Abstract
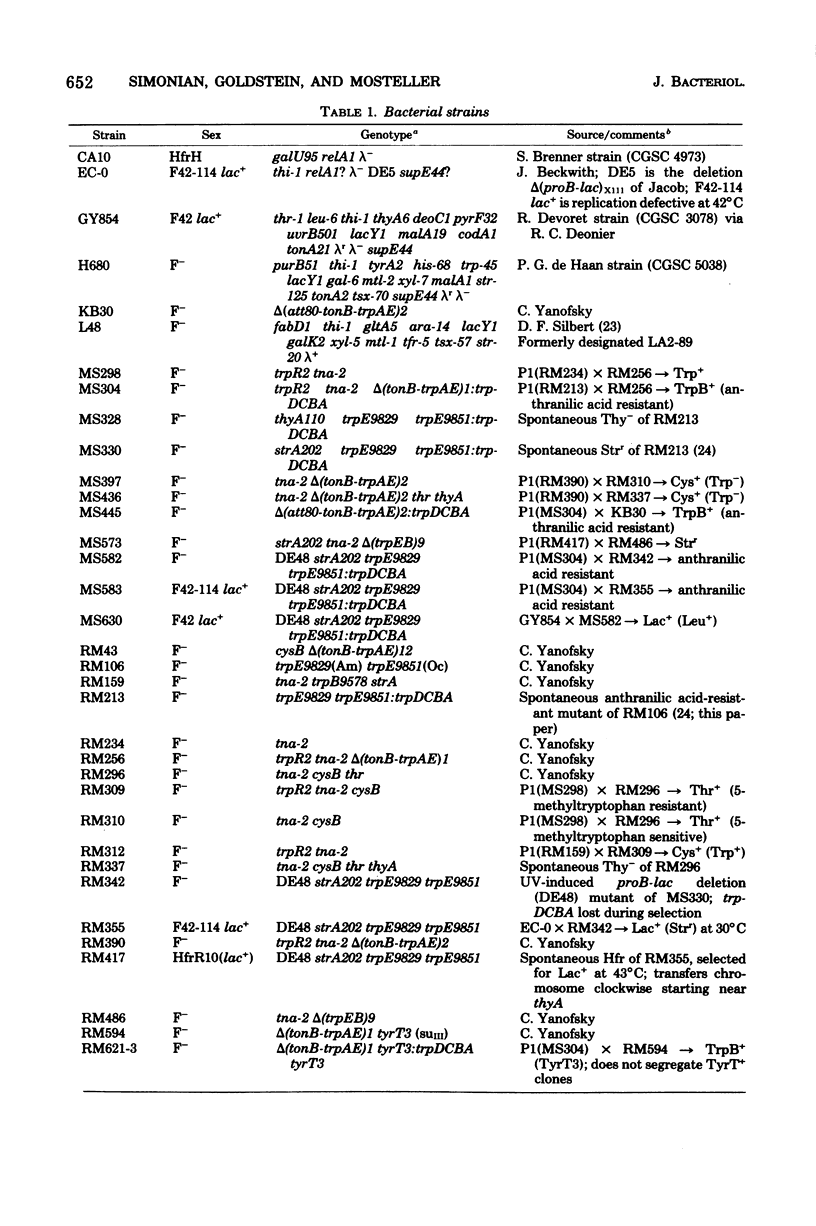
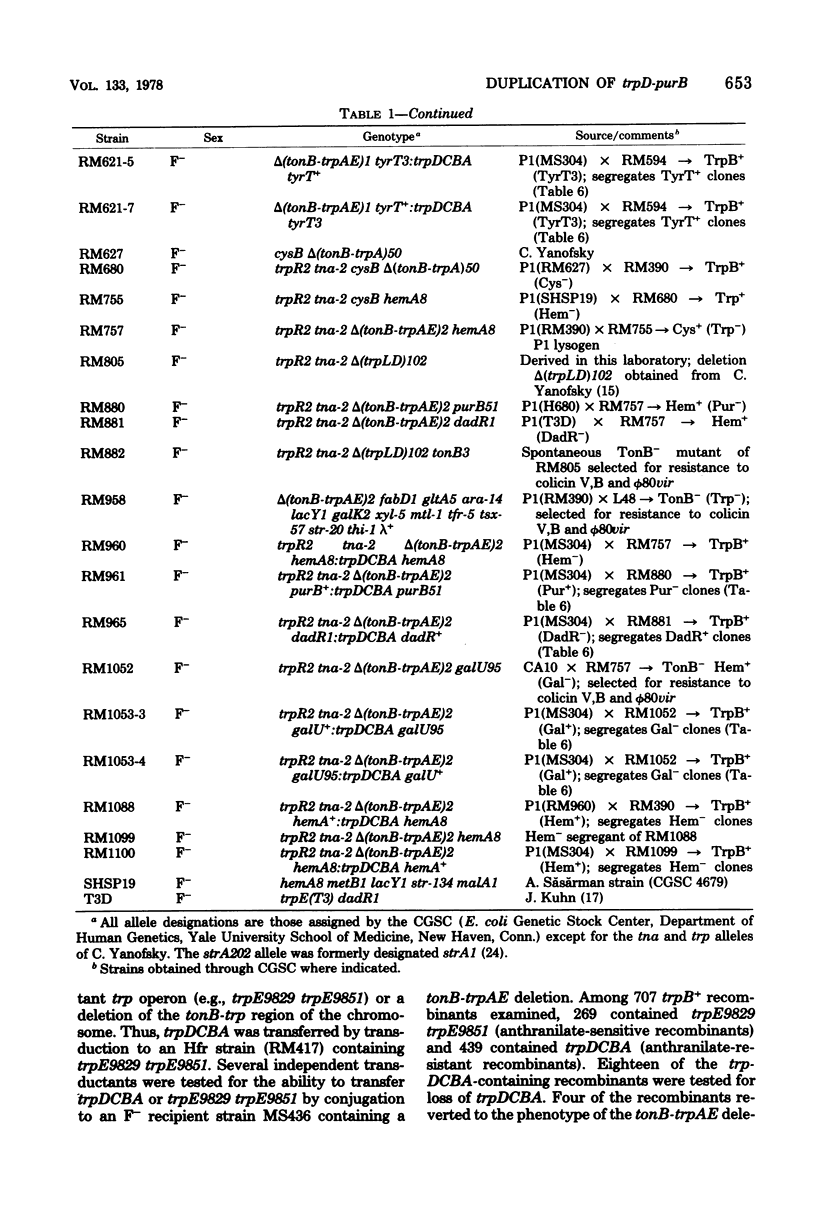
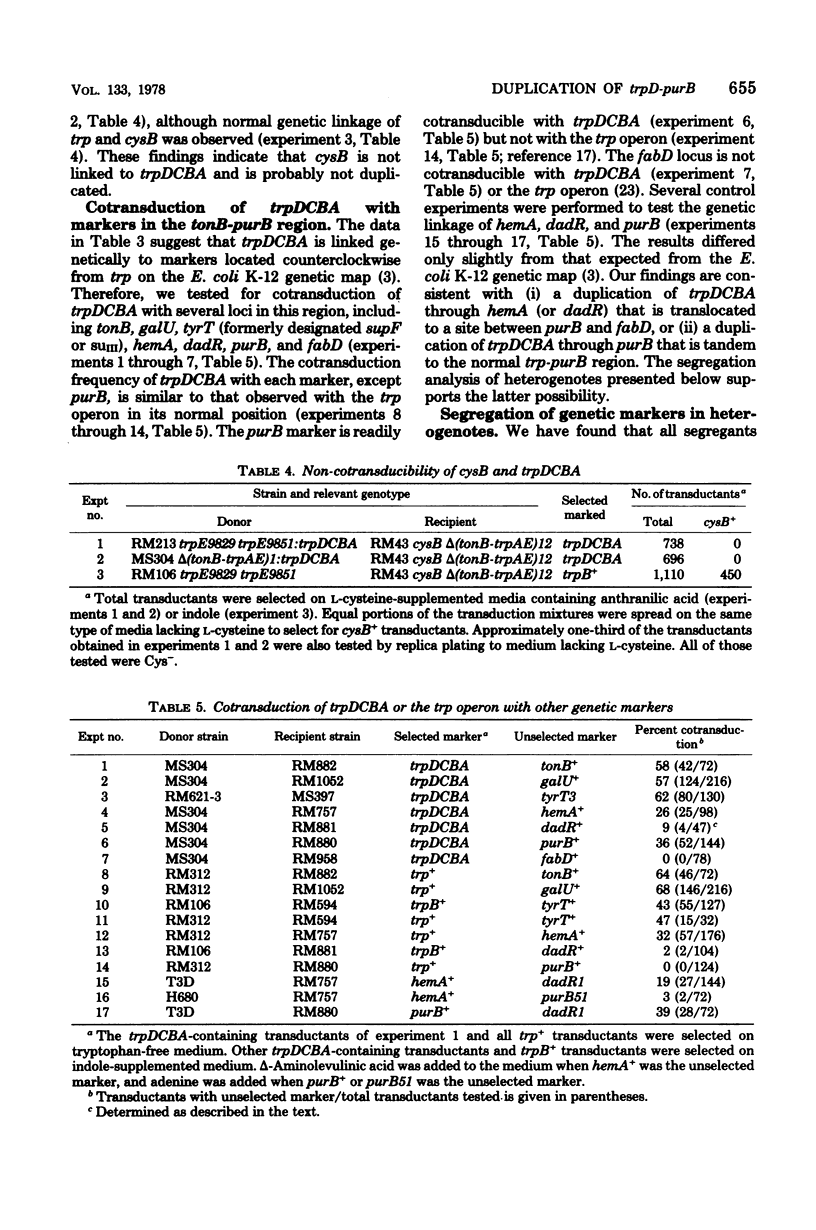
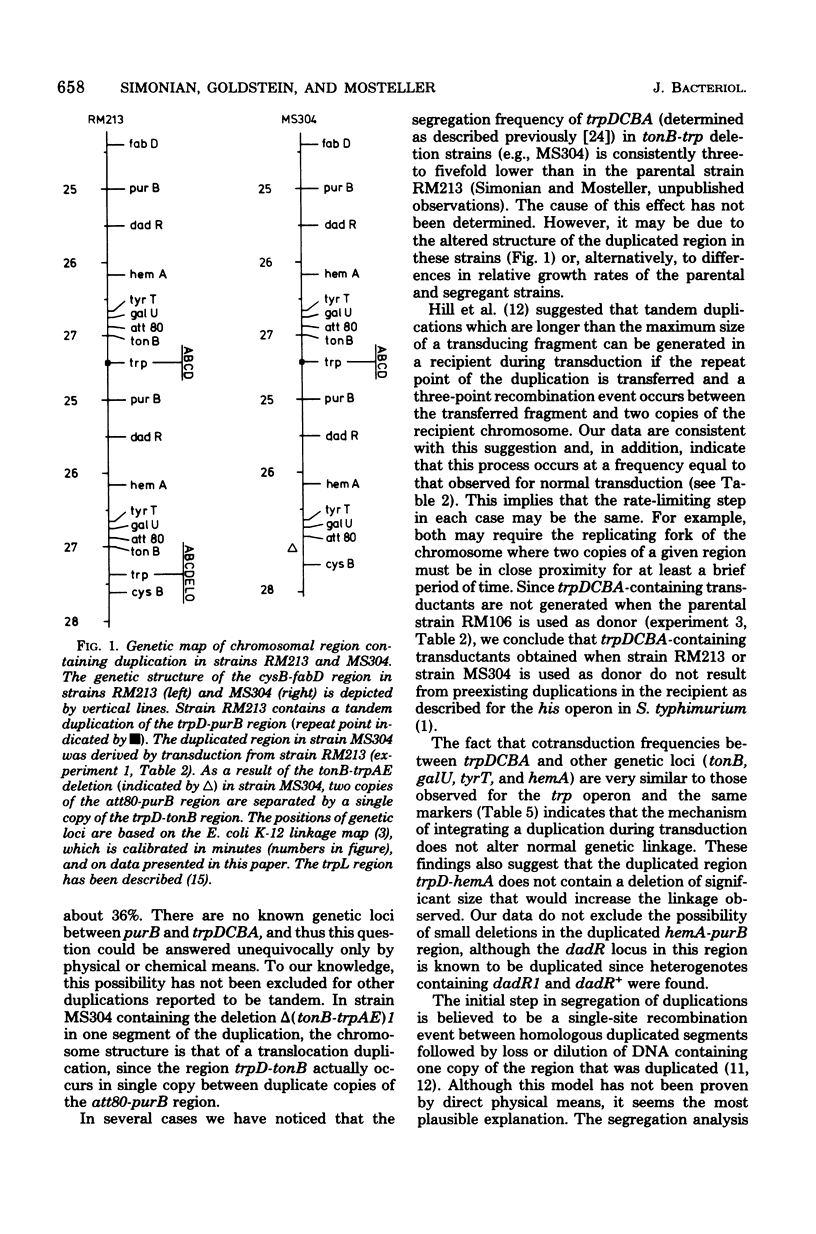
Genetic and segregation analysis of Escherichia coli strains containing a partial duplication of the trp operon reveal that the 2.5-min-long region trpD-purB is duplicated in tandem in the chromosome. The adjacent loci cysB and fabD are not duplicated. Although one copy of the duplicated region is longer than the maximum size of bacteriophage P1kc transducing fragments, the frequency at which the duplicated segment trpDCBA is transferred by transduction to tonB-trp deletion strains is equal to that observed for transfer of the normal trp operon. This suggests that three-point recombination events believed to account for transduction of long duplications occur as frequently as two-point recombination events believed to account for normal transduction. Cotransduction frequencies of trpDCBA with the duplicated loci tonB, galU, tyrT, and hemA are very similar to those for the trp operon with the same loci. This indicates that normal genetic linkage is maintained during the three-point recombination event. However, purB, which is normally unlinked to trp by transduction, is closely linked to trpDCBA and thus must be near the repeat point of the duplication. Transduction tests with point mutations in the trp operon indicated that the repeat point occurs near the normal boundary between trpE and trpD. Segregation analysis of heterogenotes constructed from tonB-trp deletion strains shows that the frequency at which a