Abstract
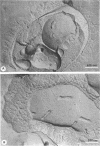
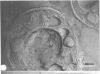
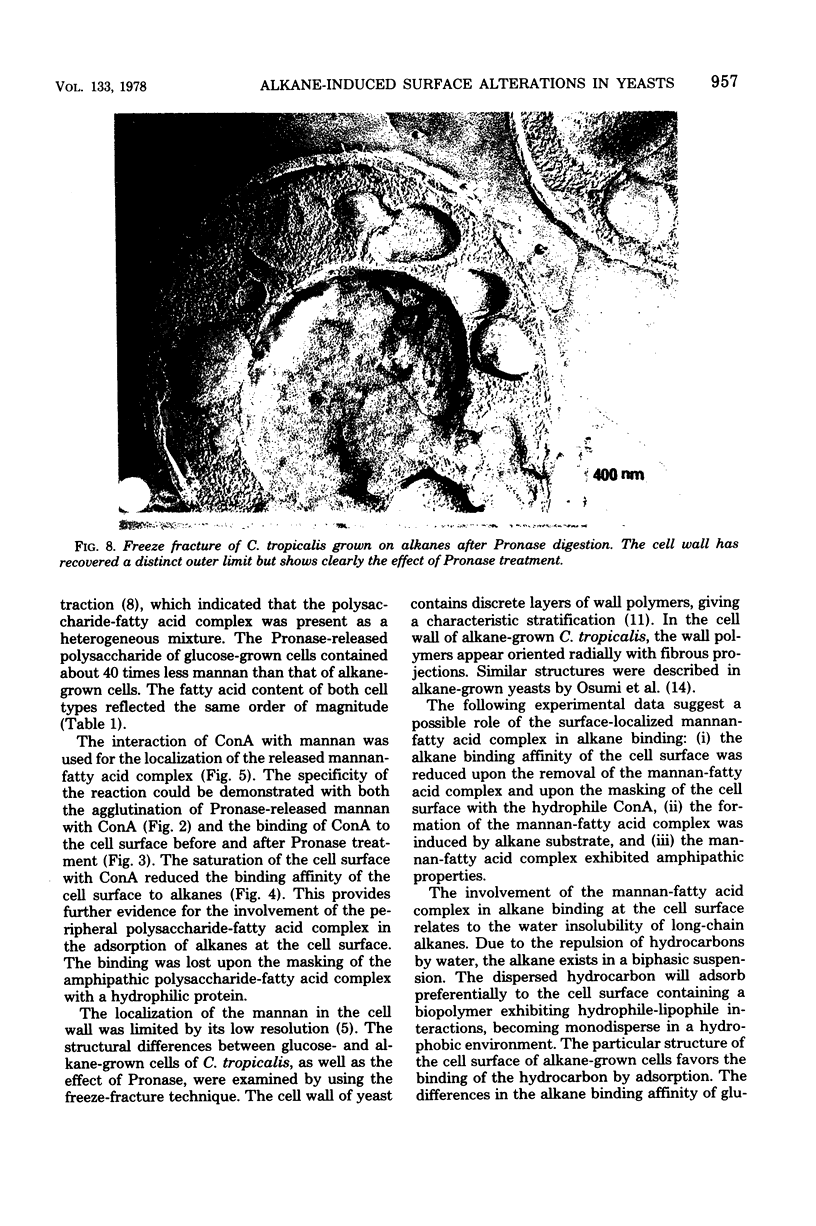
The surface-localized polysaccharide of alkane-grown cells of Candida tropicalis was identified as mannan containing approximately 4% covalently linked fatty acids. Glucose-grown cells lacked the mannan-fatty acid complex. The surface structure of alkane-grown cells showed a radial arrangement of the wall polymers, with protruding parts. The cell surface of glucose-grown cells was smooth, with a coherent outer limit. The mannan was localized by using concanavalin A. Masking of the mannan with concanavalin A reduced the binding affinity of the surface for alkane, indicating the involvement of the surface-localized mannan-fatty acid complex in the binding of alkanes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernhard W., Avrameas S. Ultrastructural visualization of cellular carbohydrate components by means of concanavalin A. Exp Cell Res. 1971 Jan;64(1):232–236. doi: 10.1016/0014-4827(71)90217-5. [DOI] [PubMed] [Google Scholar]
- Horisberger M., Bauer H., Bush D. A. Mercury-labelled concanavalin A as a marker in electron microscopy-localisation of mannan in yeast cell walls. FEBS Lett. 1971 Nov 1;18(2):311–314. doi: 10.1016/0014-5793(71)80474-x. [DOI] [PubMed] [Google Scholar]
- Kaeppeli O., Fiechter A. The mode of interaction between the substrate and cell surface of the hydrocarbon-utilizing yeast Candida tropicalis. Biotechnol Bioeng. 1976 Jul;18(7):967–974. doi: 10.1002/bit.260180709. [DOI] [PubMed] [Google Scholar]
- Käppeli O., Fiechter A. Component from the cell surface of the hydrocarbon-utilizing yeast Candida tropicalis with possible relation to hydrocarbon transport. J Bacteriol. 1977 Sep;131(3):917–921. doi: 10.1128/jb.131.3.917-921.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ludvík J., Munk V., Dostálek M. Ultrastructural changes in the yeast Candida lipolytica caused by penetration of hydrocarbons into the cell. Experientia. 1968 Oct 15;24(10):1066–1068. doi: 10.1007/BF02138754. [DOI] [PubMed] [Google Scholar]
- Miura Y., Okazaki M., Hamada S. I., Murakawa S. I., Yugen R. Assimilation of liquid hydrocarbon by microorganisms. I. Mechanism of hydrocarbon uptake. Biotechnol Bioeng. 1977 May;19(5):701–714. doi: 10.1002/bit.260190507. [DOI] [PubMed] [Google Scholar]
- So L. L., Goldstein I. J. Protein-carbohydrate interaction. 13. The interaction of concanavalin A with alpha-mannans from a variety of microorganisms. J Biol Chem. 1968 Apr 25;243(8):2003–2007. [PubMed] [Google Scholar]