Abstract
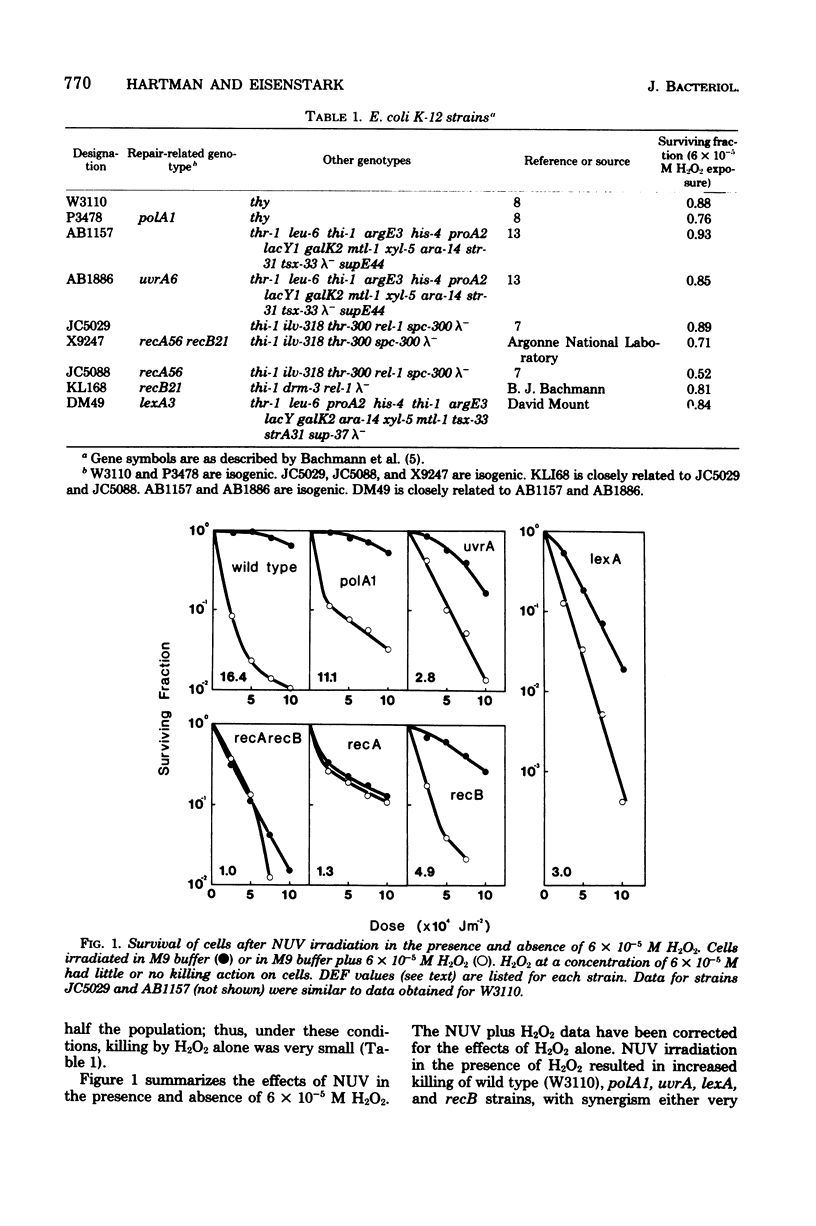
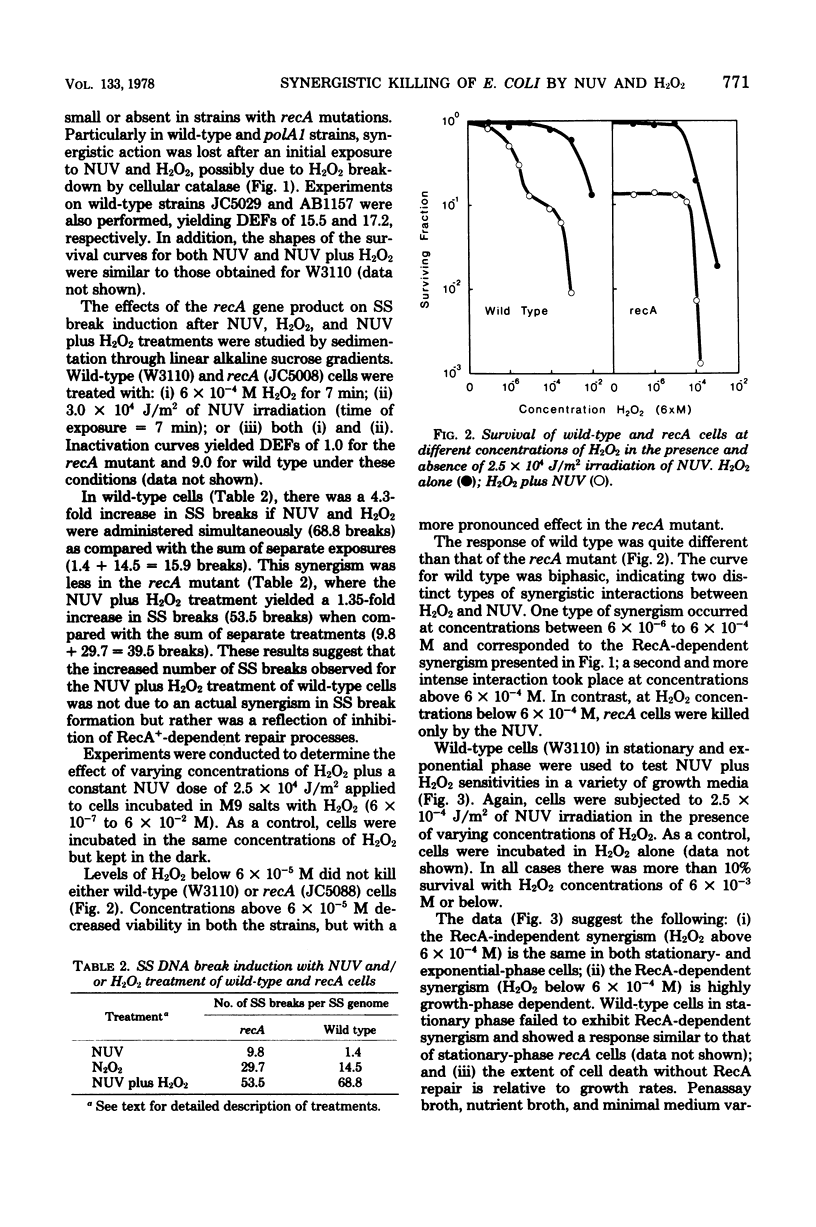
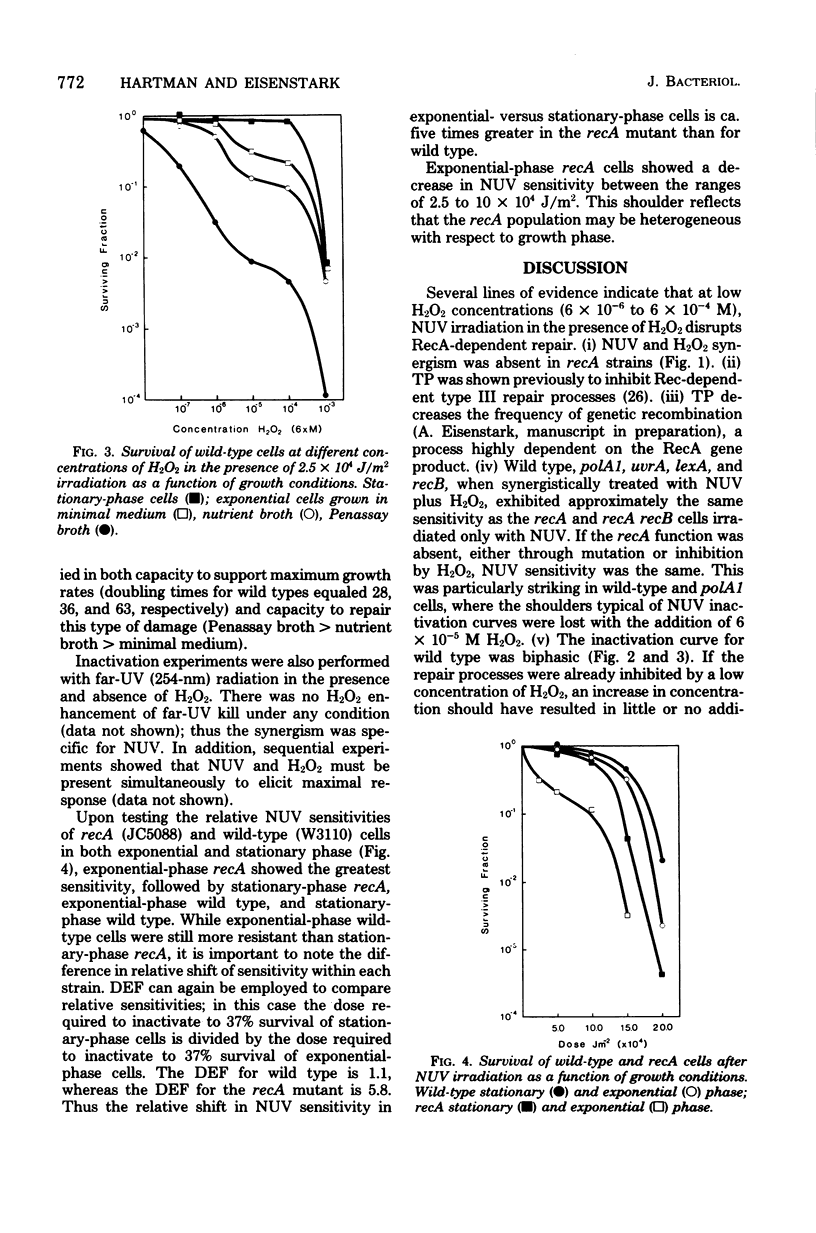
Wild-type cells and six DNA repair-deficient mutants (lexA, recA, recB, recA, recB, polA1, and uvrA) of Escherichia coli K-12 were treated with near-ultraviolet radiation plus hydrogen peroxide (H2O2). At low H2O2 concentrations (6 X 10(-6) to 6 X 10(-4) M), synergistic killing occurred in all strains except those containing a mutation in recA. This RecA-repairable damage was absent from stationary-phase cells but increased in logarithmic cells as a function of growth rate. At higher H2O2 concentrations (above 6 X 10(-4) M) plus near-ultraviolet radiation, all strains, including those with a mutation in recA, were synergistically killed; thus, at high H2O2 concentrations, the damage was not RecA repairable.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER H. I. Catalase, hydrogen peroxide, and ionizing radiation. Radiat Res. 1963;Suppl 3:110–129. [PubMed] [Google Scholar]
- Ananthaswamy H. N., Eisenstark A. Near-UV-induced breaks in phage DNA: sensitization by hydrogen peroxide (a tryptophan photoproduct). Photochem Photobiol. 1976 Nov;24(5):439–442. doi: 10.1111/j.1751-1097.1976.tb06851.x. [DOI] [PubMed] [Google Scholar]
