Abstract
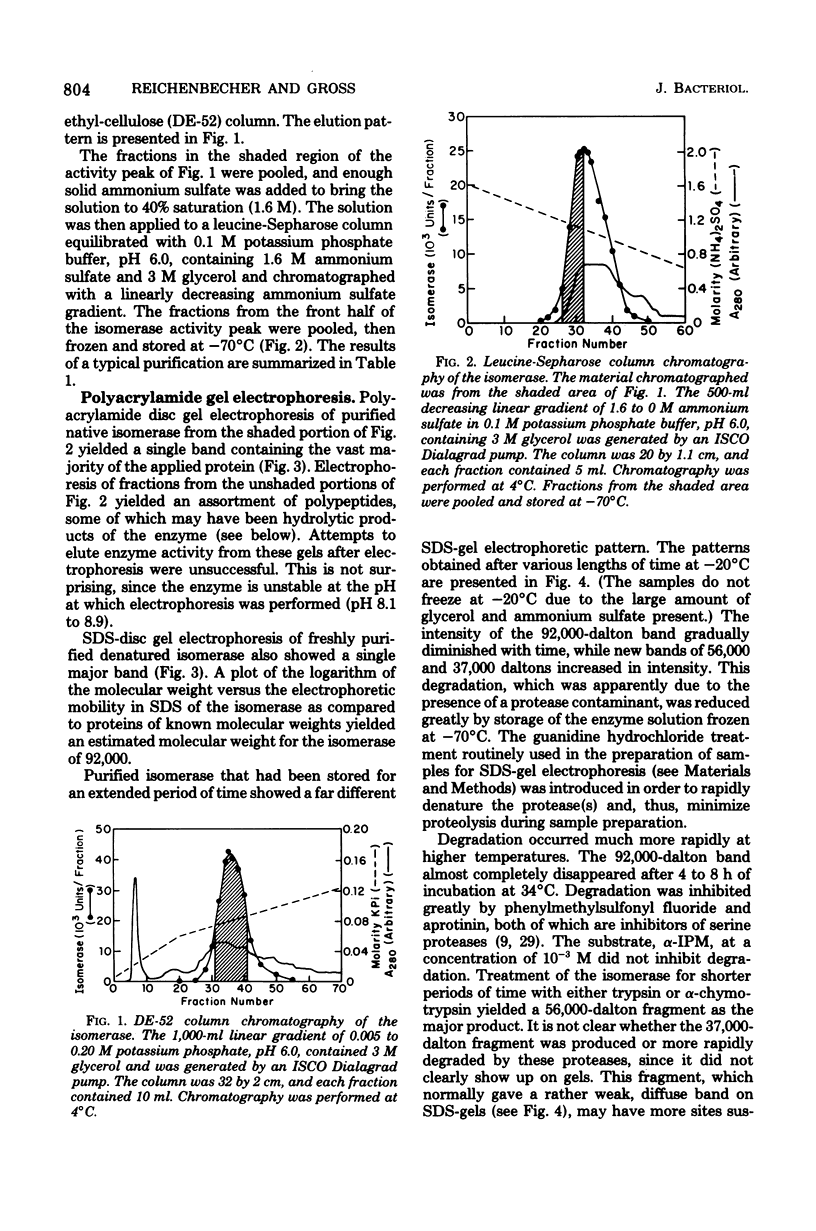
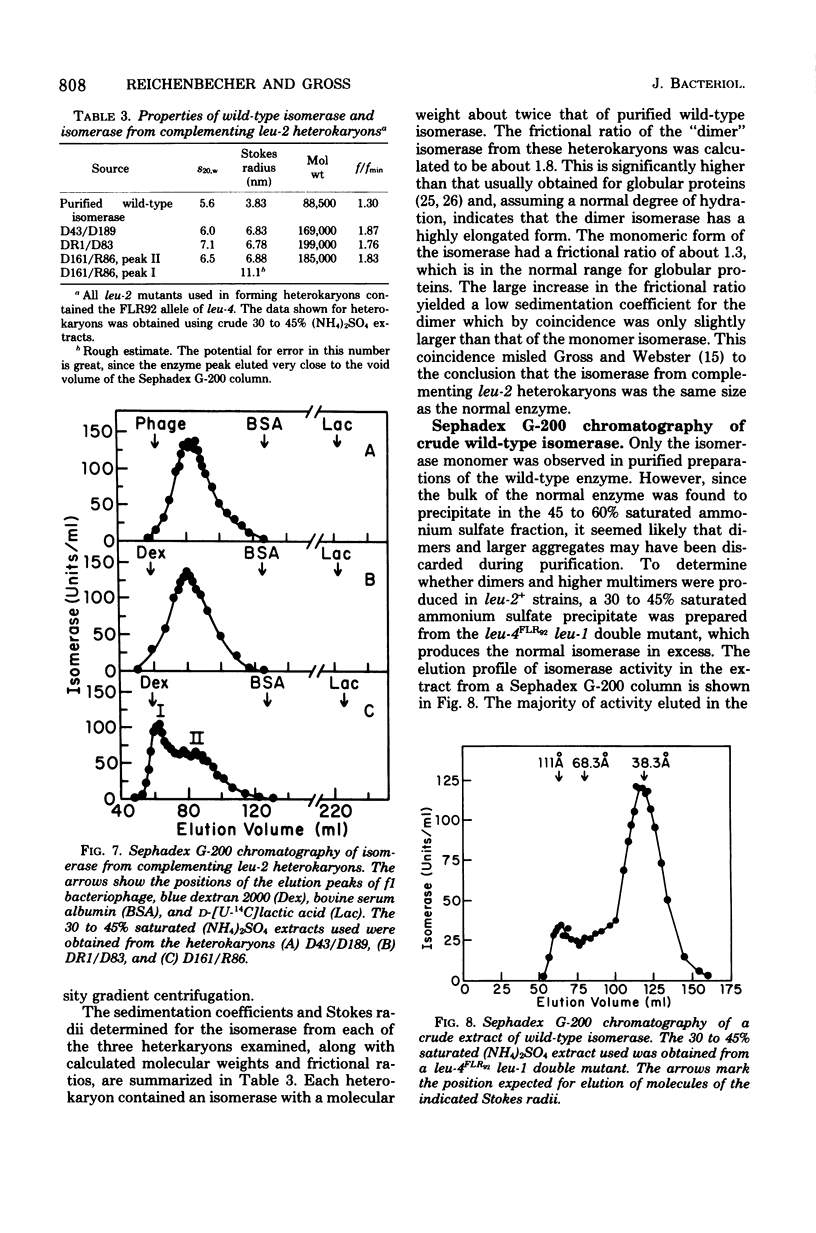
The isopropylmalate isomerase (EC 4.2.1.33) of Neurospora crassa is a globular protein consisting of a single polypeptide chain with a molecular weight of about 90,000. The isomerase cannot easily be freed of a contaminating protease which cleaves the enzyme into two major fragments, one of approximately 56,000 and the other 37,000 daltons. This suggests that the folded polypeptide chain may contain some hinge point or loop exposed on the surface which makes it susceptible to proteolytic attack. Most of the isomerase activity extracted from the wild-type strain is in monomer form. However, a small fraction of the activity in crude extracts is found in multimeric aggregates, and the active isomerase extracted from complementing leu-2 heterokaryons consists entirely of dimers and higher multimers. These observations suggest that, though active as a monomer, a significant fraction of the normal enzyme might be organized in multimeric form within the cell.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERS G. K. MOLECULAR EXCLUSION AND RESTRICTED DIFFUSION PROCESSES IN MOLECULAR-SIEVE CHROMATOGRAPHY. Biochemistry. 1964 May;3:723–730. doi: 10.1021/bi00893a021. [DOI] [PubMed] [Google Scholar]
- Bigelis R., Umbarger H. E. Purification of yeast alpha-isopropylmalate isomerase. High ionic strength hydrophobic chromatography. J Biol Chem. 1975 Jun 10;250(11):4315–4321. [PubMed] [Google Scholar]
