Abstract
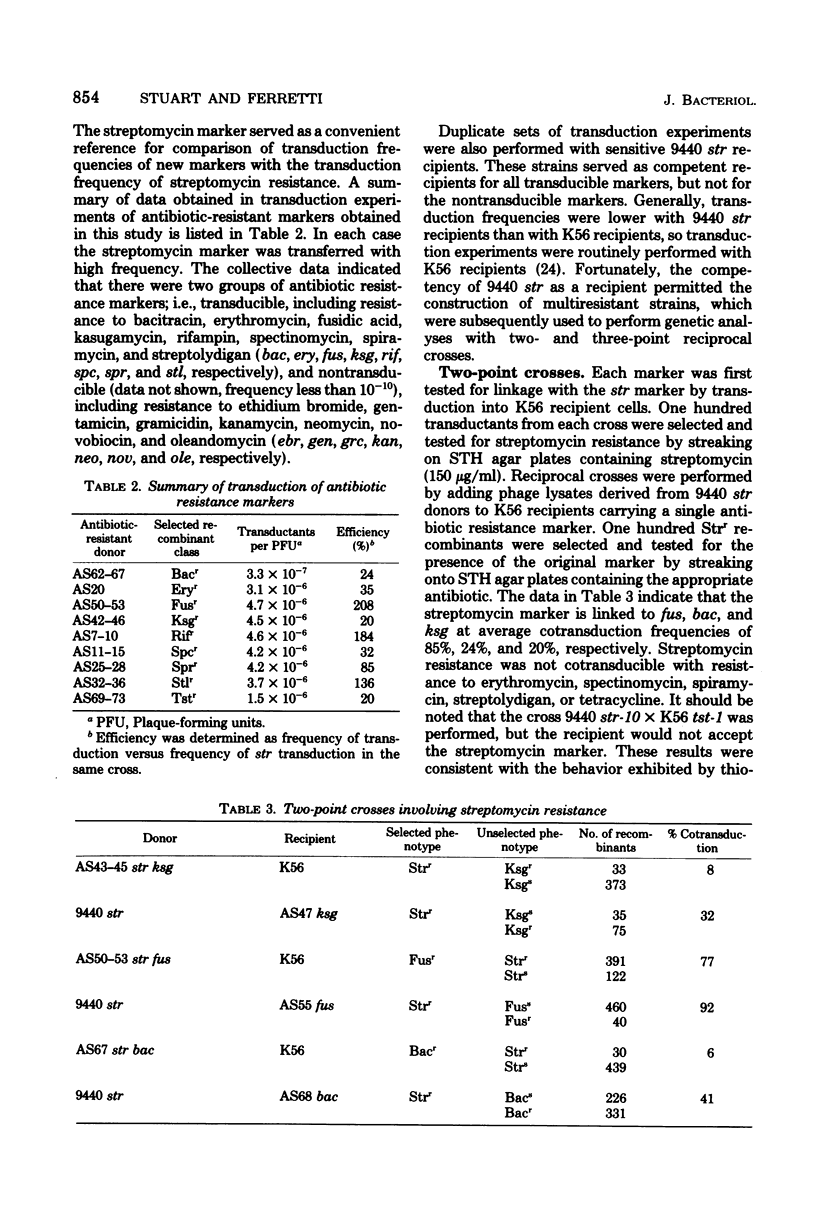
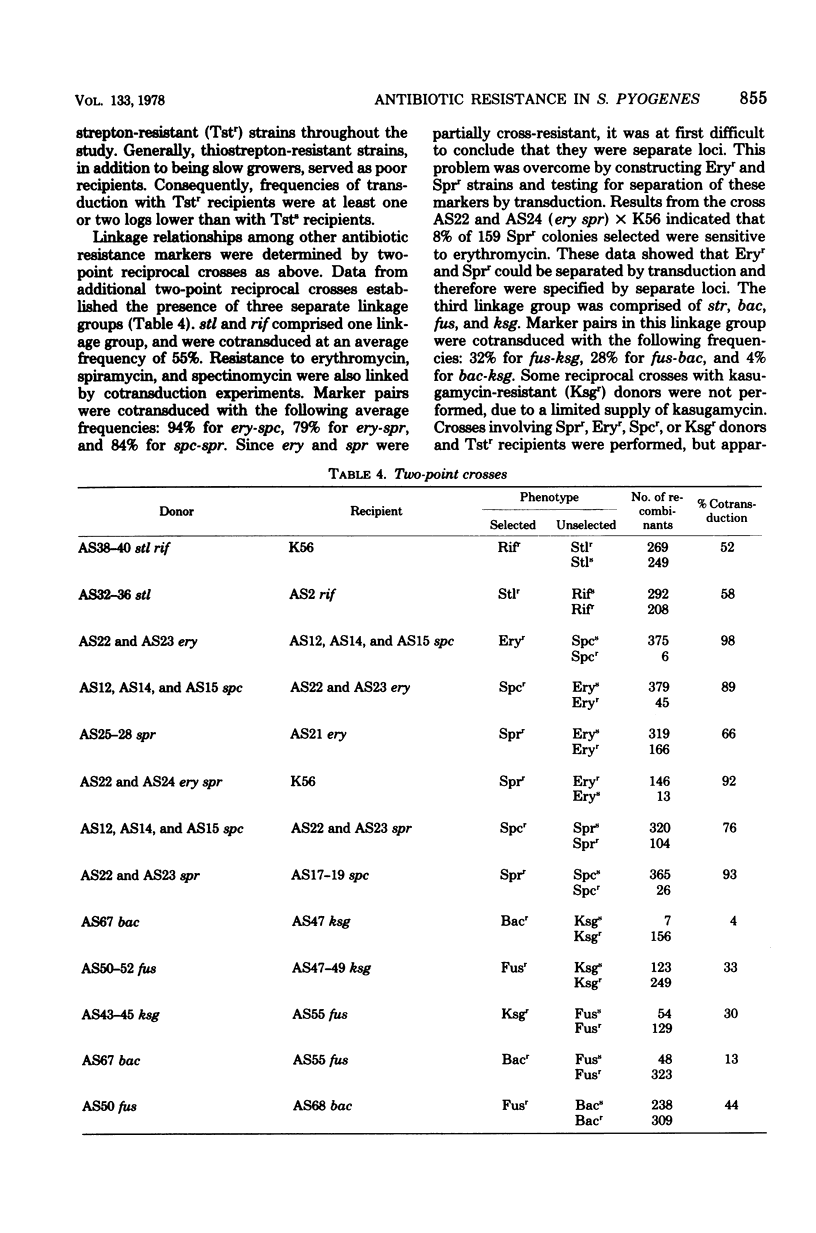
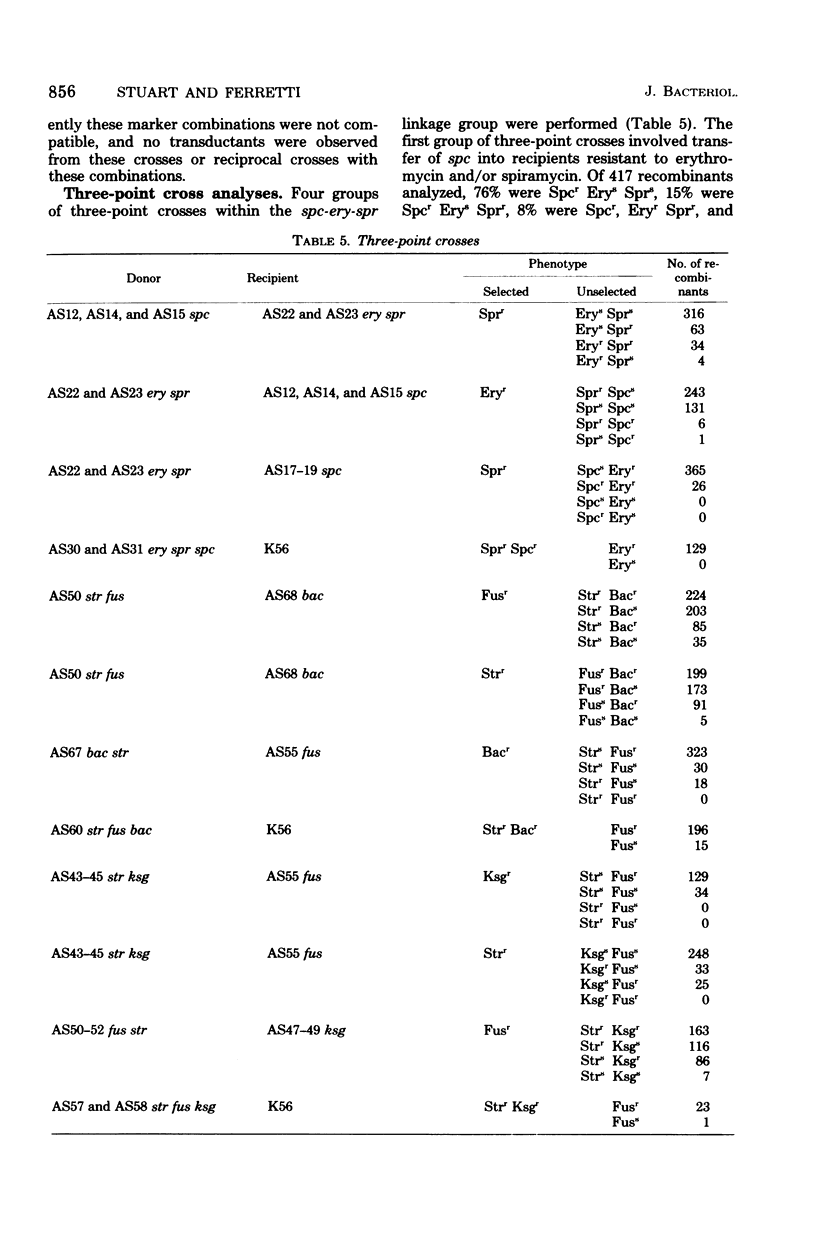
The genetics of antibiotic resistance in mutant strains of Streptococcus pyrogenes was studied. Utilizing a type 6 strain (9440) primarily resistant to strepttomycin (Strr), classes of mutant strains were isolated that were resistant to one of the following antibiotics: rifampin (Rifr), erythromycin (Eryr), thiostrepton (Tstr), spiramycin (Sprr), fusidic acid (Fusr), gramicidin (Grcr), ethidium bromide (Ebrr), kanamycin (Kanr), neomycin (Neor), oleandomycin (Oler), gentamicin (Genr), and novobiocin (Novr). Transduction experiments separated antibiotic resistance markers into two distinct groups: transducible markers, including Fusr, Bacr, Ksg+, Spcr, Eryr, Sprr, Rifr, Stlr, and Tstr (Bacr, Ksgr, Spcr, and Stlr refer to resistance to bacitracin, kasugamycin, spectinomycin, and streptolydigan, respectively), and nontransducible markers, including Grcr, Ebrr, Kanr, Neor, Oler, Genr, and Novr. By means of two- and three-point crosses, transducible markers (excluding tst) were located in three separate linkage groups. spr was found to be linked with ery and spc in the order spc-ery-spr, whereas in a separate linkage group the order was determined to be str-fus-bac-ksg. The third linkage group contained the rif and stl markers.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. N., Thornsberry C. Antimicrobial susceptibility of Streptococcus mutans isolated from patients with endocarditis. Antimicrob Agents Chemother. 1974 Mar;5(3):268–271. doi: 10.1128/aac.5.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco L., Vazquez D. Ribosomal sites involved in binding of aminoacyl-tRNA and EF 2. Mode of action of fusidic acid. FEBS Lett. 1973 May 15;32(1):152–156. doi: 10.1016/0014-5793(73)80760-4. [DOI] [PubMed] [Google Scholar]
