Abstract
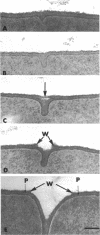
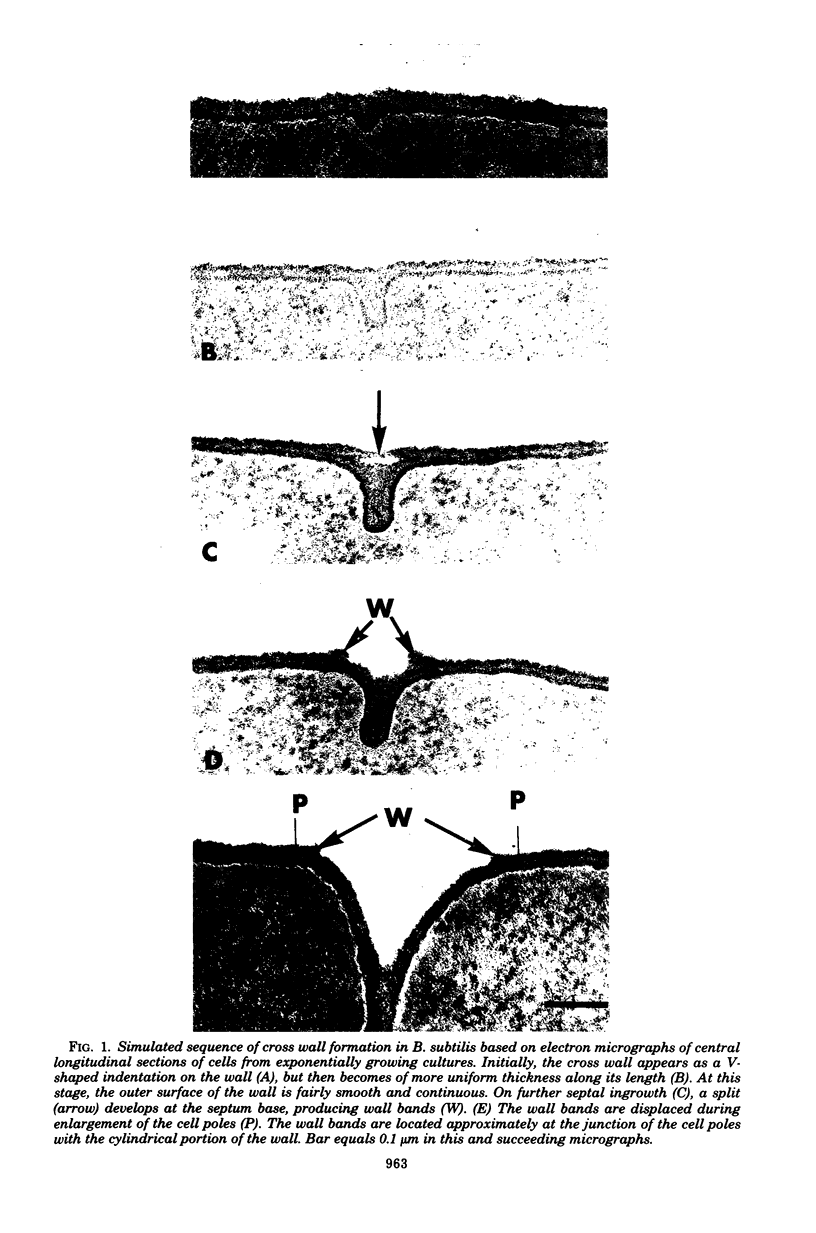
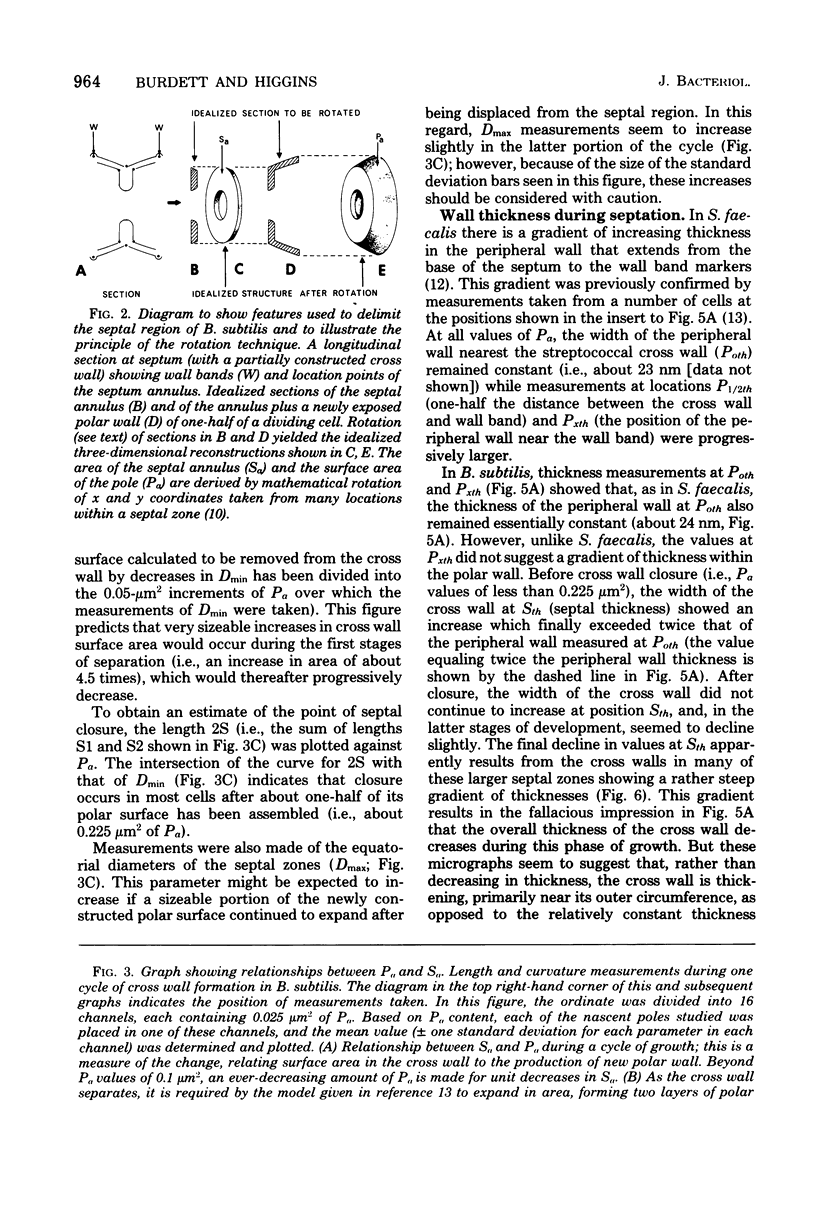
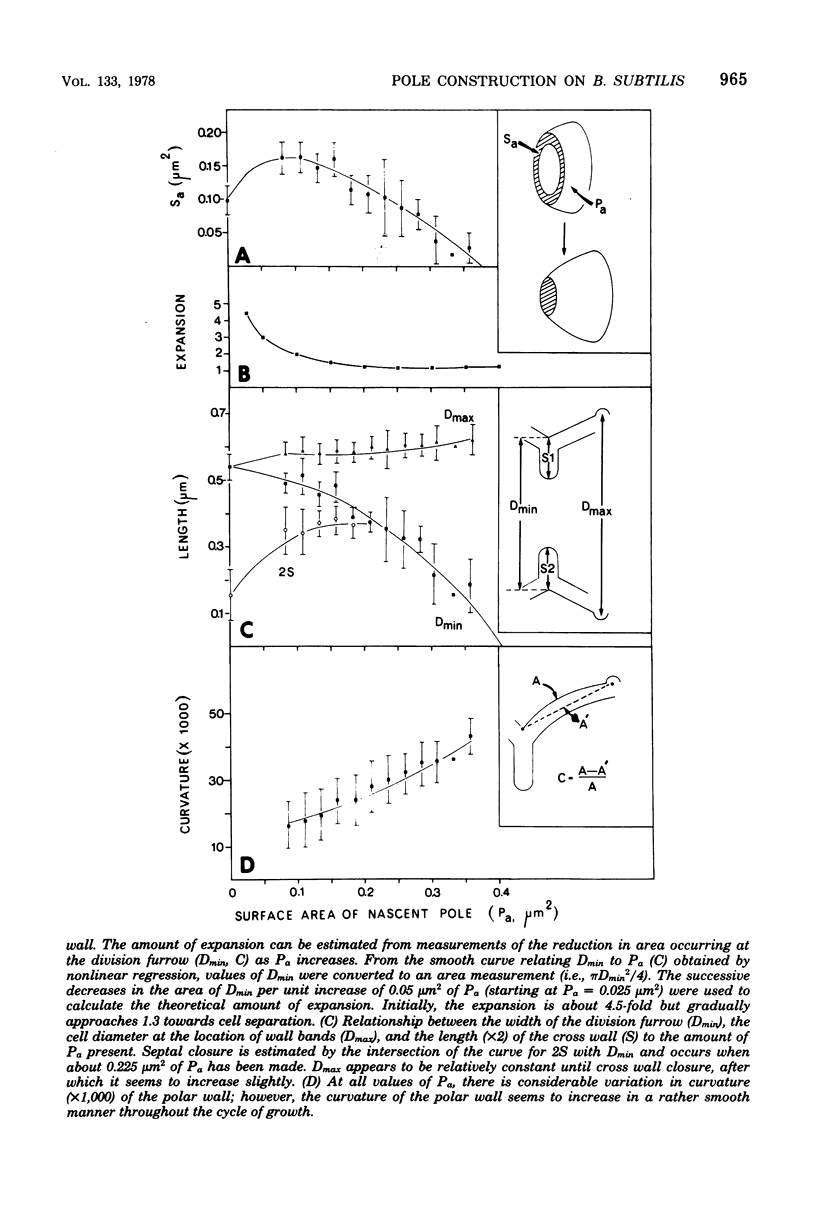
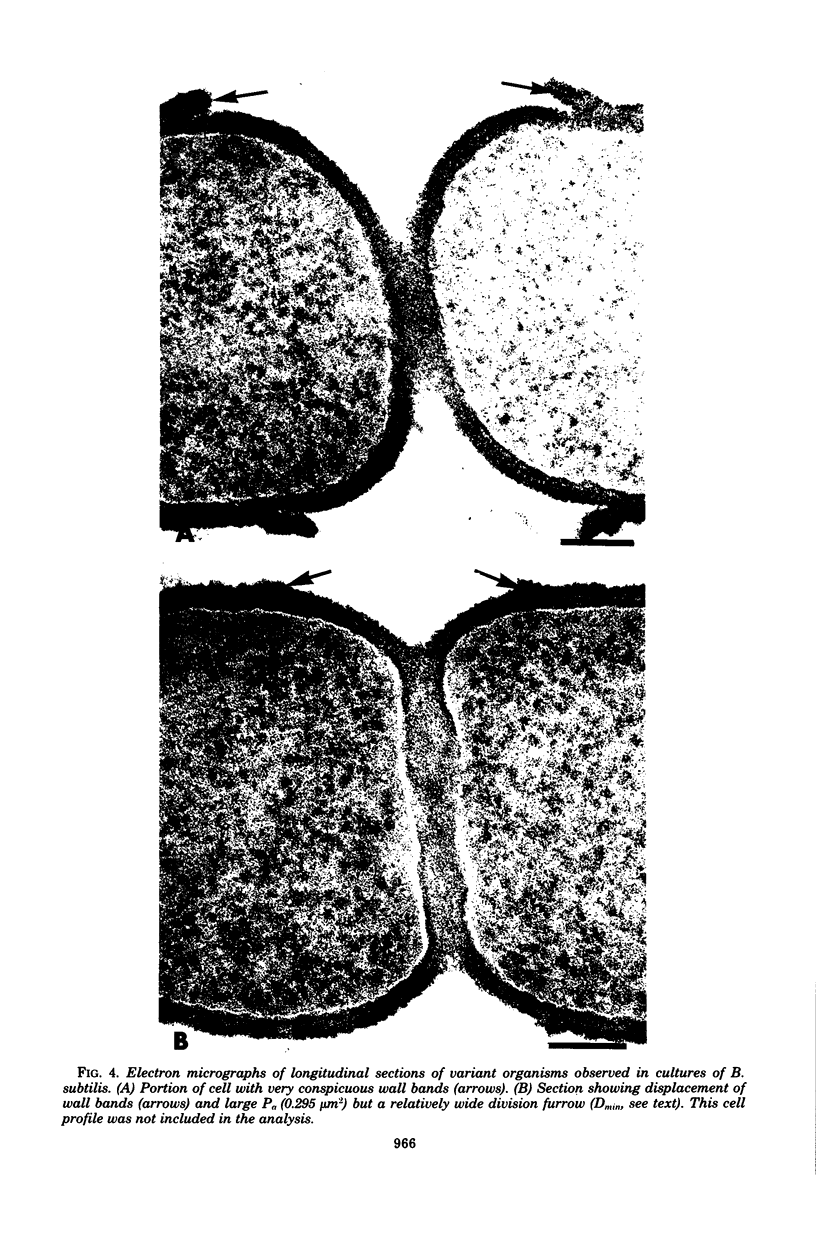
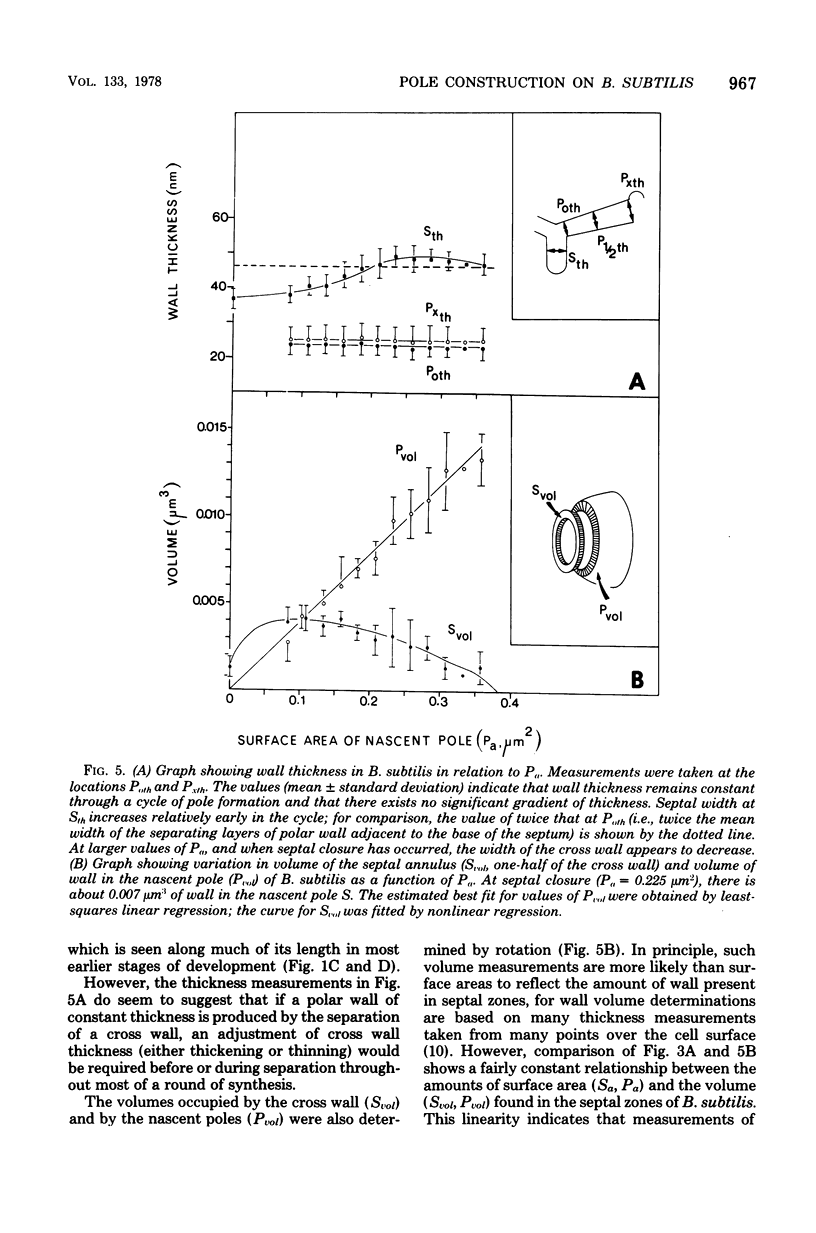
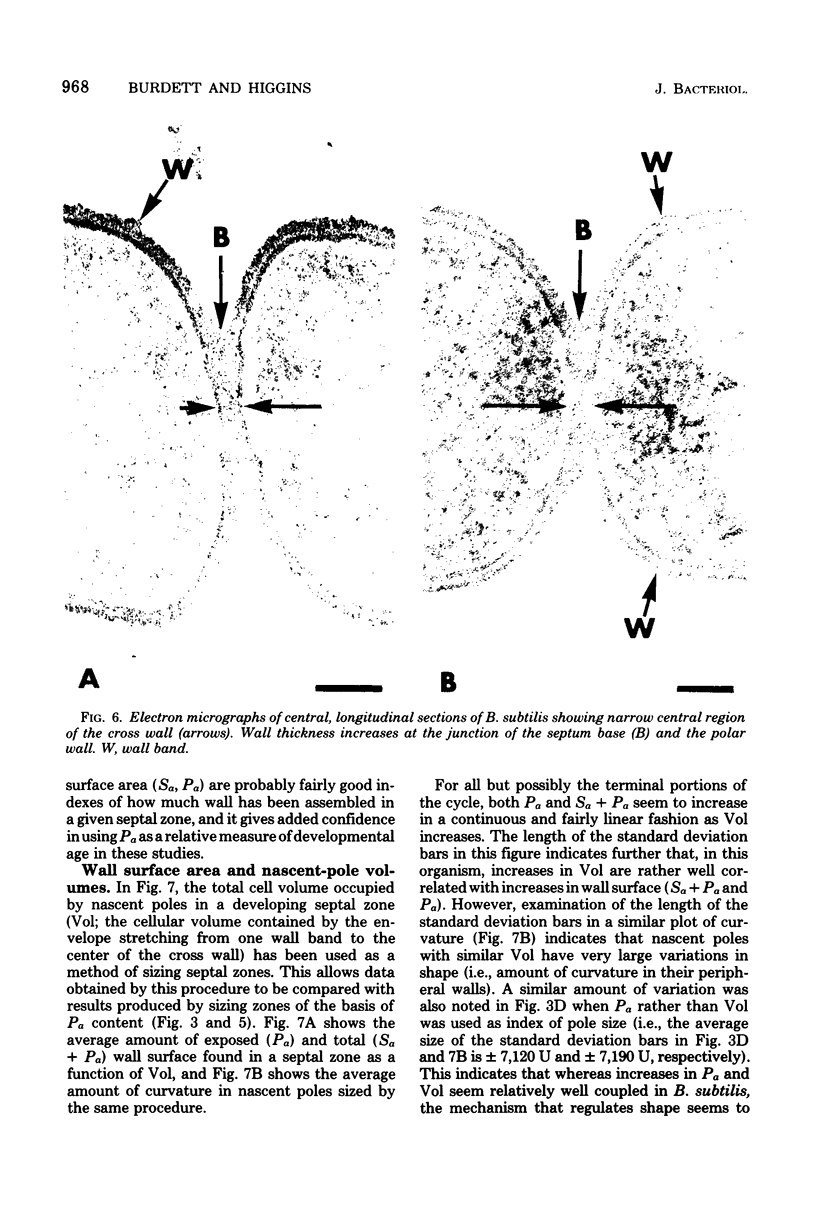
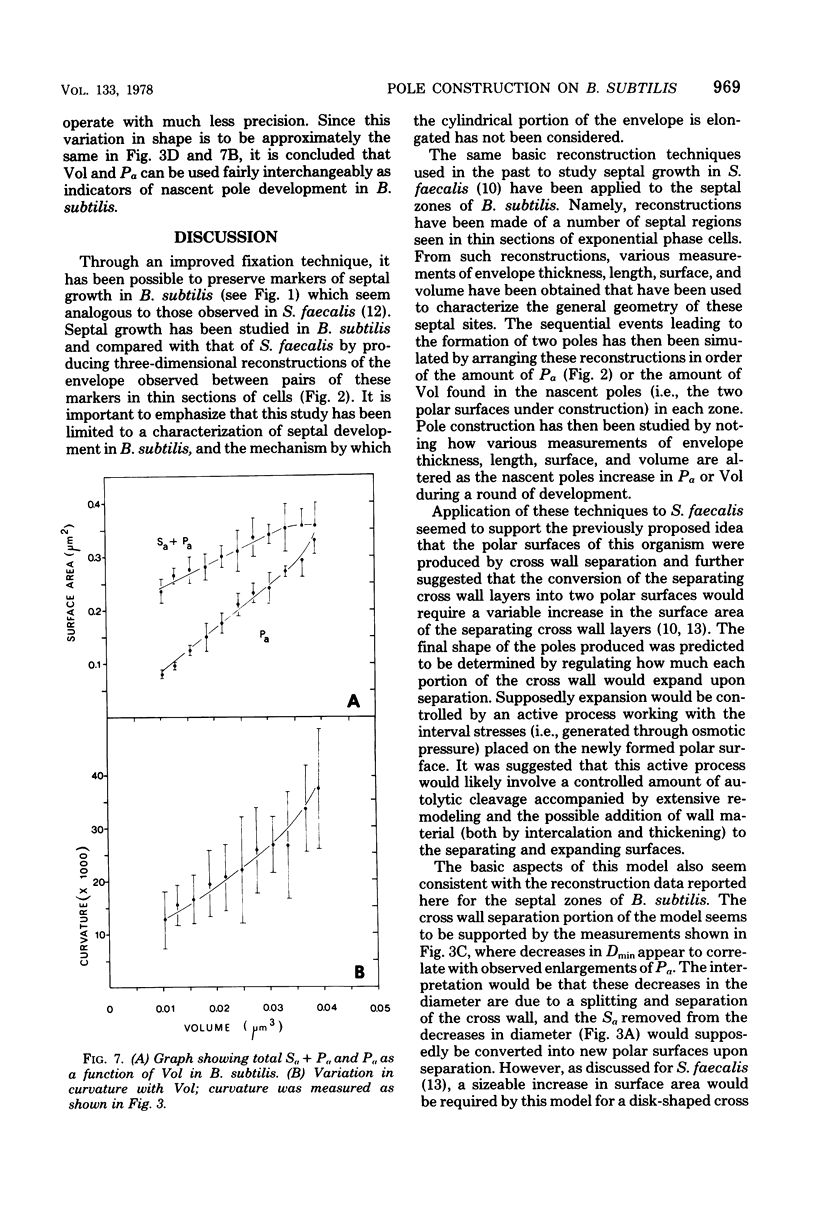
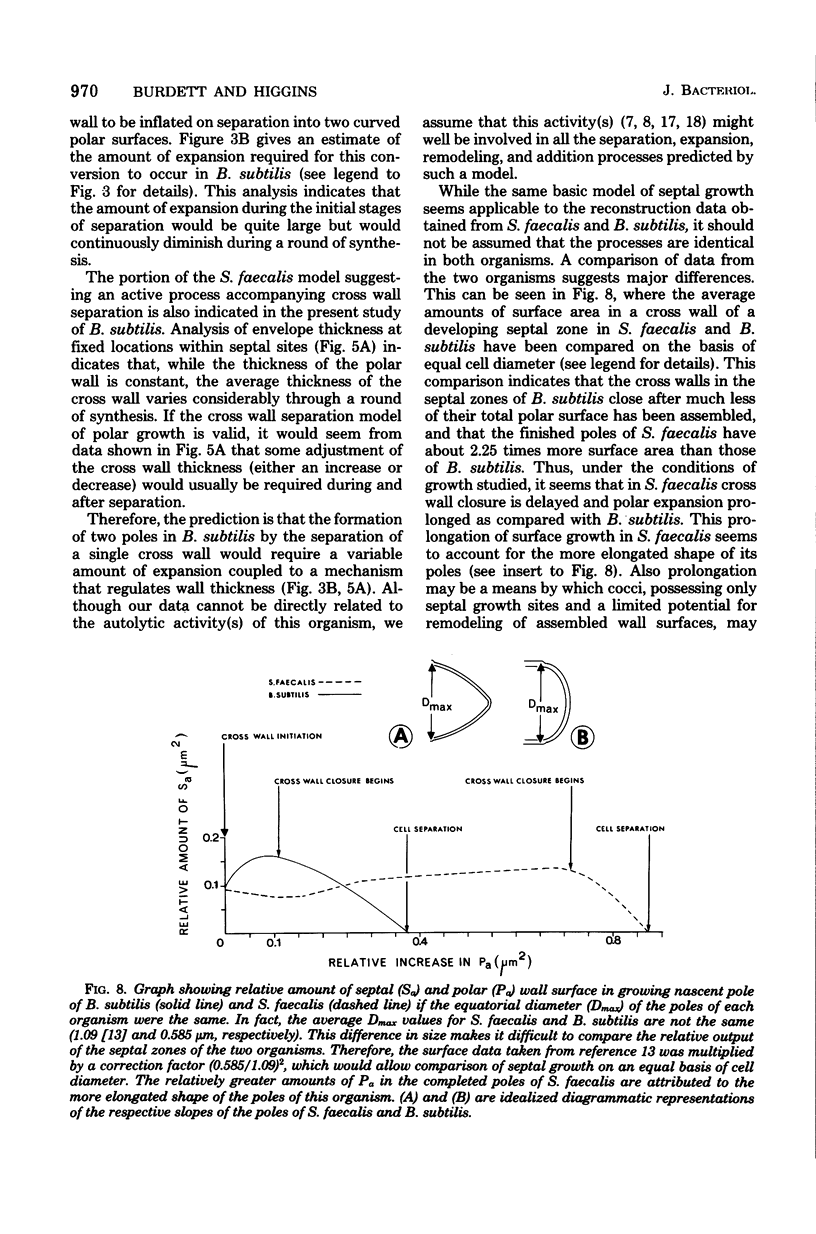
The septal growth of Bacillus subtilis 168/s has been studied by making a number of observations from thin sections of cells from exponentially growing cultures. The process was initiated by the formation of a new cross wall under a preexisting layer of cylindrical wall. An annular notch appeared to cut through the overlying wall and presumably allowed the cross wall to split into two layers of peripheral wall. During this initial notching process, two raised bands of wall material were produced which resembled those previously observed in morphological studies of Streptococcus faecalis. Through an improved fixation technique, it was possible to preserve the bands seen in B. subtilis to the