Abstract
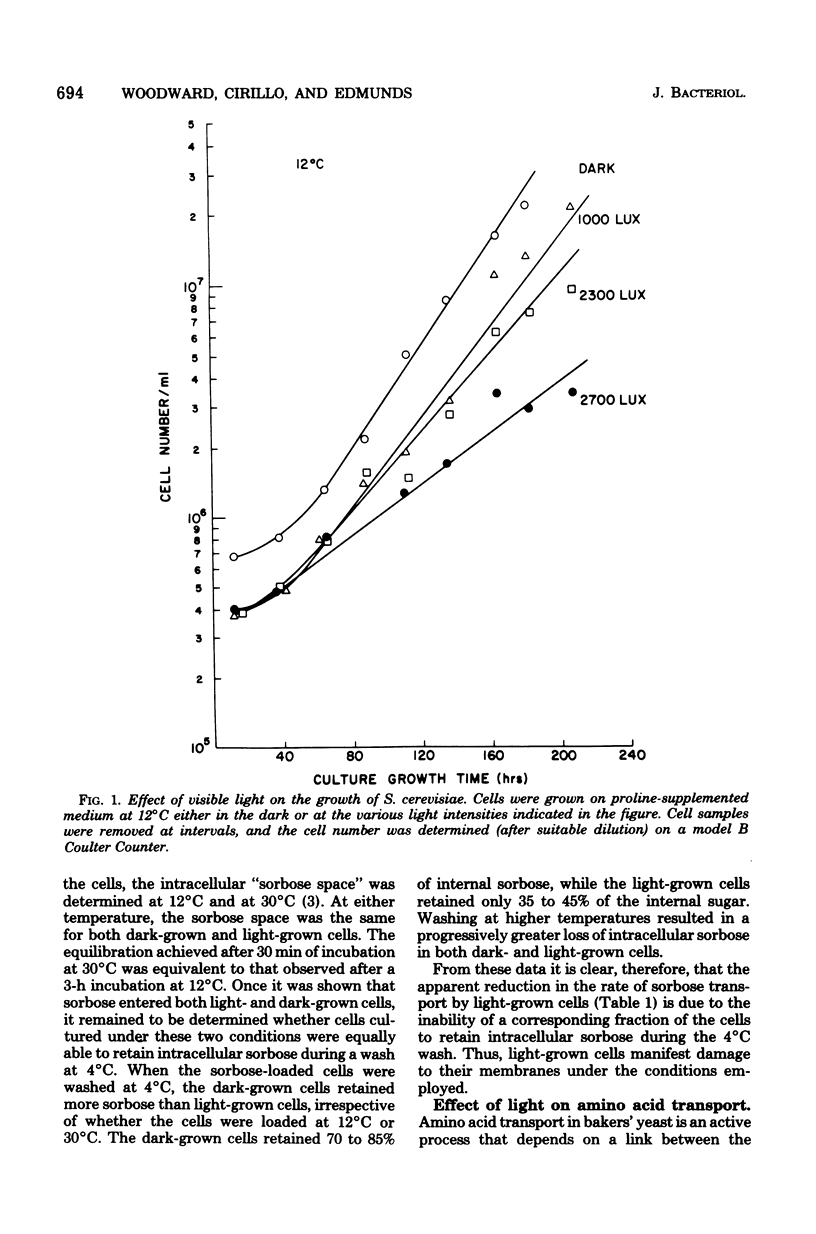
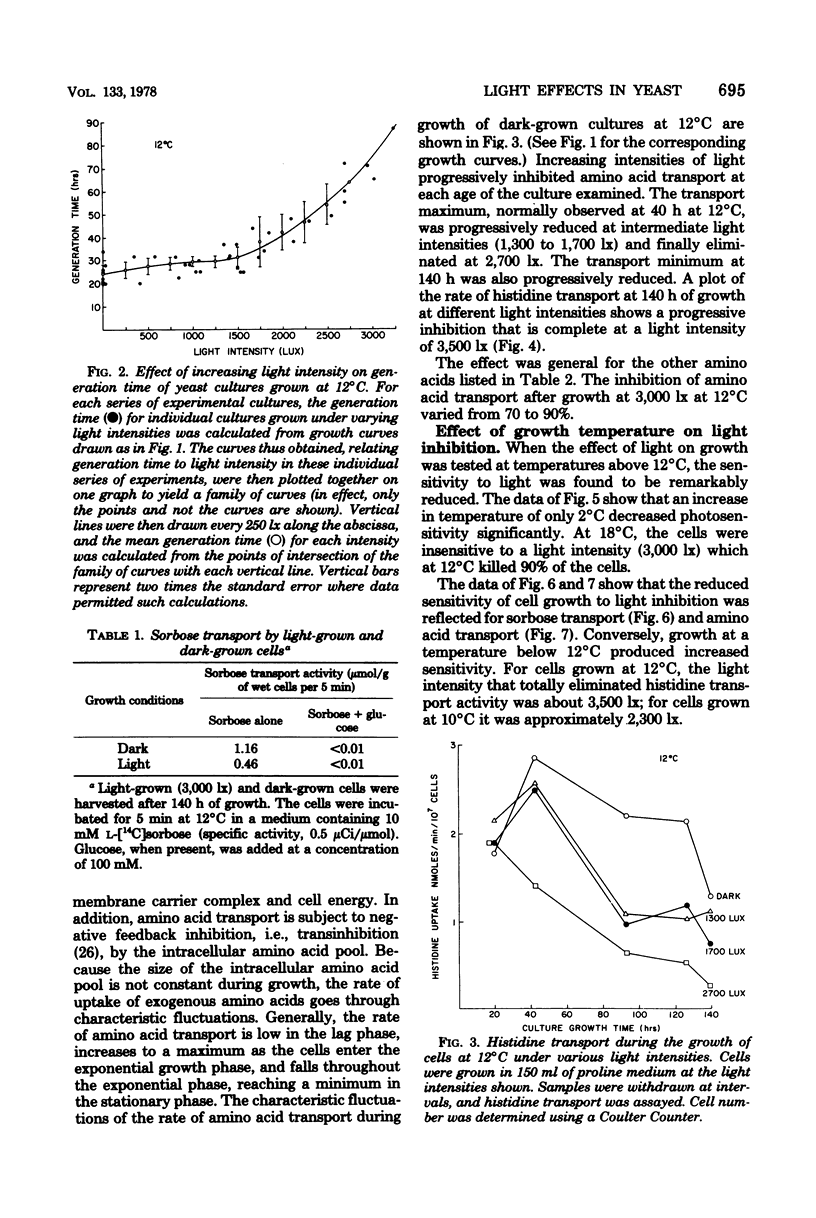
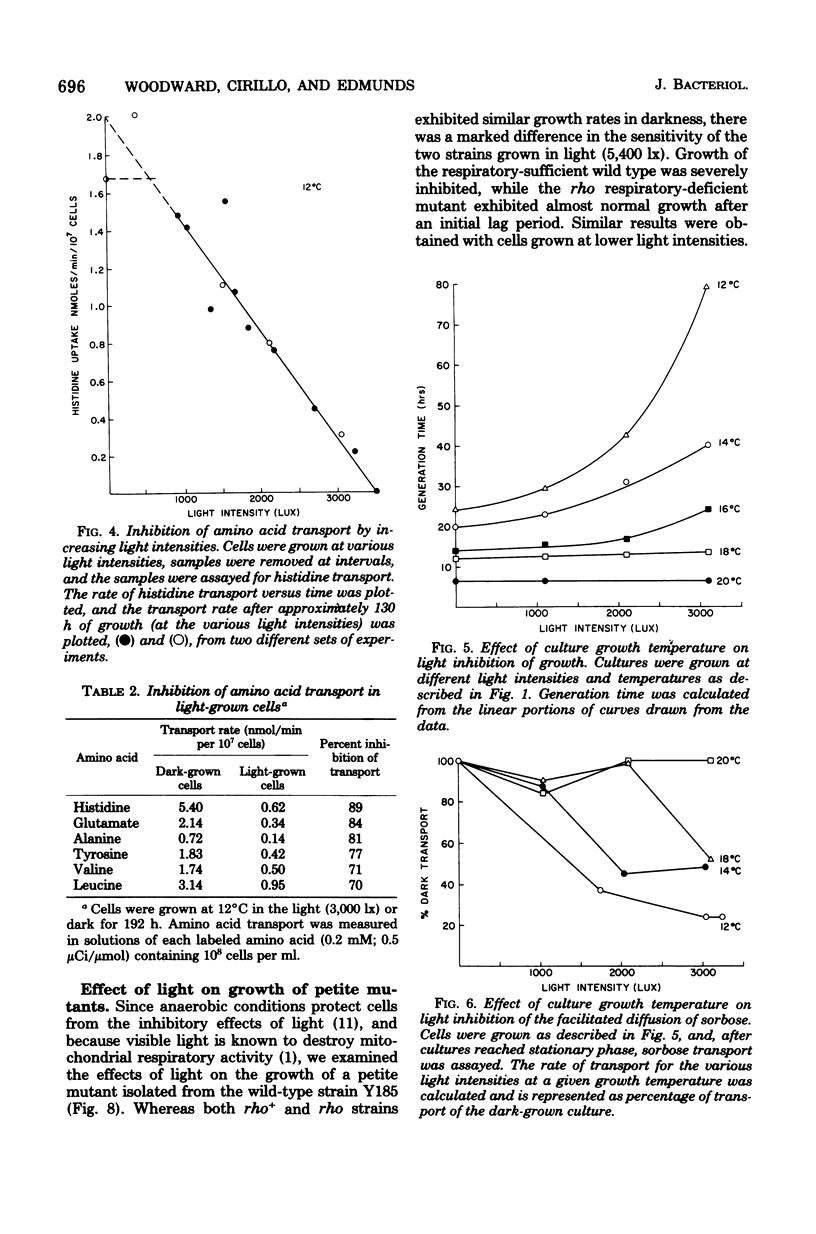
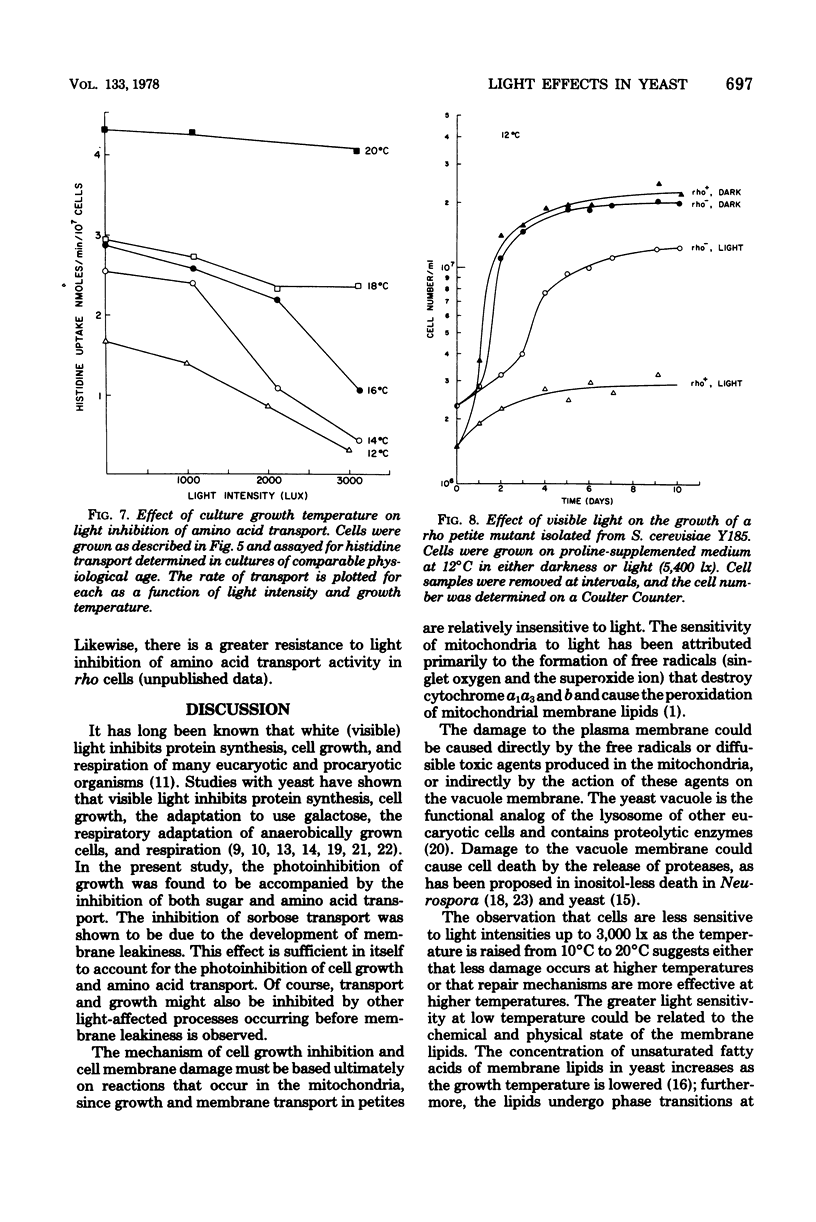
Growth rate, sugar transport, and amino acid transport of yeast cells grown at 12 degrees C were inhibited by cool-white fluorescent light. At light intensities below 1,250 lx, growth and membrane transport were only slightly inhibited. Above 1,250 lx, there was increasing inhibition of both processes. Transport of histidine was completely inhibited after 3 to 5 days in cultures grown at 12 degrees C under 3,500-lx illumination. Cells grown at 20 degrees C were not inhibited by light intensities that caused complete loss of viability and membrane transport activity in cells grown at 12 degrees C.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal B. B., Avi-Dor Y., Tinberg H. M., Packer L. Effect of visible light on the mitochondrial inner membrane. Biochem Biophys Res Commun. 1976 Mar 22;69(2):362–368. doi: 10.1016/0006-291x(76)90530-1. [DOI] [PubMed] [Google Scholar]