- Colón A. E., Cole R. M., Leonard C. G. Transduction in group A streptococci by ultraviolet-irradiated bacteriophages. Can J Microbiol. 1970 Mar;16(3):201–202. doi: 10.1139/m70-034. [DOI] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldthwaite C., Smith I. Genetic mapping of aminoglycoside and fusidic acid resistant mutations in Bacillus subtilis. Mol Gen Genet. 1972;114(3):181–189. doi: 10.1007/BF01788887. [DOI] [PubMed] [Google Scholar]
- Harford N., Sueoka N. Chromosomal location of antibiotic resistance markers in Bacillus subtilis. J Mol Biol. 1970 Jul 28;51(2):267–286. doi: 10.1016/0022-2836(70)90142-7. [DOI] [PubMed] [Google Scholar]
- Hash J. H. Antibiotic mechanisms. Annu Rev Pharmacol. 1972;12:35–56. doi: 10.1146/annurev.pa.12.040172.000343. [DOI] [PubMed] [Google Scholar]
- Haworth S. R., Brown L. R. Genetic analysis of ribonucleic acid polymerase mutants of Bacillus subtilis. J Bacteriol. 1973 Apr;114(1):103–113. doi: 10.1128/jb.114.1.103-113.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida K., Koike M. Cell wall alterations of gram-negative bacteria by aminoglycoside antibiotics. Antimicrob Agents Chemother. 1974 Jan;5(1):95–97. doi: 10.1128/aac.5.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klainer A. S. The normal and abnormal surface morphology of staphylococci. Ann N Y Acad Sci. 1974 Jul 31;236(0):63–75. doi: 10.1111/j.1749-6632.1974.tb41482.x. [DOI] [PubMed] [Google Scholar]
- Leonard C. G., Colón A. E., Cole R. M. Transduction in group A streptococcus. Biochem Biophys Res Commun. 1968 Jan 25;30(2):130–135. doi: 10.1016/0006-291x(68)90459-2. [DOI] [PubMed] [Google Scholar]