extent that they were used as markers to study the subsequent stages of septal growth. These stages included (i) the continued displacement of the two bands from the cross wall (as the two nascent polar surfaces enlarged and as the diameter of the cross wall decreased), (ii) the closure of the cross wall, and (iii) the final severance of the common cross wall connection between two completed poles. To study this process in a more quantitative manner, three-dimensional reconstructions of the envelope observed between pairs of the raised bands were made from axial thin sections of cells. The process of reconstruction was based on a technique by which x, y coordinates were taken from thin sections and were rotated around the cell's central axis. These reconstructions were used to estimate the surface area or volume of the reconstructed zones or their parts. A round of septal growth was then simulated by arranging 118 reconstructions in order of increasing surface area or volume. The topology of the process was studied by noting how various measurements of septal thickness, length, surface area, and volume varied as a function of increasing septal zone size. This analysis was based on several assumptions, of which three of the most important are: (i) the bands produced by the initial notching process are markers which separate septal from cylindrical wall growth; (ii) a septal zone observed between pairs of bands is made up of two nascent poles and a single cross wall; and (iii) as septal zones develop in terms of relative age they increase in size (volume or surface area) or amount of wall. The data suggested that the S. faecalis model of surface growth (in which polar growth occurs through a regulated constrictive separation and expansion of a cross wall) also seems applicable to the pattern of septal growth observed here for B. subtilis. This was indicated from measurements which showed that increases in the size of nascent polar surfaces were correlated with decreases in cross wall diameter. An explanation of these observations may be that decreases in cross wall diameter were due to a progressive splitting of the cross wall