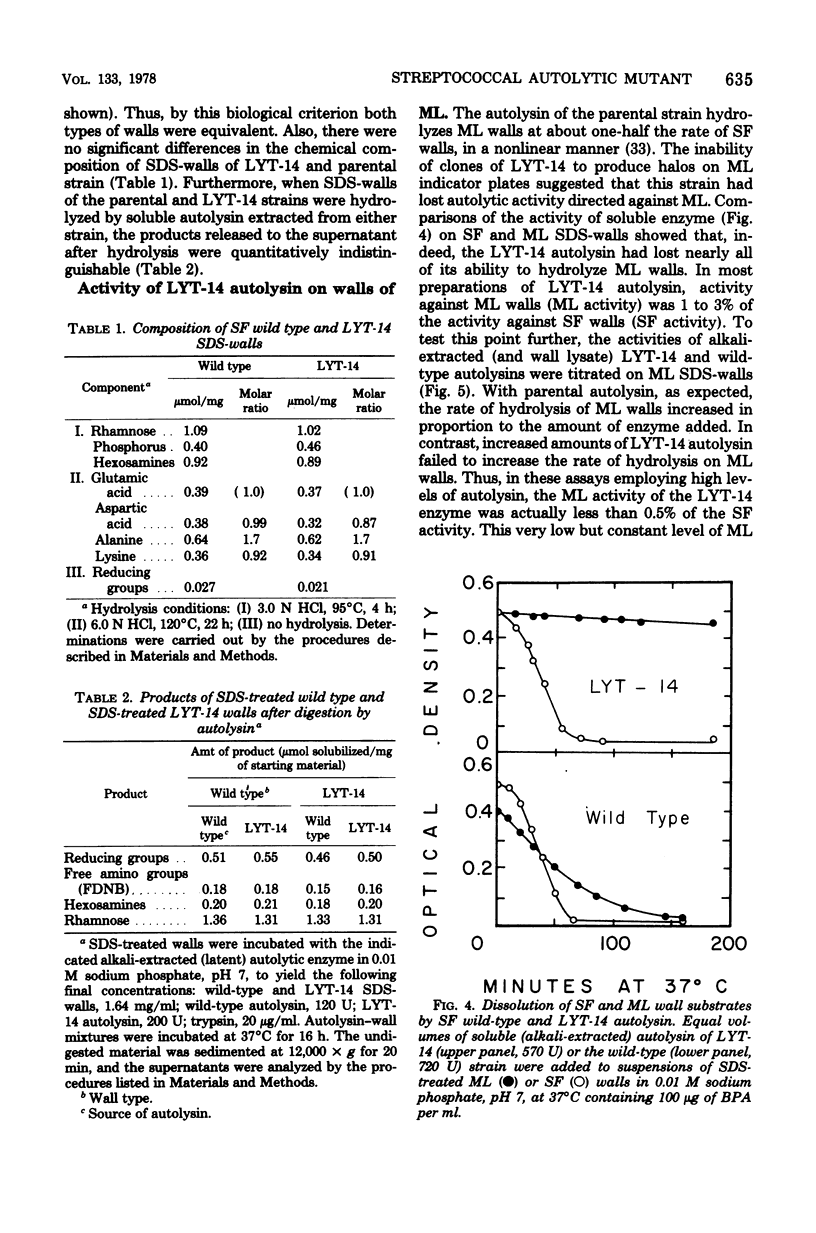
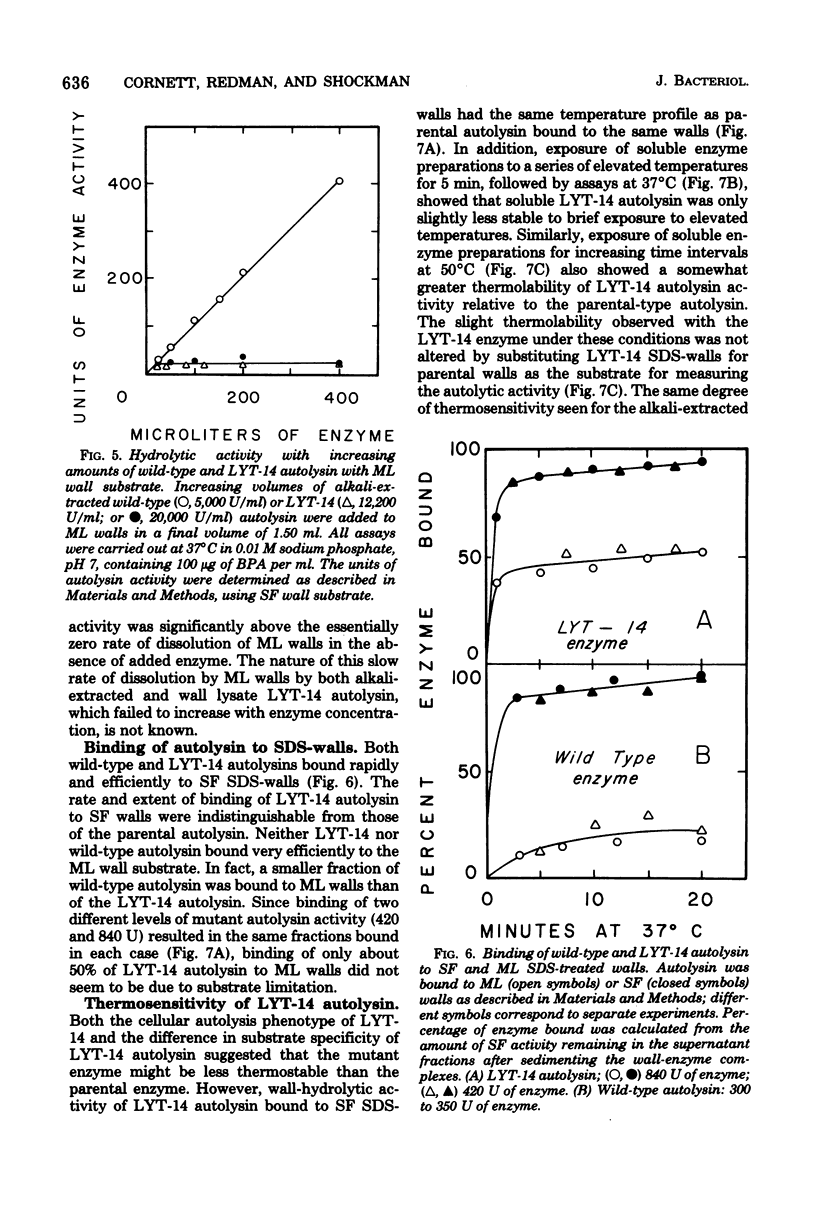
Properties of a variant of Streptococcus faecalis ATCC 9790 with defective cellular autolysis are described. The mutant strain was selected as a survivor from a mutagenized cell population simultaneously challenged with two antibiotics which inhibit cell wall biosynthesis, penicillin G and cycloserine. Compared to the parental strain, the mutant strain exhibited: (i) a thermosensitive pattern of cellular autolysis; (ii) an autolytic enzyme activity that had only a slightly increased thermolability when tested in solution in the absence of wall substrate; and (iii) an isolated autolysin that had hydrolytic activity on isolated S. faecalis wall substrate indistinguishable from that of the parental strain, but that was inactive when tested on walls of Micrococcus lysodeikticus as a substrate. These data indicate an alteration in the substrate specificity of the autolytic enzyme of the mutant which appears to result from the synthesis of an altered form of autolytic enzyme.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akrigg A., Ayad S. R. Studies on the competence-inducing factor of Bacillus subtilis. Biochem J. 1970 Apr;117(2):397–403. doi: 10.1042/bj1170397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayusawa D., Yoneda Y., Yamane K., Maruo B. Pleiotropic phenomena in autolytic enzyme(s) content, flagellation, and simultaneous hyperproduction of extracellular alpha-amylase and protease in a Bacillus subtilis mutant. J Bacteriol. 1975 Oct;124(1):459–469. doi: 10.1128/jb.124.1.459-469.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. C., Wilson C. R., Lukehart S., Young F. E., Shiflett M. A. Analysis of autolysins in temperature-sensitive morphological mutants of Bacillus subtilis. J Bacteriol. 1976 Jan;125(1):166–173. doi: 10.1128/jb.125.1.166-173.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. N., Wong W., Young F. E., Gilpin R. W. Isolation and characterization of a mutant of Staphylococcus aureus deficient in autolytic activity. J Bacteriol. 1976 Mar;125(3):961–967. doi: 10.1128/jb.125.3.961-967.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland R. F., Wicken A. J., Daneo-Moore L., Shockman G. D. Inhibition of wall autolysis in Streptococcus faecalis by lipoteichoic acid and lipids. J Bacteriol. 