Abstract
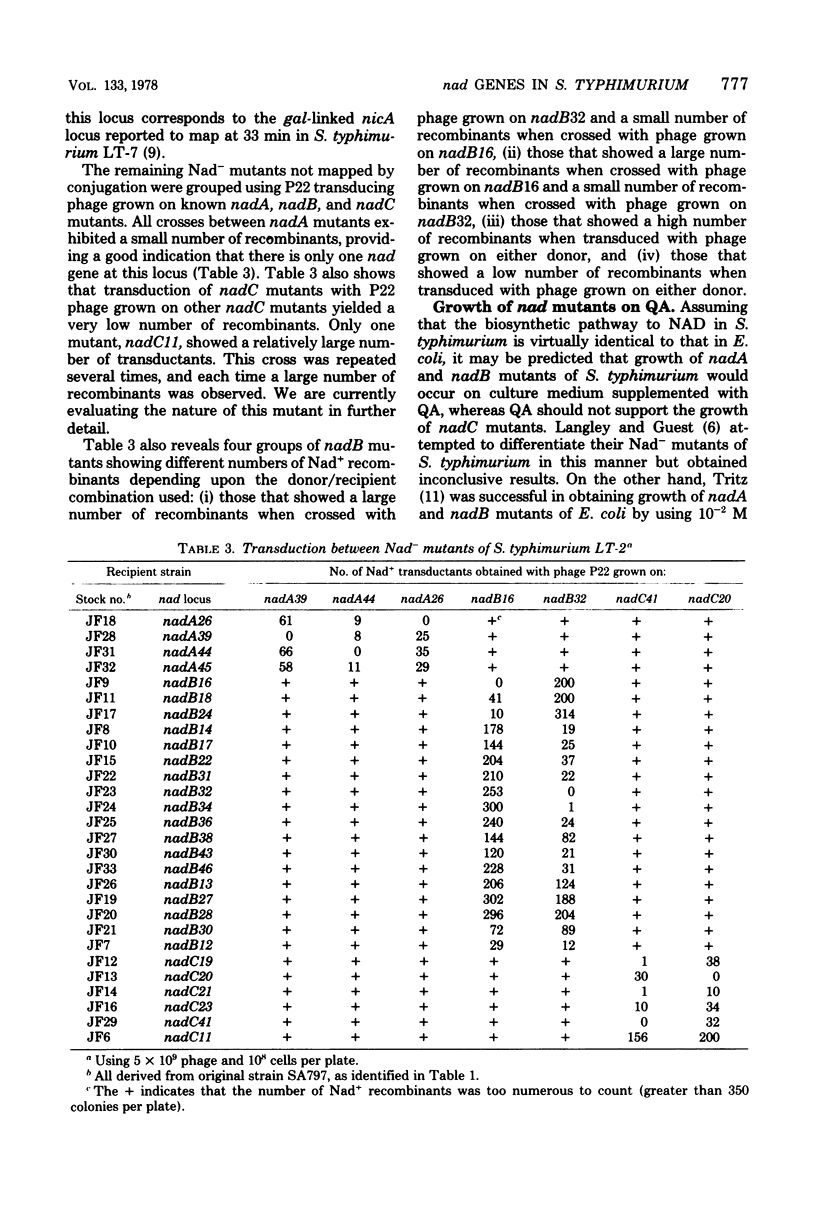
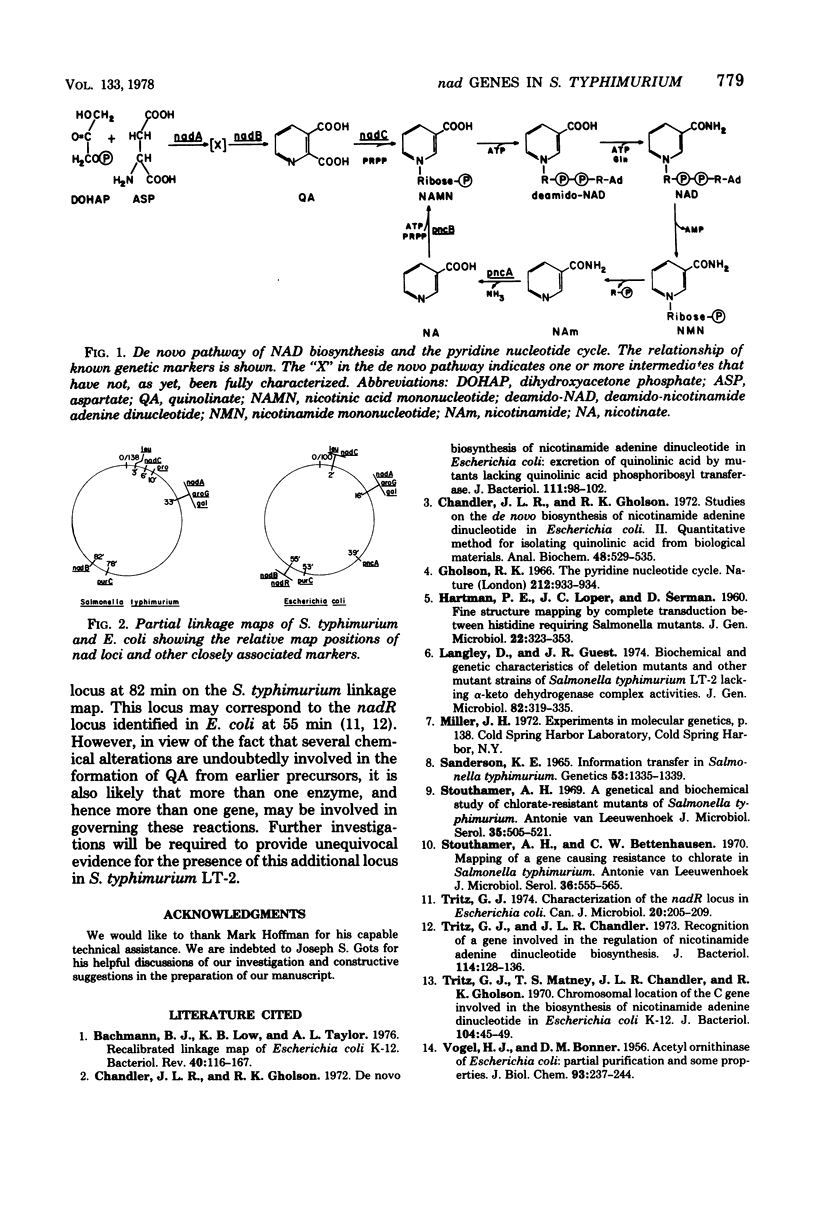
An ampicillin enrichment technique was used to isolate 39 nicotinic acid-requiring mutants of Salmonella typhimurium LT-2. Using interrupted-mating and transductional mapping procedures, three loci, designated nadA, nadB, and nadC, were identified. These loci mapped at 33, 82, and 6 min, respectively, on the S. typhimurium linkage map. The arrangement of the loci on the Salmonella linkage map corresponded closely to the nadA, nadB, and nadC loci on the Escherichia coli K-12 linkage map, indicating that the de novo pathway to nicotinamide adenine dinucleotide and the genes governing the enzymes involved in this pathway in S. typhimurium are very similar to those in E. coli. Evidence is also presented which indicates that the product of the nadC locus in S. typhimurium LT-2 is the enzyme quinolinic acid phosphoribosyltransferase. All nadC mutants of S. typhimurium secreted between 2 and 8 mumol of quinolinic acid per 100 ml of secretion medium. In addition, none of the nadC mutants isolated were able to grow in 10(-3) M quinolinic acid, whereas all nadA and nadB mutants of S. typhimurium grew well in the presence of quinolinic acid. Transductional crosses between nadB mutants provided evidence suggestive of more than one locus in the nadB region.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler J. L., Gholson R. K. De novo biosynthesis of nicotinamide adenine dinucleotide in Escherichia coli: excretion of quinolinic acid by mutants lacking quinolinate phosphoribosyl transferase. J Bacteriol. 1972 Jul;111(1):98–102. doi: 10.1128/jb.111.1.98-102.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler J. L., Gholson R. K. Studies on the de novo biosynthesis of NAD in Escherichia coli. II. Quantitative method for isolating quinolinic acid from biological materials. Anal Biochem. 1972 Aug;48(2):529–535. doi: 10.1016/0003-2697(72)90108-x. [DOI] [PubMed] [Google Scholar]