- Ananthaswamy H. N., Eisenstark A. Repair of hydrogen peroxide-induced single-strand breaks in Escherichia coli deoxyribonucleic acid. J Bacteriol. 1977 Apr;130(1):187–191. doi: 10.1128/jb.130.1.187-191.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E. H. Growth Requirements of Virus-Resistant Mutants of Escherichia Coli Strain "B". Proc Natl Acad Sci U S A. 1946 May;32(5):120–128. doi: 10.1073/pnas.32.5.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A., Grossman L. An endonuclease from Escherichia coli that acts preferentially on UV-irradiated DNA and is absent from the uvrA and uvrB mutants. Proc Natl Acad Sci U S A. 1974 May;71(5):1838–1842. doi: 10.1073/pnas.71.5.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J. The beginning of a genetic analysis of recombination proficiency. J Cell Physiol. 1967 Oct;70(2 Suppl):165–180. doi: 10.1002/jcp.1040700412. [DOI] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. Model for regulation of Escherichia coli DNA repair functions. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2330–2334. doi: 10.1073/pnas.72.6.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison A. P., Jr Survival of bacteria. Harmful effects of light, with some comparisons with other adverse physical agents. Annu Rev Microbiol. 1967;21:143–156. doi: 10.1146/annurev.mi.21.100167.001043. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martignoni K. D., Smith K. C. The synergistic action of ultraviolet and x radiation on mutants of Escherichia coli K-12. Photochem Photobiol. 1973 Jul;18(1):1–8. doi: 10.1111/j.1751-1097.1973.tb06385.x. [DOI] [PubMed] [Google Scholar]