1976 Apr;126(1):192–197. doi: 10.1128/jb.126.1.192-197.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyette J., Shockman G. D. Some properties of the autolytic N-acetylmuramidase of Lactobacillus acidophilus. J Bacteriol. 1973 Apr;114(1):34–41. doi: 10.1128/jb.114.1.34-41.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P. Autolysin(s) of Bacillus subtilis as dechaining enzyme. J Bacteriol. 1970 Aug;103(2):494–499. doi: 10.1128/jb.103.2.494-499.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P., Beckman M. M., Cunningham W. P. Ultrastructural studies on a mutant of Bacillus subtilis whose growth is inhibited due to insufficient autolysin production. J Bacteriol. 1972 Mar;109(3):1247–1257. doi: 10.1128/jb.109.3.1247-1257.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P., Beckman M. M. Mutant of Bacillus subtilis demonstrating the requirement of lysis for growth. J Bacteriol. 1971 Feb;105(2):629–636. doi: 10.1128/jb.105.2.629-636.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P., Beckmann M. M. Mutant of Bacillus subtilis with a temperature-sensitive autolytic amidase. J Bacteriol. 1973 May;114(2):798–803. doi: 10.1128/jb.114.2.798-803.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein J. E., Rogers H. J. Autolytic enzyme-deficient mutants of Bacillus subtilis 168. J Bacteriol. 1976 Sep;127(3):1427–1442. doi: 10.1128/jb.127.3.1427-1442.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Rogers H. J. Characterization of Bacillus licheniformis 6346 mutants which have altered lytic enzyme activities. J Bacteriol. 1974 May;118(2):358–368. doi: 10.1128/jb.118.2.358-368.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghuysen J. M. Use of bacteriolytic enzymes in determination of wall structure and their role in cell metabolism. Bacteriol Rev. 1968 Dec;32(4 Pt 2):425–464. [PMC free article] [PubMed] [Google Scholar]
- Gilpin R. W., Chatterjee A. N., Young F. E. Autolysis of microbial cells: salt activation of autolytic enzymes in a mutant of Staphylococcus aureus. J Bacteriol. 1972 Jul;111(1):272–283. doi: 10.1128/jb.111.1.272-283.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerola N., Ingraham J. L., Cerdá-Olmedo E. Induction of closely linked multiple mutations by nitrosoguanidine. Nat New Biol. 1971 Mar 24;230(12):122–125. doi: 10.1038/newbio230122a0. [DOI] [PubMed] [Google Scholar]
