Abstract
Most physiological effects of σ1 receptor ligands are sensitive to pertussis toxin, suggesting a coupling with cell membrane-bound G proteins. However, the cloning of the σ1 receptor has allowed the identification of an intracellular protein anchored on the endoplasmic reticulum. Here, we show, using the isolated adult guinea pig brainstem preparation, that activation of the σ1 receptor results in its translocation from the cytosol to the vicinity of the cell membrane and induces a robust and rapid decrease in hypoglossal activity, which is mediated by phospholipase C. The subsequent activation of protein kinase C β1 and β2 isoforms and the phosphorylation of a protein of the same molecular weight as the cloned σ1 receptor lead to a desensitization of the σ1 motor response. Our results indicate that the intracellular σ1 receptor regulates several components implicated in plasma membrane-bound signal transduction. This might be an example of a mechanism by which an intracellular receptor modulates metabotropic responses.
Keywords: respiratory activity, motor control
The existence of σ receptors has been inferred from a unique profile of pharmacological properties (1, 2). Highly selective σ drugs have suggested the existence of at least two different classes of σ receptors (2). The receptor termed σ1 binds (+)benzomorphans [e.g., (+)N-allylnormetazocine [(+)SKF-10,047] and (+)pentazocine (PTZ)], 1,3-di-tolyl-guanidine (DTG), the antipsychotic haloperidol, and N-[2-(3,4-dichlorophenyl) ethyl]-N-methyl-2-(dimethylamino)ethylamine (BD-1047) with high affinity, and σ2 receptor binds (+)benzomorphans with low affinity and DTG, haloperidol, BD-1047, and 1[2-(3,4-dichlorophenyl)ethyl]-4-methylpiperazine (BD-1063) with high affinity. Cloning of the σ1 receptor identified an intracellular (28-kDa) protein (3–5), allowing us to study its mechanism of action with regard to the clinical relevance of σ1 ligands currently developed for the treatment of affective and memory disorders (6, 7).‖ The σ1 receptor is a 223-aa protein that has a single, putative transmembrane segment anchored in the endoplasmic reticulum membrane (3–5). These characteristics, although alleviating the controversy on the σ1 receptor molecular reality, do not fit with its currently proposed mechanisms of action on potassium conductances and coupling with heterotrimeric membrane-bound G proteins (8–10).
Autoradiographic studies performed in mammalian brain provide evidence that the highest density of σ1 receptors is in brainstem motor function-related nuclei (1). We thus have used the adult guinea pig brainstem preparation, which has the advantage of a persistent respiratory-related rhythmic motor activity on hypoglossal nerve (XII), independent of peripheral and cortical inputs (11). Combining a pharmacological approach to the study of motor activity and biochemical characterization of the intracellular mechanisms involved in the motor response has allowed us to propose a new mode of recruitment for membrane-bound signal transducers by the intracellular σ1 receptor.
MATERIALS AND METHODS
Electrophysiological Assessment.
One hundred and thirty-five isolated brainstem preparations from young adult male guinea pigs (150–250 g) were used (11) in accordance with the institutional guidelines that follow national and European laws and policies (European Economic Community council directive 86/609). Hypoglossal spontaneous rhythmic motor activity (XII), which consisted of a single burst of action potentials followed by low-amplitude tonic activity, was monitored from hypoglossal and/or C1-C2 roots through a glass-suction electrode. The integrated XII activity was digitized to calculate the amplitude, burst frequency, and interburst and burst durations of the neural activity. Each of the σ ligands (PTZ, (+)SKF-10,047, DTG, haloperidol, BD-1047, and BD-1063) was perfused once for 3 min at concentrations ranging from 1 to 100 nM through the basilar artery cannula. Long-lasting (30-min) or successive 3-min perfusions every 20 min of σ ligands (10–100 nM) were carried out in a second series of experiments. To assess whether phospholipase C (PLC) and protein kinases were involved in the σ1 receptor-mediated effect and subsequent neural desensitization, PLC and protein kinase inhibitors were perfused through the catheter before selective σ1 ligands. Note that 3-[1-[3-(dimethylamino)propyl]-1H-indol-3yl]-4-(1H-indol-3yl)-1H-pyrrole-2,5-dione (GF-109,203x) (50 nM for 5 min) was preadministered before the PLC inhibitor (U-73, 122; 300 nM) or the σ1 antagonist [N,N-dipropyl-2-[4-methoxy-3-(211phenylethoxy) phenyl]-ethylamine monohydrochloride (NE-100); 100 nM] to prevent desensitization.
Autoradiographic and Western Blot Assessment.
