Abstract
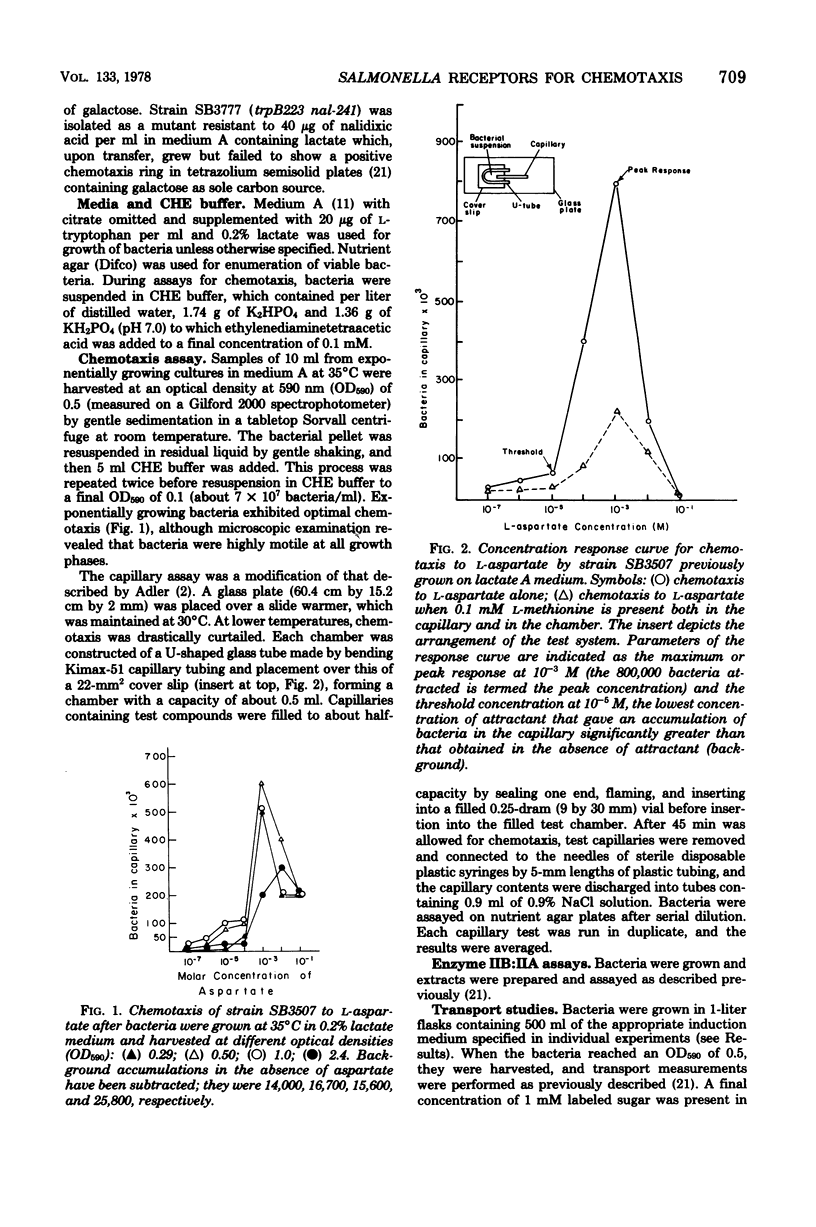
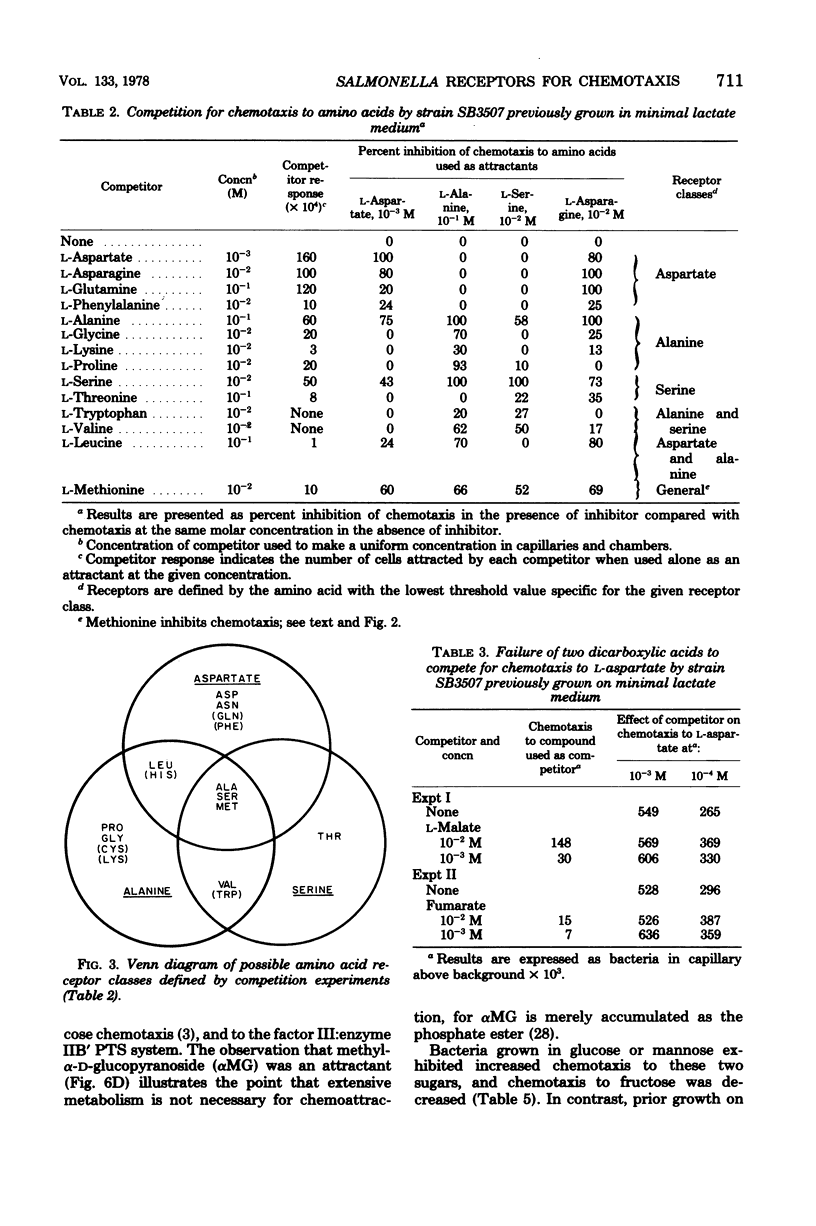
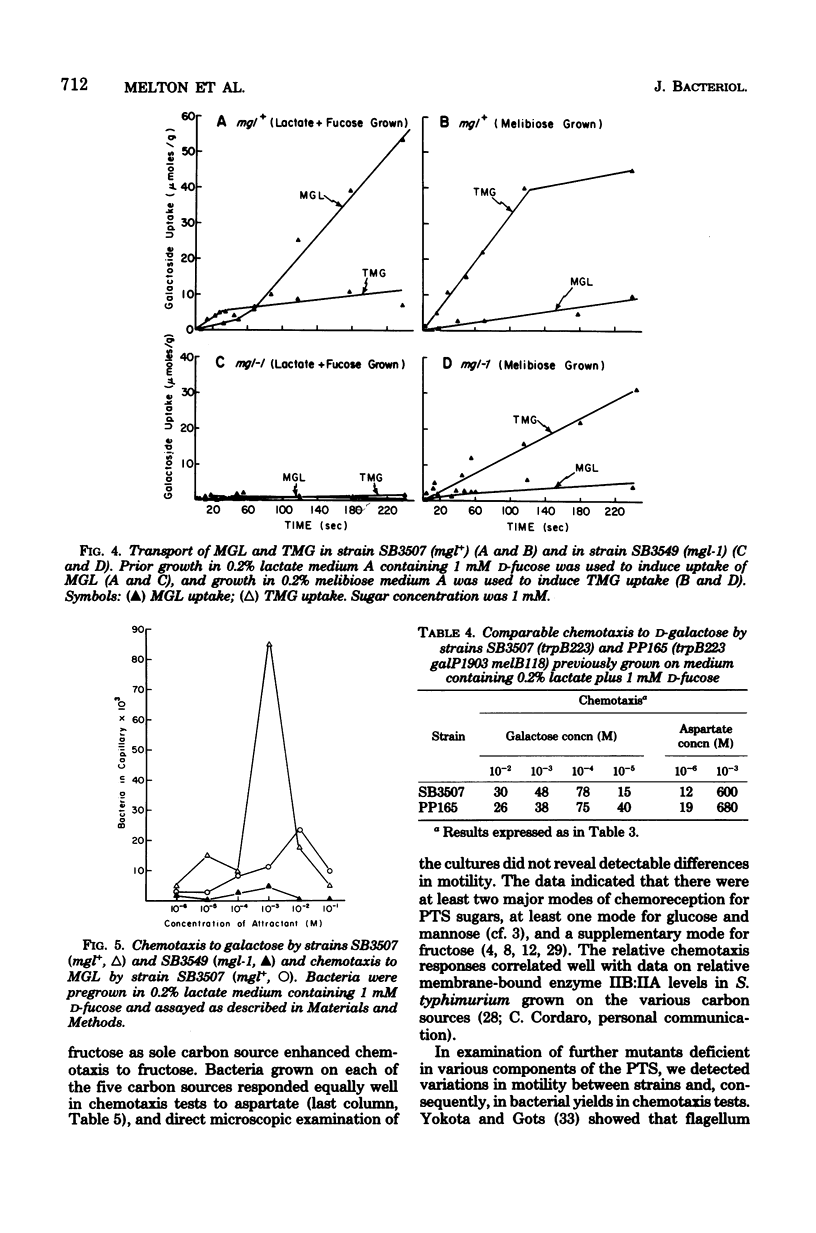
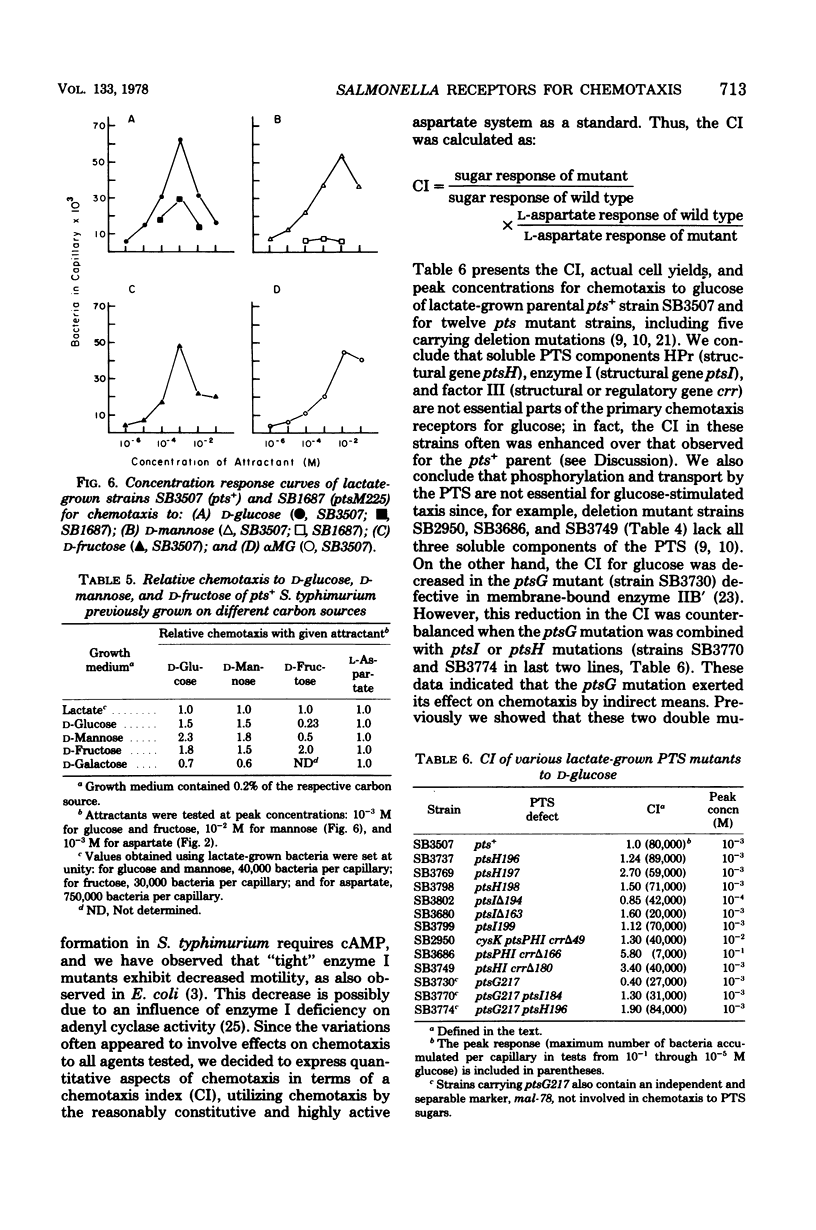
Patterns of chemotaxis by Salmonella typhimurium strain LT-2 to l-amino acids and to several sugars were quantitated by the Adler capillary procedure. Competition experiments indicated that LT-2 possesses three predominant receptors, or interacting sets of receptors, for amino acids. These were termed the aspartate, serine, and alanine classes, respectively. Studies with strains carrying point and deletion mutations affecting components of the phosphoenolpyruvate: glycose phosphotransferase system (PTS) made unlikely a role in primary reception of d-glucose by the three soluble PTS components, namely HPr, enzyme I, and factor III. A ptsG mutant defective in membrane-bound enzyme IIB′ of the high-affinity glucose transport system was shown to exhibit normal chemotaxis providing pleiotropic effects of the mutation were eliminated by its genotypic combination with other pts mutations or, phenotypically, by addition of cyclic AMP and substrate. A correlation was demonstrated between chemotaxis to glucose and activity of the low-affinity glucose transport complex, membrane-bound enzymes IIB:IIA, and an enzyme IIB:IIA mutant was shown to have a preponderant defect in chemotaxis to glucose and mannose. Of four systems capable of galactose transport, only the β-methylgalactoside transport system was implicated in chemotaxis to galactose. Some properties of a mutant possibly defective in processing of signals for chemotaxis to sugars is described.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973 Jan;74(1):77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- Adler J. Chemoreceptors in bacteria. Science. 1969 Dec 26;166(3913):1588–1597. doi: 10.1126/science.166.3913.1588. [DOI] [PubMed] [Google Scholar]