- McCormick J. P., Fischer J. R., Pachlatko J. P., Eisenstark A. Characterization of a cell-lethal product from the photooxidation of tryptophan: hydrogen peroxide. Science. 1976 Feb 6;191(4226):468–469. doi: 10.1126/science.1108203. [DOI] [PubMed] [Google Scholar]
- Peak M. J., Peak J. G., Webb R. B. Synergism between different near-ultraviolet wavelenghts in the inactivation of transforming DNA. Photochem Photobiol. 1975 Feb;21(2):129–131. doi: 10.1111/j.1751-1097.1975.tb06639.x. [DOI] [PubMed] [Google Scholar]
- Peak M. J. Some observations on the lethal effects of near-ultraviolet light on Escherichia coli, compared with the lethal effects of far-ultraviolet light. Photochem Photobiol. 1970 Jul;12(1):1–8. doi: 10.1111/j.1751-1097.1970.tb06031.x. [DOI] [PubMed] [Google Scholar]
- Stoien J. D., Wang R. J. Effect of near-ultraviolet and visible light on mammalian cells in culture II. Formation of toxic photoproducts in tissue culture medium by blacklight. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3961–3965. doi: 10.1073/pnas.71.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell R. M., Moss S. H., Davies D. J. The variation in UV sensitivity of four K12 strains of Escherichia coli as a function of their stage of growth. Mutat Res. 1972 Sep;16(7):1–12. doi: 10.1016/0027-5107(72)90058-9. [DOI] [PubMed] [Google Scholar]
- Tyrrell R. M. Rec A+-dependent synergism between 365 NM and ionizing radiation in log-phase Escherichia coli: a model for oxygen-dependent near-UV inactivation by disruption of DNA repair. Photochem Photobiol. 1976 Jan;23(1):13–20. doi: 10.1111/j.1751-1097.1976.tb06764.x. [DOI] [PubMed] [Google Scholar]