marker is lost is approximately proportional to its distance from the repeat point. This finding is consistent with a random, singlesite crossover event during segregation. Several observations indicate that non-reciprocal genetic exchange also occurs between copies of the duplication. Analysis of heterogenotes containing dadR1 and dadR+ demonstrate that the mutant allele is transdominant.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. P., Miller C. G., Roth J. R. Tandem duplications of the histidine operon observed following generalized transduction in Salmonella typhimurium. J Mol Biol. 1976 Aug 5;105(2):201–218. doi: 10.1016/0022-2836(76)90107-8. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeftinck F., Cunin R., Glansdorff N. Arginine gene duplications in recombination proficient and deficient strains of Escherichia coli K 12. Mol Gen Genet. 1974;132(3):241–253. doi: 10.1007/BF00269397. [DOI] [PubMed] [Google Scholar]
- Coukell M. B., Yanofsky C. Increased frequency of deletions in DNA polymerase mutants of Escherichia coli. Nature. 1970 Nov 14;228(5272):633–635. doi: 10.1038/228633a0. [DOI] [PubMed] [Google Scholar]
- Cunin R., Elseviers D., Glansdorff N. De novo gene duplication versus reactivation of cryptic genes in Escherichia coli K-12. Mol Gen Genet. 1970;108(2):154–157. doi: 10.1007/BF02430521. [DOI] [PubMed] [Google Scholar]
- Folk W. R., Berg P. Duplication of the structural gene for glycyl-transfer RNA synthetase in Escherichia coli. J Mol Biol. 1971 Jun 14;58(2):595–610. doi: 10.1016/0022-2836(71)90374-3. [DOI] [PubMed] [Google Scholar]
