Abstract
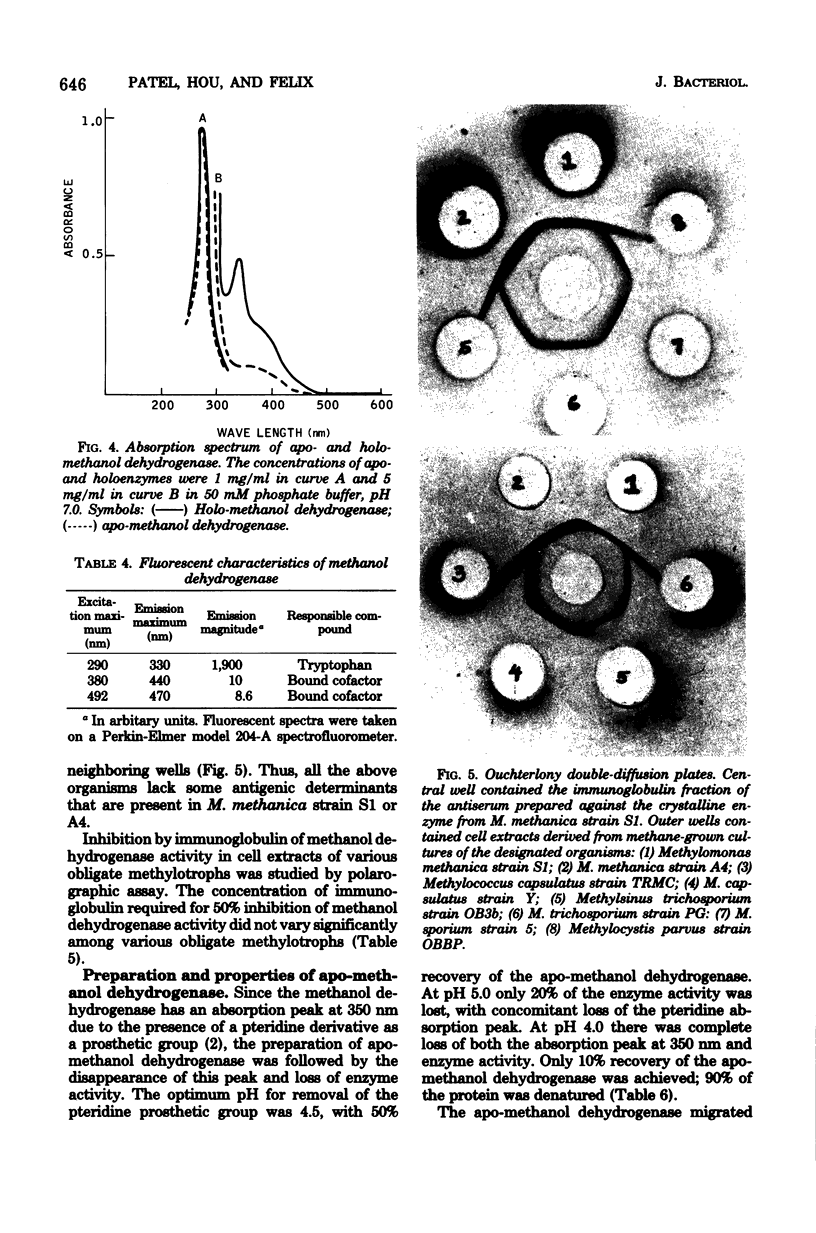
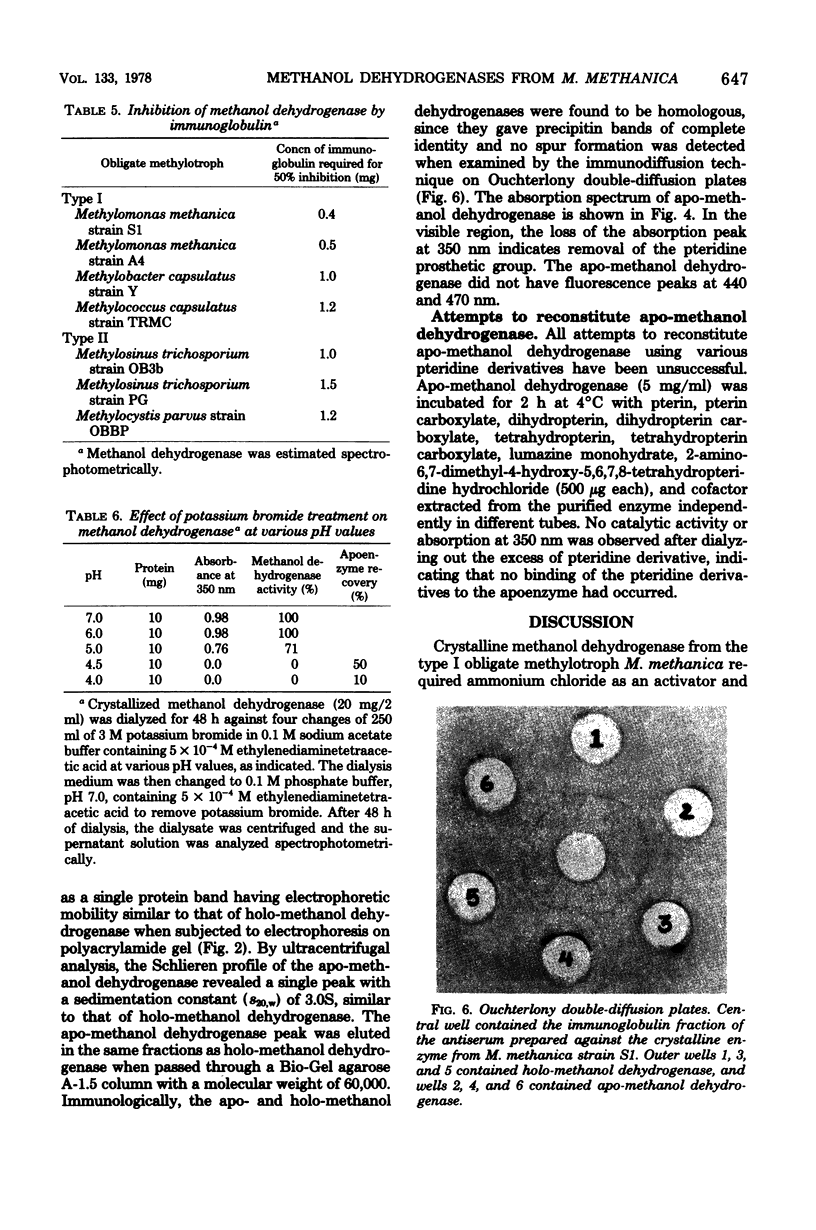
Procedures are described for the purification and crystallization of methanol dehydrogenase from the soluble fraction of the type I obligate methylotroph Methylomonas methanica strain S1. The crystallized enzyme is homogeneous as judged by acrylamide gel electrophoresis and ultracentrifugation. The enzyme had a high pH optimum (9.5) and required ammonium salt as an activator. In the presence of phenazine methosulfate as an electron acceptor, the enzyme catalyzed the oxidation of primary alcohols and formaldehyde. Secondary, tertiary, and aromatic alcohols were not oxidized. The molecular weight as well as subunit size of methanol dehydrogenase was 60,000, indicating that it is monomeric. The sedimentation constant (s20,w) was 3.1S. The amino acid composition of the crystallized enzyme is also presented. Antisera prepared against the crystalline enzyme were nonspecific; they cross-reacted with and inhibited the isofunctional enzyme from other obligate methylotrophic bacteria. The crystalline methanol dehydrogenase had an absorption peak at 350 nm in the visible region and weak fluorescence peaks at 440 and 470 nm due to the presence of a pteridine derivative as the prosthetic group. A procedure was developed for the preparation of apo-methanol dehydrogenase. The molecular weights, sedimentation constants, electrophoretic mobilities, and immunological properties of apo- and holo-methanol dehydrogenases are identical. Apo-methanol dehydrogenase lacked the absorption peak at 350 nm and the fluorescence peaks at 440 and 470 nm and was catalytically inactive. All attempts to reconstitute an active enzyme from apo-methanol dehydrogenase, using various pteridine derivatives, were unsuccessful.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony C., Zatman L. J. The microbial oxidation of methanol. Purification and properties of the alcohol dehydrogenase of Pseudomonas sp. M27. Biochem J. 1967 Sep;104(3):953–959. doi: 10.1042/bj1040953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony C., Zatman L. J. The microbial oxidation of methanol. The prosthetic group of the alcohol dehydrogenase of Pseudomonas sp. M27: a new oxidoreductase prosthetic group. Biochem J. 1967 Sep;104(3):960–969. doi: 10.1042/bj1040960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Zatman L. J. Hexose phosphate synthese and tricarboxylic acid-cycle enzymes in bacterium 4B6, an obligate methylotroph. Biochem J. 1972 Aug;128(5):1373–1376. doi: 10.1042/bj1281373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey J. F., Whittenbury R., Wilkinson J. F. The distribution in the methylobacteria of some key enzymes concerned with intermediary metabolism. Arch Mikrobiol. 