- Barran L. R., Daoust J. Y., Labelle J. L., Martin W. G., Schneider H. Differential effects of visible light on active transport in E. coli. Biochem Biophys Res Commun. 1974 Jan 23;56(2):522–528. doi: 10.1016/0006-291x(74)90874-2. [DOI] [PubMed] [Google Scholar]
- CIRILLO V. P., HARSCH M., LAMPEN J. O. ACTION OF THE POLYENE ANTIBIOTICS FILIPIN, NYSTATIN AND N-ACETYLCANDIDIN ON THE YEAST CELL MEMBRANE. J Gen Microbiol. 1964 May;35:249–259. doi: 10.1099/00221287-35-2-249. [DOI] [PubMed] [Google Scholar]
- CIRILLO V. P. Mechanism of glucose transport across the yeast cell membrane. J Bacteriol. 1962 Sep;84:485–491. doi: 10.1128/jb.84.3.485-491.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo V. P. Relationship between sugar structure and competition for the sugar transport system in Bakers' yeast. J Bacteriol. 1968 Feb;95(2):603–611. doi: 10.1128/jb.95.2.603-611.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmunds L. N., Jr, Cirillo V. P. On the interplay among cell cycle, biological clock and membrane transport control systems. Int J Chronobiol. 1974;2(3):233–246. [PubMed] [Google Scholar]