that removed surface from the outer circumference of the cross wall and converted it into new polar surface. Calculations further suggested that if the poles of B. subtilis were made by this model a sizeable and variable increase in surface area of the cross wall would also be required to convert these separating cross wall layers into two curved polar structures. Measurements of wall thickness taken from various locations within septal zones indicated that while the thickness of the polar wall of B. subtilis was constant over its surface, the width of the cross wall varied considerably during a round of synthesis. Again, one of the simplest explanations compatible with these observations and those previously made in S. faecalis is that the B. subtilis cross wall is brought to a constant thickness (possibly by remodeling or precursor addition) before or during separation. Although most observations made from the reconstruction of the septal zones of B. subtilis may fit the S. faecalis model of surface growth, differences in the pattern of septal growth were seen when the two organisms were compared. These have been discussed in terms of differences in the regulation of their respective septal growth sites and basic mechanisms of wall assembly and modification.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amako K., Umeda A. Bacterial surfaces as revealed by the high resolution scanning electron microscope. J Gen Microbiol. 1977 Jan;98(1):297–299. doi: 10.1099/00221287-98-1-297. [DOI] [PubMed] [Google Scholar]
- Boothby D., Daneo-Moore L., Higgins M. L., Coyette J., Shockman G. D. Turnover of bacterial cell wall peptidoglycans. J Biol Chem. 1973 Mar 25;248(6):2161–2169. [PubMed] [Google Scholar]
- Burdett I. D., Murray R. G. Septum formation in Escherichia coli: characterization of septal structure and the effects of antibiotics on cell division. J Bacteriol. 1974 Jul;119(1):303–324. doi: 10.1128/jb.119.1.303-324.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen E. L., Marquis R. E. Passive electrical properties of microorganisms. 