- Adler J., Epstein W. Phosphotransferase-system enzymes as chemoreceptors for certain sugars in Escherichia coli chemotaxis. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2895–2899. doi: 10.1073/pnas.71.7.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler J., Hazelbauer G. L., Dahl M. M. Chemotaxis toward sugars in Escherichia coli. J Bacteriol. 1973 Sep;115(3):824–847. doi: 10.1128/jb.115.3.824-847.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksamit R. R., Howlett B. J., Koshland D. E., Jr Soluble and membrane-bound aspartate-binding activities in Salmonella typhimurium. J Bacteriol. 1975 Sep;123(3):1000–1005. doi: 10.1128/jb.123.3.1000-1005.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner B. R., Savageau M. A. Generalized indicator plate for genetic, metabolic, and taxonomic studies with microorganisms. Appl Environ Microbiol. 1977 Feb;33(2):434–444. doi: 10.1128/aem.33.2.434-444.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordaro C. Genetics of the bacterial phosphoenolpyruvate: glycose phosphotransferase system. Annu Rev Genet. 1976;10:341–359. doi: 10.1146/annurev.ge.10.120176.002013. [DOI] [PubMed] [Google Scholar]
- Cordaro J. C., Melton T., Stratis J. P., Atagün M., Gladding C., Hartman P. E., Roseman S. Fosfomycin resistance: selection method for internal and extended deletions of the phosphoenolpyruvate:sugar phosphotransferase genes of Salmonella typhimurium. J Bacteriol. 1976 Dec;128(3):785–793. doi: 10.1128/jb.128.3.785-793.