Abstract
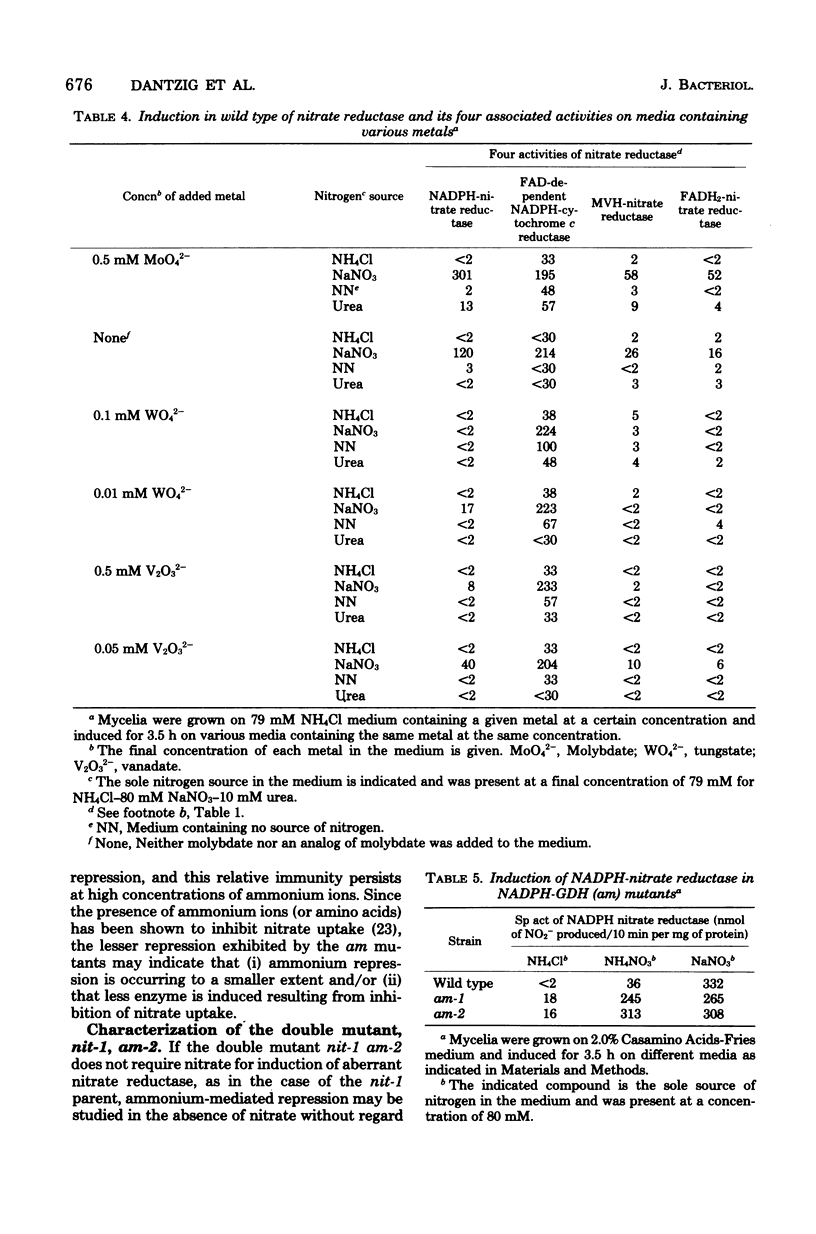
Synthesis of wild-type Neurospora crassa assimilatory nitrate reductase is induced in the presence of nitrate ions and repressed in the presence of ammonium ions. Effects of several Neurospora mutations on the regulation of this enzyme are shown: (i) the mutants, nit-1 and nit-3, involving separate lesions, lack reduced nicotinamide adenine dinucleotide (NADPH)-nitrate reductase activity and at least one of three other activities associated with the wild-type enzyme. The two mutants do not require the presence of nitrate for induction of their aberrant nitrate reductases and are constitutive for their component nitrate reductase activities in the absence of ammonium ions. (ii) An analog of the wild-type enzyme (similar to the nit-1 enzyme) is formed when wild type is grown in a medium in which molybdenum has been replaced by vanadium or tungsten; the resulting enzyme lacks NADPH-nitrate reductase activity. Unlike nit-1, wild type produced this analog only in the presence of nitrate. Contaminating nitrate does not appear to be responsible for the observed mutants' activities. Nitrate reductase is proposed to be autoregulated. (iii) Mutants (am) lacking NADPH-dependent glutamate dehydrogenase activity partially escape ammonium repression of nitrate reductase. The presence of nitrate is required for the enzyme's induction. (iv) A double mutant, nit-1 am-2, proved to be an ideal test system to study the repressive effects of nitrogen-containing metabolites on the induction of nitrate reductase activity. The double mutant does not require nitrate for induction of nitrate reductase, and synthesis of the enzyme is not repressed by the presence of high concentrations of ammonium ions. It is, however, repressed by the presence of any one of six amino acids. Nitrogen metabolites (other than ammonium) appear to be responsible for the mediation of “ammonium repression.”