3. Conductivity of isolated bacterial cell walls. Biophys J. 1968 May;8(5):536–548. doi: 10.1016/s0006-3495(68)86506-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K. L. Influence of cell-wall thickness on cell division: electron microscopic study with Bacillus cereus. Can J Microbiol. 1973 Feb;19(2):217–221. doi: 10.1139/m73-033. [DOI] [PubMed] [Google Scholar]
- Fein J. E., Rogers H. J. Autolytic enzyme-deficient mutants of Bacillus subtilis 168. J Bacteriol. 1976 Sep;127(3):1427–1442. doi: 10.1128/jb.127.3.1427-1442.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C., Rogers H. J. Autolytic enzymes in growth of bacteria. Nature. 1971 Jan 22;229(5282):272–273. doi: 10.1038/229272a0. [DOI] [PubMed] [Google Scholar]
- Higgins M. L., Daneo-Moore L., Boothby D., Shockman G. D. Effect of inhibition of deoxyribonucleic acid and protein synthesis on the direction of cell wall growth in Streptococcus faecalis. J Bacteriol. 1974 May;118(2):681–692. doi: 10.1128/jb.118.2.681-692.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Model for cell wall growth of Streptococcus faecalis. J Bacteriol. 1970 Feb;101(2):643–648. doi: 10.1128/jb.101.2.643-648.