- Joseph R., Shockman G. D. Autolytic formation of protoplasts (autoplasts) of Streptococcus faecalis; location of active and latent autolysin. J Bacteriol. 1976 Sep;127(3):1482–1493. doi: 10.1128/jb.127.3.1482-1493.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- Lindsay B., Glaser L. Characterization of the N-acetylmuramic acid L-alanine amidase from Bacillus subtilis. J Bacteriol. 1976 Aug;127(2):803–811. doi: 10.1128/jb.127.2.803-811.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck J., Chan L., Glaser L. Turnover of the cell wall of Gram-positive bacteria. J Biol Chem. 1971 Mar 25;246(6):1820–1827. [PubMed] [Google Scholar]
- Pooley H. M., Porres-Juan J. M., Shockman G. D. Dissociation of an autolytic enzyme-cell wall complex by treatment with unusually high concentrations of salt. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1134–1140. doi: 10.1016/0006-291x(70)90357-8. [DOI] [PubMed] [Google Scholar]
- Pooley H. M., Shockman G. D., Higgins M. L., Porres-Juan J. Some properties of two autolytic-defective mutants of Streptococcus faecalis ATCC 9790. J Bacteriol. 1972 Jan;109(1):423–431. doi: 10.1128/jb.109.1.423-431.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley H. M., Shockman G. D. Relationship between the latent form and the active form of the autolytic enzyme of Streptococcus faecalis. J Bacteriol. 1969 Nov;100(2):617–624. doi: 10.1128/jb.100.2.617-624.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranhand J. M., Leonard C. G., Cole R. M. Autolytic activity associated with competent group H streptococci. J Bacteriol. 1971 Apr;106(1):257–268. doi: 10.1128/jb.106.1.257-268.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J. Bacterial growth and the cell envelope. Bacteriol Rev. 1970 Jun;34(2):194–214. doi: 10.1128/br.34.2.194-214.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J., Forsberg C. W. Role of autolysins in the killing of bacteria by some bactericidal antibiotics. J Bacteriol. 1971 Dec;108(3):1235–1243. doi: 10.1128/jb.108.3.1235-1243.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J., Pooley H. M., Thurman P. F., Taylor C. Wall and membrane growth in bacilli and their mutants. Ann Microbiol (Paris) 1974 Sep;125 B(2):135–147. [PubMed] [Google Scholar]