1972;87(4):359–366. doi: 10.1007/BF00409135. [DOI] [PubMed] [Google Scholar]
- Ferenci T., Strom T., Quayle J. R. Oxidation of carbon monoxide and methane by Pseudomonas methanica. J Gen Microbiol. 1975 Nov;91(1):79–91. doi: 10.1099/00221287-91-1-79. [DOI] [PubMed] [Google Scholar]
- Foster J. W., Davis R. H. A methane-dependent coccus, with notes on classification and nomenclature of obligate, methane-utilizing bacteria. J Bacteriol. 1966 May;91(5):1924–1931. doi: 10.1128/jb.91.5.1924-1931.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman S. Metabolism of the phenylalanine hydroxylation cofactor. J Biol Chem. 1967 Sep 10;242(17):3934–3943. [PubMed] [Google Scholar]
- Kekwick R. A. The serum proteins in multiple myelomatosis. Biochem J. 1940 Sep;34(8-9):1248–1257. doi: 10.1042/bj0341248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Meagher R. B., Ornston L. N. Relationships among enzymes of the beta-ketoadipate pathway. I. Properties of cis,cis-muconate-lactonizing enzyme and muconolactone isomerase from Pseudomonas putida. Biochemistry. 1973 Aug 28;12(18):3523–3530. doi: 10.1021/bi00742a027. [DOI] [PubMed] [Google Scholar]
- Patel R. N., Bose H. R., Mandy W. J., Hoare D. S. Physiological studies of methane- and methanol-oxidizing bacteria: comparison of a primary alcohol dehydrogenase from Methylococcus capsulatus (Texas strain) and Pseudomonas species M27. J Bacteriol. 1972 May;110(2):570–577. doi: 10.1128/jb.110.2.570-577.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. N., Felix A. Microbial oxidation of methane and methanol: crystallization and properties of methanol dehydrogenase from Methylosinus sporium. J Bacteriol. 1976 Oct;128(1):413–424. doi: 10.1128/jb.128.1.413-424.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. N., Mandy W. J., Hoare D. S. Physiological studies of methane- and methanol-oxidizing bacteria: immunological comparison of a primary alcohol dehydrogenase from Methylococcus capsulatus and Pseudomonas sp. M27. J Bacteriol. 1973 Feb;113(2):937–945. doi: 10.1128/jb.113.2.937-945.