- Glansdorff N., Sand G. Duplication of a gene belonging to an arginine operon of Escherichia coli K-12. Genetics. 1968 Oct;60(2):257–268. doi: 10.1093/genetics/60.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERSHEY A. D. An upper limit to the protein content of the germinal substance of bacteriophage T2. Virology. 1955 May;1(1):108–127. doi: 10.1016/0042-6822(55)90009-x. [DOI] [PubMed] [Google Scholar]
- HORIUCHI T., HORIUCHI S., NOVICK A. The genetic basis of hyper-synthesis of beta-galactosidase. Genetics. 1963 Feb;48:157–169. doi: 10.1093/genetics/48.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. W., Combriato G. Genetic duplications induced at very high frequency by ultraviolet irradiation in Escherichia coli. Mol Gen Genet. 1973 Dec 31;127(3):197–214. doi: 10.1007/BF00333760. [DOI] [PubMed] [Google Scholar]
- Hill C. W., Foulds J., Soll L., Berg P. Instability of a missense suppressor resulting from a duplication of genetic material. J Mol Biol. 1969 Feb 14;39(3):563–581. doi: 10.1016/0022-2836(69)90146-6. [DOI] [PubMed] [Google Scholar]
- Hill C. W., Schiffer D., Berg P. Transduction of merodiploidy: induced duplication of recipient genes. J Bacteriol. 1969 Jul;99(1):274–278. doi: 10.1128/jb.99.1.274-278.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson E. N., Yanofsky C. Duplication-translocations of tryptophan operon genes in Escherichia coli. J Bacteriol. 1973 Oct;116(1):33–40. doi: 10.1128/jb.116.1.33-40.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson E. N., Yanofsky C. Localization of two functions of the phosphoribosyl anthranilate transferase of Escherichia coli to distinct regions of the polypeptide chain. J Bacteriol. 