- Tyrrell R. M., Webb R. B. Reduced dimer excision in bacteria following near ultraviolet (365 nm) radiation. Mutat Res. 1973 Sep;19(3):361–364. doi: 10.1016/0027-5107(73)90238-8. [DOI] [PubMed] [Google Scholar]
- Yoakum G. H. Tryptophan photoproduct(s): sensitized induction of strand breaks (or alkali-labile bonds) in bacterial deoxyribonucleic acid during near-ultraviolet irradiation. J Bacteriol. 1975 Apr;122(1):199–205. doi: 10.1128/jb.122.1.199-205.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoakum G., Eisenstark A. Toxicity of L-Tryptophan photoproduct on recombinationless (rec) mutants of Salmonella typhimurium. J Bacteriol. 1972 Oct;112(1):653–655. doi: 10.1128/jb.112.1.653-655.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoakum G., Eisenstark A., Webb R. B. Near-UV photoproduct(s) of L-typtophan: an inhibitor of medium-dependent repair of X-ray-induced single-strand breaks in DNA which also inhibits replication-gap closure in Escherichia coli DNA. Basic Life Sci. 1975;5B:453–458. doi: 10.1007/978-1-4684-2898-8_5. [DOI] [PubMed] [Google Scholar]
- Youngs D. A., Smith K. C. Genetic control of multiple pathways of post-replicational repair in uvrB strains of Escherichia coli K-12. J Bacteriol. 1976 Jan;125(1):102–110. doi: 10.1128/jb.125.1.102-110.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