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoine A. D. Purification and properties of the nitrate reductase isolated from Neurospora crassa mutant nit-3. Kinetics, molecular weight determination, and cytochrome involvement. Biochemistry. 1974 May 21;13(11):2289–2294. doi: 10.1021/bi00708a008. [DOI] [PubMed] [Google Scholar]
- Arst H. N., Jr, MacDonald D. W. A mutant of Asperigillus nidulans lacking NADP-linked glutamate dehydrogenase. Mol Gen Genet. 1973 May 9;122(3):261–265. doi: 10.1007/BF00278601. [DOI] [PubMed] [Google Scholar]
- Chang H. C., Sorger G. J. Effect of ammonium ions on the induction of nitrite reductase in Neurospora crassa. J Bacteriol. 1976 May;126(2):1002–1004. doi: 10.1128/jb.126.2.1002-1004.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cove D. J., Pateman J. A. Autoregulation of the synthesis of nitrate reductase in Aspergillus nidulans. J Bacteriol. 1969 Mar;97(3):1374–1378. doi: 10.1128/jb.97.3.1374-1378.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett R. H., Nason A. Further purification and properties of Neurospora nitrate reductase. J Biol Chem. 1969 Jun 10;244(11):2870–2882. [PubMed] [Google Scholar]
- Garrett R. H., Nason A. Involvement of a B-type cytochrome in the assimilatory nitrate reductase of Neurospora crassa. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1603–1610. doi: 10.1073/pnas.58.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenson M., Hou C. Ammonia inhibition of the general amino acid permease and its suppression in NADPH-specific glutamate dehydrogenaseless mutants of saccharomyces cerevisiae. Biochem Biophys Res Commun. 1972 Aug 21;48(4):749–756. doi: 10.1016/0006-291x(72)90670-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee K. Y., Erickson R., Pan S. S., Jones G., May F., Nason A. Effect of tungsten and vanadium on the in vitro assembly of assimilatory nitrate reductase utilizing Neurospora mutant nit-1. J Biol Chem. 1974 Jun 25;249(12):3953–3959. [PubMed] [Google Scholar]
- NASON A., EVANS H. J. Triphosphopyridine nucleotide-nitrate reductase in Neurospora. J Biol Chem. 1953 Jun;202(2):655–673. [PubMed] [Google Scholar]
- Nason A., Antoine A. D., Ketchum P. A., Frazier W. A., 3rd, Lee D. K. Formation of assimilatory nitrate reductase by in vitro inter-cistronic complementation in Neurospora crassa. Proc Natl Acad Sci U S A. 1970 Jan;65(1):137–144. doi: 10.1073/pnas.65.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nason A., Lee K. Y., Pan S. S., Ketchum P. A., Lamberti A., DeVries J. Invitro formation of assimilatory reduced nicotinamide adenine dinucleotide phosphate: nitrate reductase from a Neurospora mutant and a component of molybdenum-enzymes. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3242–3246. doi: 10.1073/pnas.68.12.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pateman J. A., Cove D. J. Regulation of nitrate reduction in Aspergillus nidulans. Nature. 1967 Sep 16;215(5107):1234–1237. doi: 10.1038/2151234a0. [DOI] [PubMed] [Google Scholar]
- Pateman J. A., Rever B. M., Cove D. J. Genetic and biochemical studies of nitrate reduction in Aspergillus nidulans. Biochem J. 1967 Jul;104(1):103–111. doi: 10.1042/bj1040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne W. J. Reduction of nitrogenous oxides by microorganisms. Bacteriol Rev. 1973 Dec;37(4):409–452. doi: 10.1128/br.37.4.409-452.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert W. R., Marzluf G. A. Genetic and metabolic control of the purine catabolic enzymes of Neurospora crasse. Mol Gen Genet. 1975 Aug 5;139(1):39–55. doi: 10.1007/BF00267994. [DOI] [PubMed] [Google Scholar]
- SANWAL B. D., LATA M. The regulation of glutamic dehydrogenases and an antigenically related protein in amination deficient mutants of Neurospora. Arch Biochem Biophys. 1962 Sep;98:420–426. doi: 10.1016/0003-9861(62)90207-2. [DOI] [PubMed] [Google Scholar]
- Schloemen R. H., Garrett R. H. Nitrate transport system in Neurospora crassa. J Bacteriol. 1974 Apr;118(1):259–269. doi: 10.1128/jb.118.1.259-269.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger G. J., Giles N. H. Genetic control of nitrate reductase in Neurospora crassa. Genetics. 1965 Oct;52(4):777–788. doi: 10.1093/genetics/52.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootton J. C., Chambers G. K., Taylor J. G., Fincham J. R. Amino-acid sequence homologies between the NADP-dependent glutamate dehydrogenase of Neurospora and the bovine enzyme. Nat New Biol. 1973 Jan 10;241(106):42–43. doi: 10.1038/newbio241042a0. [DOI] [PubMed] [Google Scholar]