1974 Feb;117(2):502–508. doi: 10.1128/jb.117.2.502-508.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson E. N., Yanofsky C. Thr region between the operator and first structural gene of the tryptophan operon of Escherichia coli may have a regulatory function. J Mol Biol. 1973 May 5;76(1):89–101. doi: 10.1016/0022-2836(73)90082-x. [DOI] [PubMed] [Google Scholar]
- Kuhn J., Somerville R. L. Mutant strains of Escherichia coli K12 that use D-amino acids. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2484–2487. doi: 10.1073/pnas.68.10.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Levinthal M., Yeh J. The pi-histidine factor of Salmonella typhimurium: a demonstration that pi-histidine factor integrates into the chromosome. J Bacteriol. 1972 Mar;109(3):993–1000. doi: 10.1128/jb.109.3.993-1000.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. G., Roth J. R. Recessive-lethal nonsense suppressors in Salmonella typhimurium. J Mol Biol. 1971 Jul 14;59(1):63–75. doi: 10.1016/0022-2836(71)90413-x. [DOI] [PubMed] [Google Scholar]
- Russell R. L., Abelson J. N., Landy A., Gefter M. L., Brenner S., Smith J. D. Duplicate genes for tyrosine transfer RNA in Escherichia coli. J Mol Biol. 1970 Jan 14;47(1):1–13. doi: 10.1016/0022-2836(70)90397-9. [DOI] [PubMed] [Google Scholar]