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordaro J. C., Roseman S. Deletion mapping of the genes coding for HPr and enzyme I of the phosphoenolpyruvate: sugar phosphotransferase system in Salmonella typhimurium. J Bacteriol. 1972 Oct;112(1):17–29. doi: 10.1128/jb.112.1.17-29.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenci T., Kornberg H. L. The role of phosphotransferase-mediated syntheses of fructose 1-phosphate and fructose 6-phosphate in the growth of Escherichia coli on fructose. Proc R Soc Lond B Biol Sci. 1974 Sep 17;187(1087):105–119. doi: 10.1098/rspb.1974.0065. [DOI] [PubMed] [Google Scholar]
- Gutnick D., Calvo J. M., Klopotowski T., Ames B. N. Compounds which serve as the sole source of carbon or nitrogen for Salmonella typhimurium LT-2. J Bacteriol. 1969 Oct;100(1):215–219. doi: 10.1128/jb.100.1.215-219.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelbauer G. L., Adler J. Role of the galactose binding protein in chemotaxis of Escherichia coli toward galactose. Nat New Biol. 1971 Mar 24;230(12):101–104. doi: 10.1038/newbio230101a0. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr A response regulator model in a simple sensory system. Science. 1977 Jun 3;196(4294):1055–1063. doi: 10.1126/science.870969. [DOI] [PubMed] [Google Scholar]
- Kundig W., Roseman S. Sugar transport. II. Characterization of constitutive membrane-bound enzymes II of the Escherichia coli phosphotransferase system. J Biol Chem. 1971 Mar 10;246(5):1407–1418. [PubMed] [Google Scholar]
- Lengeler J. Mutations affecting transport of the hexitols D-mannitol, D-glucitol, and galactitol in Escherichia coli K-12: isolation and mapping. J Bacteriol. 1975 Oct;124(1):26–38. doi: 10.1128/jb.124.1.26-38.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler J. Nature and properties of hexitol transport systems in Escherichia coli. J Bacteriol. 