- Edmunds L. N., Jr Phasing effects of light on cell division in exponentially increasing cultures of Tetrahymena grown at low temperatures. Exp Cell Res. 1974 Feb;83(2):367–379. doi: 10.1016/0014-4827(74)90351-6. [DOI] [PubMed] [Google Scholar]
- Ehrenberg M. Wirjungen sichtbaren Lichtes auf Saccharomyces cerevisiae. II. Die Gärung unter den Einfluss von Pasteur-Effekt und Licht-Effekt. Arch Mikrobiol. 1966 Oct 19;55(1):26–30. [PubMed] [Google Scholar]
- Epel B. L. Inhibition of growth and respiration by visible and near-visible light. Photophysiology. 1973;0(0):209–229. doi: 10.1016/b978-0-12-282608-5.50013-8. [DOI] [PubMed] [Google Scholar]
- Epel B., Krauss R. W. The inhibitory effect of light on growth of Prototheca zopfii Kruger. Biochim Biophys Acta. 1966 May 12;120(1):73–83. doi: 10.1016/0926-6585(66)90278-0. [DOI] [PubMed] [Google Scholar]
- Guerin B., Jacques R. Photoinhibition de l'adaptation respiratoire chez Saccharomyces cerevisiae. II. Le spectre d'action. Biochim Biophys Acta. 1968 Jan 15;153(1):138–142. doi: 10.1016/0005-2728(68)90154-0. [DOI] [PubMed] [Google Scholar]
- Guerin B., Sulkowski E. Photoinhibition de l'adaptation respiratoire chez Saccharomyces cerevisiae. I. Variations de la sensibilite à l'inhibition. Biochim Biophys Acta. 1966 Oct 24;129(1):193–200. [PubMed] [Google Scholar]