- Semple K. S., Silbert D. F. Mapping of the fabD locus for fatty acid biosynthesis in Escherichia coli. J Bacteriol. 1975 Mar;121(3):1036–1046. doi: 10.1128/jb.121.3.1036-1046.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonian M. H., Mosteller R. D. Increased loss of duplicated genes in streptomycin-resistant (strA) mutants of Escherichia coli k-12. J Bacteriol. 1976 Jan;125(1):382–384. doi: 10.1128/jb.125.1.382-384.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stodolsky M. Recipient gene duplication during generalized transduction. Genetics. 1974 Nov;78(3):809–822. doi: 10.1093/genetics/78.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus D. S., D'Ari Straus L. Large overlapping tandem genetic duplications in Salmonella typhimurium. J Mol Biol. 1976 May 5;103(1):143–153. doi: 10.1016/0022-2836(76)90056-5. [DOI] [PubMed] [Google Scholar]
- Straus D. S. Induction by mutagens of tandem gene duplications in the glyS region of the Escherichia coli chromosome. Genetics. 1974 Nov;78(3):823–830. doi: 10.1093/genetics/78.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus D. S. Selection for a large genetic duplication in Salmonella typhimurium. Genetics. 1975 Jun;80(2):227–237. doi: 10.1093/genetics/80.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wang C. C., Newton A. Iron transport in Escherichia coli: relationship between chromium sensitivity and high iron requirement in mutants of Escherichia coli. J Bacteriol. 1969 Jun;98(3):1135–1141. doi: 10.1128/jb.98.3.1135-1141.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C., Horn V., Bonner M., Stasiowski S. Polarity and enzyme functions in mutants of the first three genes of the tryptophan operon of Escherichia coli. Genetics. 1971 Dec;69(4):409–433. doi: 10.1093/genetics/69.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]