1975 Oct;124(1):39–47. doi: 10.1128/jb.124.1.39-47.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo T. C., Sanwal B. D. Genetic analysis of mutants of Escherichia coli defective in dicarboxylate transport. Mol Gen Genet. 1975 Oct 22;140(4):303–307. doi: 10.1007/BF00267321. [DOI] [PubMed] [Google Scholar]
- Melton T., Kundig W., Hartman P. E., Meadow N. 3-Deoxy-3-fluoro-D-glucose-resistant Salmonella typhimurium mutants defective in the phosphoenolpyruvate:glycose phosphotransferase system. J Bacteriol. 1976 Dec;128(3):794–800. doi: 10.1128/jb.128.3.794-800.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesibov R., Adler J. Chemotaxis toward amino acids in Escherichia coli. J Bacteriol. 1972 Oct;112(1):315–326. doi: 10.1128/jb.112.1.315-326.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordal G. W., Adler J. Isolation and complementation of mutants in galactose taxis and transport. J Bacteriol. 1974 Feb;117(2):509–516. doi: 10.1128/jb.117.2.509-516.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordal G. W., Adler J. Properties of mutants in galactose taxis and transport. J Bacteriol. 1974 Feb;117(2):517–526. doi: 10.1128/jb.117.2.517-526.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Interaction of enzyme I of the phosphoenolpyruvate:sugar phosphotransferase system with adenylate cyclase of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2920–2924. doi: 10.1073/pnas.72.8.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W. Galactose transport in Salmonella typhimurium. J Bacteriol. 1977 Feb;129(2):630–639. doi: 10.1128/jb.129.2.630-639.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W. Involvement of the phosphotransferase system in galactose transport in Salmonella typhimurium. FEBS Lett. 1976 Jan 1;61(1):49–53. doi: 10.1016/0014-5793(76)80169-x. [DOI] [PubMed] [Google Scholar]