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Study of cycle of cell wall assembly in Streptococcus faecalis by three-dimensional reconstructions of thin sections of cells. J Bacteriol. 1976 Sep;127(3):1346–1358. doi: 10.1128/jb.127.3.1346-1358.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L. Three-dimensional reconstruction of whole cells of Streptococcus faecalis from thin sections of cells. J Bacteriol. 1976 Sep;127(3):1337–1345. doi: 10.1128/jb.127.3.1337-1345.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. C., Stokes E. Cell wall growth in Bacillus licheniformis followed by immunofluorescence with mucopeptide-specific antiserum. J Bacteriol. 1971 May;106(2):694–696. doi: 10.1128/jb.106.2.694-696.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. C., Tanner P. J., Stokes E. Cell-wall thickening in Bacillus subtilis. Comparison of thickened and normal walls. Biochem J. 1970 Nov;120(1):159–170. doi: 10.1042/bj1200159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley H. M. Layered distribution, according to age, within the cell wall of bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1139–1147. doi: 10.1128/jb.125.3.1139-1147.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley H. M. Turnover and spreading of old wall during surface growth of Bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1127–1138. doi: 10.1128/jb.125.3.1127-1138.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G. Synchronous cultures of Bacillus subtilis obtained by filtration with glass fiber filters. J Bacteriol. 1973 Nov;116(2):736–740. doi: 10.1128/jb.116.2.736-740.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt P. J. Cell wall thickness, size distribution, refractive index ratio and dry weight content of living bacteria (Staphylococcus aureus). Nature. 1970 Apr 18;226(5242):277–279. doi: 10.1038/226277a0. [DOI] [PubMed] [Google Scholar]
- de Chastellier C., Hellio R., Ryter A. Study of cell wall growth in Bacillus megaterium by high-resolution autoradiography. J Bacteriol. 1975 Sep;123(3):1184–1196. doi: 10.1128/jb.123.3.1184-1196.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]