- Bigelis R., Umbarger H. E. Yeast alpha-isopropylmalate isomerase. Factors affecting stability and enzyme activity. J Biol Chem. 1976 Jun 25;251(12):3545–3552. [PubMed] [Google Scholar]
- CRICK F. H., ORGEL L. E. THE THEORY OF INTER-ALLELIC COMPLEMENTATION. J Mol Biol. 1964 Jan;8:161–165. doi: 10.1016/s0022-2836(64)80156-x. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- GROSS S. R., BURNS R. O., UMBARGER H. E. THE BIOSYNTHESIS OF LEUCINE. II. THE ENZYMIC ISOMERIZATION OF BETA-CARBOXY-BETA-HYDROXYISOCAPROATE AND ALPHA-HYDROXY-BETA-CARBOXYISOCAPROATE. Biochemistry. 1963 Sep-Oct;2:1046–1052. doi: 10.1021/bi00905a023. [DOI] [PubMed] [Google Scholar]
- GROSS S. R. On the mechanism of complementation at the LEU-2 locus of Neurospora. Proc Natl Acad Sci U S A. 1962 Jun 15;48:922–930. doi: 10.1073/pnas.48.6.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross S. R. The regulation of synthesis of leucine biosynthetic enzymes in Neurospora. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1538–1546. doi: 10.1073/pnas.54.6.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashmiri S. V., Gross S. R. Mutations affecting the regulation of production of the enzymes of leucine synthesis in Neurospora. Genetics. 1970 Mar-Apr;64(3):423–440. [PMC free article] [PubMed] [Google Scholar]
- Koenig R., Stegemann H., Francksen H., Paul H. L. Protein subunits in the potato virus X group. Determination of the molecular weights by polyacrylamide electrophoresis. Biochim Biophys Acta. 1970 Apr 28;207(1):184–189. doi: 10.1016/0005-2795(70)90150-9. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Olshan A. R., Gross S. R. Role of the leu-3 cistron in the regulation of the synthesis of isoleucine and valine biosynthetic enzymes of Neurospora. J Bacteriol. 1974 May;118(2):374–384. doi: 10.1128/jb.118.2.374-384.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacco J. C., Gross S. R. The product of the leu-3 cistron as a regulatory element for the production of the leucine biosynthetic enzymes of Neurospora. Genetics. 1973 Jul;74(3):443–459. doi: 10.1093/genetics/74.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyanarayana T., Umbarger H. E., Lindegren G. Biosynthesis of branched-chain amino acids in yeast: correlation of biochemical blocks and genetic lesions in leucine auxotrophs. J Bacteriol. 1968 Dec;96(6):2012–2017. doi: 10.1128/jb.96.6.2012-2017.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Siepen D., Kula M. R. Specificity of five intracellular proteinases of Neurospora crassa. FEBS Lett. 1976 Jul 1;66(1):31–34. doi: 10.1016/0014-5793(76)80578-9. [DOI] [PubMed] [Google Scholar]
- Siepen D., Yu P. H., Kula M. R. Proteolytic enzymes of Neurospora crassa. Purification and some properties of five intracellular proteinases. Eur J Biochem. 1975 Aug 1;56(1):271–281. doi: 10.1111/j.1432-1033.1975.tb02230.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weiner A. M., Platt T., Weber K. Amino-terminal sequence analysis of proteins purified on a nanomole scale by gel electrophoresis. J Biol Chem. 1972 May 25;247(10):3242–3251. [PubMed] [Google Scholar]