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R., Hoare L., Hoare D. S., Taylor B. F. Incomplete tricarboxylic acid cycle in a type I methylotroph, Methylococcus capsulatus. J Bacteriol. 1975 Jul;123(1):382–384. doi: 10.1128/jb.123.1.382-384.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patt T. E., Cole G. C., Bland J., Hanson R. S. Isolation and characterization of bacteria that grow on methane and organic compounds as sole sources of carbon and energy. J Bacteriol. 1974 Nov;120(2):955–964. doi: 10.1128/jb.120.2.955-964.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAYMOND S. A convenient apparatus for vertical gel electrophoresis. Clin Chem. 1962 Sep-Oct;8:455–470. [PubMed] [Google Scholar]
- Ribbons D. W., Harrison J. E., Wadzinski A. M. Metabolism of single carbon compounds. Annu Rev Microbiol. 1970;24:135–158. doi: 10.1146/annurev.mi.24.100170.001031. [DOI] [PubMed] [Google Scholar]
- Spencer R. L., Wold F. A new convenient method for estimation of total cystine-cysteine in proteins. Anal Biochem. 1969 Oct 15;32(1):185–190. doi: 10.1016/0003-2697(69)90123-7. [DOI] [PubMed] [Google Scholar]
- Sperl G. T., Forrest H. S., Gibson D. T. Substrate specificity of the purified primary alcohol dehydrogenases from methanol-oxidizing bacteria. J Bacteriol. 1974 May;118(2):541–550. doi: 10.1128/jb.118.2.541-550.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Wachter D., Gasser C., Wilson A. C. Comparative immunological studies of two Pseudomonas enzymes. J Bacteriol. 1970 May;102(2):351–362. doi: 10.1128/jb.102.2.351-362.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushibara T., Forrest H. S., Hoare D. S., Patel R. N. Pteridines produced by Methylococcus capsulatus. Isolation and identification of a neopterin 2':3'-phosphate. Biochem J. 1971 Nov;125(1):141–146. doi: 10.1042/bj1250141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadzinski A. M., Ribbons D. W. Oxidation of C1 compounds by particulate fractions from Methylococcus capsulatus: properties of methanol oxidase and methanol dehydrogenase. J Bacteriol. 1975 Jun;122(3):1364–1374. doi: 10.1128/jb.122.3.1364-1374.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Whittenbury R., Phillips K. C., Wilkinson J. F. Enrichment, isolation and some properties of methane-utilizing bacteria. J Gen Microbiol. 1970 May;61(2):205–218. doi: 10.1099/00221287-61-2-205. [DOI] [PubMed] [Google Scholar]