- Malke H., Jacob H. E., Störl K. Characterization of the antibiotic resistance plasmid ERL1 from Streptococcus pyogenes. Mol Gen Genet. 1976 Mar 30;144(3):333–338. doi: 10.1007/BF00341732. [DOI] [PubMed] [Google Scholar]
- Malke H. Linkage relationships of mutations endowing Streptococcus pyogenes with resistance to antibiotics that affect the ribosome. Mol Gen Genet. 1972;116(4):299–308. doi: 10.1007/BF00270087. [DOI] [PubMed] [Google Scholar]
- Malke H. Transduction of Streptococcus pyogenes K 56 by temperature-sensitive mutants of the transducing phage A 25. Z Naturforsch B. 1969 Dec;24(12):1556–1561. doi: 10.1515/znb-1969-1214. [DOI] [PubMed] [Google Scholar]
- Malke H. Transfer of a plasmid mediating antibiotic resistance between strains of Streptococcus pyogenes in mixed cultures. Z Allg Mikrobiol. 1975;15(8):645–649. doi: 10.1002/jobm.3630150810. [DOI] [PubMed] [Google Scholar]
- Pestka S. Inhibitors of ribosome functions. Annu Rev Microbiol. 1971;25:487–562. doi: 10.1146/annurev.mi.25.100171.002415. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E. Linkage map of Salmonella typhimurium, edition IV. Bacteriol Rev. 1972 Dec;36(4):558–586. doi: 10.1128/br.36.4.558-586.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarubbi F. A., Jr, Blackman E., Sparling P. F. Genetic mapping of linked antibiotic resistance loci in Neisseria gonorrhoeae. J Bacteriol. 1974 Dec;120(3):1284–1292. doi: 10.1128/jb.120.3.1284-1292.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I., Smith H. Location of the SPO2 attachment site and the bryamycin resistance marker on the Bacillus subtilis chromosome. J Bacteriol. 1973 Jun;114(3):1138–1142. doi: 10.1128/jb.114.3.1138-1142.