- Gholson R. K. The pyridine nucleotide cycle. Nature. 1966 Nov 26;212(5065):933–935. doi: 10.1038/212933a0. [DOI] [PubMed] [Google Scholar]
- HARTMAN P. E., LOPER J. C., SERMAN D. Fine structure mapping by complete transduction between histidine-requiring Salmonella mutants. J Gen Microbiol. 1960 Apr;22:323–353. doi: 10.1099/00221287-22-2-323. [DOI] [PubMed] [Google Scholar]
- Langley D., Guest J. R. Biochemical and genetic characterics of deletion and other mutant strains of Salmonella typhimurium LT2 lacking alpha-keto acid dehydrogenase complex activities,. J Gen Microbiol. 1974 Jun;82(2):319–335. doi: 10.1099/00221287-82-2-319. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E. Information transfer in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1335–1340. doi: 10.1073/pnas.53.6.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouthamer A. H. A genetical and biochemical study of chlorate-resistant mutants of Salmonella typhimurium. Antonie Van Leeuwenhoek. 1969;35(4):505–521. doi: 10.1007/BF02219168. [DOI] [PubMed] [Google Scholar]
- Stouthamer A. H., Bettenhaussen C. W. Mapping of a gene causing resistance to chlorate in Salmonella typhimurium. Antonie Van Leeuwenhoek. 1970;36(4):555–565. doi: 10.1007/BF02069058. [DOI] [PubMed] [Google Scholar]
- Tritz G. J., Chandler J. L. Recognition of a gene involved in the regulation of nicotinamide adenine dinucleotide biosynthesis. J Bacteriol. 1973 Apr;114(1):128–136. doi: 10.1128/jb.114.1.128-136.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritz G. J. Characterization of the nadR locus in Escherichia coli. Can J Microbiol. 1974 Feb;20(2):205–209. doi: 10.1139/m74-031. [DOI] [PubMed] [Google Scholar]
- Tritz G. J., Matney T. S., Chandler J. L., Gholson R. K. Chromosomal location of the C gene involved in the biosynthesis of nicotinamide adenine dinucleotide in Escherichia coli K-12. J Bacteriol. 1970 Oct;104(1):45–49. doi: 10.1128/jb.104.1.45-49.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]