- Simoni R. D., Roseman S., Saier M. H., Jr Sugar transport. Properties of mutant bacteria defective in proteins of the phosphoenolpyruvate: sugar phosphotransferase system. J Biol Chem. 1976 Nov 10;251(21):6584–6597. [PubMed] [Google Scholar]
- Strange P. G., Koshland D. E., Jr Receptor interactions in a signalling system: competition between ribose receptor and galactose receptor in the chemotaxis response. Proc Natl Acad Sci U S A. 1976 Mar;73(3):762–766. doi: 10.1073/pnas.73.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Drift C., De Jong M. H. Chemotaxis toward amino acids in Bacillus subtilis. Arch Mikrobiol. 1974 Mar 4;96(2):83–92. [PubMed] [Google Scholar]
- Yokota T., Gots J. S. Requirement of adenosine 3', 5'-cyclic phosphate for flagella formation in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1970 Aug;103(2):513–516. doi: 10.1128/jb.103.2.513-516.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukin R. S., Strange P. G., Heavey R., Koshland D. E. Properties of the galactose binding protein of Salmonella typhimurium and Escherichia coli. Biochemistry. 1977 Feb 8;16(3):381–386. doi: 10.1021/bi00622a007. [DOI] [PubMed] [Google Scholar]
- de Jong M. H., van der Drift C., Vogels G. D. Receptors for chemotaxis in Bacillus subtilis. J Bacteriol. 1975 Sep;123(3):824–827. doi: 10.1128/jb.123.3.824-827.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Drift C., Duiverman J., Bexkens H., Krijnen A. Chemotaxis of a motile Streptococcus toward sugars and amino acids. J Bacteriol. 1975 Dec;124(3):1142–1147. doi: 10.1128/jb.124.3.1142-1147.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