- STRAUSS B. S. Response of Escherichia coli auxotrophs to heat after treatment with mutagenic alkyl methanesulfonates. J Bacteriol. 1962 Feb;83:241–249. doi: 10.1128/jb.83.2.241-249.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto H., Tomasz A. Protoplast formation and leakage of intramembrane cell components: induction by the competence activator substance of pneumococci. J Bacteriol. 1975 Jan;121(1):344–353. doi: 10.1128/jb.121.1.344-353.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Daneo-Moore L., Higgins M. L. Problems of cell wall and membrane growth, enlargement, and division. Ann N Y Acad Sci. 1974 May 10;235(0):161–197. doi: 10.1111/j.1749-6632.1974.tb43265.x. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Thompson J. S., Conover M. J. The autolytic enzyme system of Streptococcus faecalis. II. Partial characterization of the autolysin and its substrate. Biochemistry. 1967 Apr;6(4):1054–1065. doi: 10.1021/bi00856a014. [DOI] [PubMed] [Google Scholar]
- Soper J. W., Winter C. G. Role of cell wall antolysin in chain formation by a mutant strain of Streptococcus faecalis. Biochim Biophys Acta. 1973 Feb 28;297(2):333–342. doi: 10.1016/0304-4165(73)90080-9. [DOI] [PubMed] [Google Scholar]
- Thompson J. S., Shockman G. D. A modification of the Park and Johnson reducing sugar determination suitable for the assay of insoluble materials: its application to bacterial cell walls. Anal Biochem. 1968 Feb;22(2):260–268. doi: 10.1016/0003-2697(68)90315-1. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Albino A., Zanati E. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature. 1970 Jul 11;227(5254):138–140. doi: 10.1038/227138a0. [DOI] [PubMed] [Google Scholar]
- Tomasz A. Biological consequences of the replacement of choline by ethanolamine in the cell wall of Pneumococcus: chanin formation, loss of transformability, and loss of autolysis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):86–93. doi: 10.1073/pnas.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson W. D., Foster J. F. Conformation-dependent limited proteolysis of bovine plasma albumin by an enzyme present in commercial albumin preparations. Biochemistry. 1971 May 11;10(10):1772–1780. doi: 10.1021/bi00786a007. [DOI] [PubMed] [Google Scholar]
- Wong W., Young F. E., Chatterjee A. N. Regulation of bacterial cell walls: turnover of cell wall in Staphylococcus aureus. J Bacteriol. 1974 Nov;120(2):837–843. doi: 10.1128/jb.120.2.837-843.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