- Hayashi E., Hasegawa R., Tomita T. Accumulation of neutral lipids in Saccharomyces carlsbergensis by myo-inositol deficiency and its mechanism. Reciprocal regulation of yeast acetyl-CoA carboxylase by fructose bisphosphate and citrate. J Biol Chem. 1976 Sep 25;251(18):5759–5769. [PubMed] [Google Scholar]
- KATES M., BAXTER R. M. Lipid composition of mesophilic and psychrophilic yeasts (Candida species) as influenced by environmental temperature. Can J Biochem Physiol. 1962 Sep;40:1213–1227. [PubMed] [Google Scholar]
- Koch A. L., Doyle R. J., Kubitschek H. E. Inactivation of membrane transport in Escherichia coli by near-ultraviolet light. J Bacteriol. 1976 Apr;126(1):140–146. doi: 10.1128/jb.126.1.140-146.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matile P. Inositol deficiency resulting in death: an explanation of its occurrence in Neurospora crassa. Science. 1966 Jan 7;151(3706):86–88. doi: 10.1126/science.151.3706.86. [DOI] [PubMed] [Google Scholar]
- Matile P., Wiemken A. The vacuole as the lysosome of the yeast cell. Arch Mikrobiol. 1967 Feb 20;56(2):148–155. doi: 10.1007/BF00408765. [DOI] [PubMed] [Google Scholar]
- Ninnemann H., Butler W. L., Epel B. L. Inhibition of respiration in yeast by light. Biochim Biophys Acta. 1970 Jun 30;205(3):499–506. doi: 10.1016/0005-2728(70)90115-5. [DOI] [PubMed] [Google Scholar]
- SULKOWSKI E., GUERIN B., DEFAYE J., SLONIMSKI P. P. INHIBITION OF PROTEIN SYNTHESIS IN YEAST BY LOW INTENSITIES OF VISIBLE LIGHT. Nature. 1964 Apr 4;202:36–39. doi: 10.1038/202036a0. [DOI] [PubMed] [Google Scholar]
- Sullivan J. L., Debusk A. G. Inositol-less death in Neurospora and cellular ageing. Nat New Biol. 1973 May 16;243(124):72–74. [PubMed] [Google Scholar]
- Wickerham L. J. A Critical Evaluation of the Nitrogen Assimilation Tests Commonly Used in the Classification of Yeasts. J Bacteriol. 1946 Sep;52(3):293–301. [PMC free article] [PubMed] [Google Scholar]
- Woodward J. R., Cirillo V. P. Amino acid transport and metabolism in nitrogen-starved cells of Saccharomyces cerevisiae. J Bacteriol. 1977 May;130(2):714–723. doi: 10.1128/jb.130.2.714-723.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