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling P. F., Ikeya Y., Elliot D. Two genetic loci for resistance to kasugamycin in Escherichia coli. J Bacteriol. 1973 Feb;113(2):704–710. doi: 10.1128/jb.113.2.704-710.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone K. J., Strominger J. L. Mechanism of action of bacitracin: complexation with metal ion and C 55 -isoprenyl pyrophosphate. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3223–3227. doi: 10.1073/pnas.68.12.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart J. G., Ferretti J. J. Transduction of rifampin resistance in group A streptococci. J Bacteriol. 1973 Aug;115(2):709–710. doi: 10.1128/jb.115.2.709-710.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubukata K., Konno M., Fujii R. Transduction of drug resistance to tetracycline, chloramphenicol, macrolides, lincomycin and clindamycin with phages induced from Streptococcus pyogenes. J Antibiot (Tokyo) 1975 Sep;28(9):681–688. doi: 10.7164/antibiotics.28.681. [DOI] [PubMed] [Google Scholar]
- Wannamaker L. W., Almquist S., Skjold S. Intergroup phage reactions and transduction between group C and group A streptococci. J Exp Med. 1973 Jun 1;137(6):1338–1353. doi: 10.1084/jem.137.6.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum B., Demohn V. Thiostrepton, an inhibitor of 5OS ribosome subunit function. J Bacteriol. 1970 Mar;101(3):1073–1075. doi: 10.1128/jb.101.3.1073-1075.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa M., Okuyama A., Tanaka N. A third kasugamycin resistance locus, ksgC, affecting ribosomal protein S2 in Escherichia coli K-12. J Bacteriol. 1975 May;122(2):796–797. doi: 10.1128/jb.122.2.796-797.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