Six Guinea pig brainstems (11) were perfused with Krebs–Ringer’s solution plus 32P (orthophosphate H3PO4; specific activity, 2 MBq/ml; ICN) for 45 min via the artery. GF-109,203x was (n = 3) or was not (n = 3) added simultaneously to [32P] to the Krebs–Ringer’s solution 15 min before PTZ (100 nM, 30 min). The brainstem then was processed as described (22). Western blots were stained with the polyclonal antibody raised against the cloned isoform of the guinea pig liver σ1 receptor in the presence or absence of the synthetic peptide anti-PBP45 (pbp45, 0.1 mg/ml) directed against the σ1-binding site [kindly donated by H. Glossmann and F. Moebius, Institut für Biochemische Pharmakologie, Innsbruck, Austria (3)].
Reverse transcription–PCR experiments were performed from mARN preparations purified from the guinea pig liver or brainstem. The primers were designed to amplify the total coding sequence of the guinea pig σ1 receptor according to the GenBank report (accession no. Z66537). The primers were 5′-CGAAGTGATGCAGTGG-3′ for the sense and 5′-GGTCAAGGGTCTTTGCCG-3′ for the antisense. The PCR was carried out with the following 30 cycles: 60°C, 1 min; 72°C, 1 min; and 94°C, 1 min.
Confocal Microscopy Techniques and Immunofluorescence Staining.
Ten guinea pig brainstems were prepared (11). After a 30-min perfusion of PTZ (100 nM, n = 6) to obtain desensitization or of Krebs–Ringer’s solution (control, n = 4), each brainstem was fixed for 30 min in 2% paraformaldehyde buffer and then blocked for 36 hr in sucrose at 4°C. For immunofluorescence staining, adjacent coronal sections (40-μm thick) were processed with specific antibodies directed against the guinea pig liver σ1 receptor (3) and the carboxyl-terminal portions of the conventional protein kinase C (cPKC) isoforms α, β1, β2, and γ (anti-rabbit; Sigma). Overnight incubation with each antibody (1/100) was followed with that of the rhodamine isomer goat anti-rabbit IgG (CY3-conjugated AffiniPure antibody; Jackson ImmunoResearch) for 2 hr. The rhodamine was excited by using a helium–neon laser (λ = 543 nm), and emission was measured on an LSM-410 laser-scan microscope (Zeiss) through a LP-570 filter.
Data Analysis.
Drug effects were expressed as the drug-induced relative increase in the interburst duration. All data are given as mean ± SEM for several preparations. Statistical significance was assessed by using ANOVA and Krustal–Wallis tests (P ≤ 0.01).
Drugs were dissolved in Krebs–Ringer’s solution. PTZ, BD-1047, and BD-1063 were gifts from W. D. Bowen (National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda); (+)SKF-10,047 was from F. J. Roman (Institut de Recherche Jouveinal, Fresnes, France); and NE-100 was from S. Okuyama (Taisho Pharmaceutical, Omiya, Japan). DTG and isoproterenol were purchased from Sigma; haloperidol was purchased from McNeil Laboratories; and the selective PLC inhibitor 1-[6-[[(17β)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione (U-73,122), the inactive analog of U-73, 122 1-[6-[[(17β)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-2,5-pyrrolidinedione (U-73, 343), and the protein kinase inhibitors 1-(5-isoquinolinesulfonyl)-2-methyl piperazine dihydrochloride (H-7), N-(2-guanidinoethyl)-5-isoquinolinesulfonamide hydrochloride (H-1004), GF-109,203x, and 12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo [2,3-a] pyrrolo [3,4-c] carbazole (Gö-6976) were purchased from Calbiochem.
RESULTS
Functional Identification of σ1 Receptor in the Hypoglossal Nucleus.
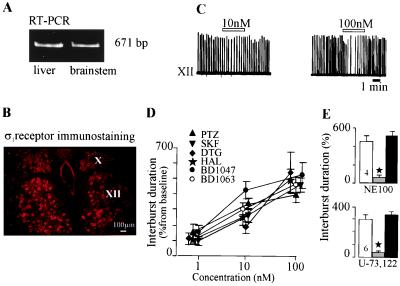
Fig. 1A shows that the σ1 receptor mRNA is expressed in the guinea pig brainstem and in the liver, the tissue of reference (3–5). Immunofluorescence mapping indicated the presence of the σ1 receptor in the motor hypoglossal nucleus (Fig. 1B) with a preferential location in the cell plasma membrane, in aggregated form (see Fig. 3).
Figure 1.
σ ligands modulate the respiratory activity. (A) Reverse transcription–PCR indicates the presence of the σ1 receptor mRNA (671 bp) in both brainstem and liver tissues. (B) Immunofluorescent staining of the σ1 receptor in both hypoglossal (XII) and vagus (X) nuclei. (C) Integrated XII activity before, during, and after a 3-min perfusion of (+)pentazocine (open bar). Note the increase in the interburst duration and the rapid recovery. (D) Concentration–response curves for the effects of σ ligands on the XII interval-burst duration. (E) NE-100 (100 nM) and U-73,122 (300 nM) blocked the inhibition of the XII by (+)pentazocine.
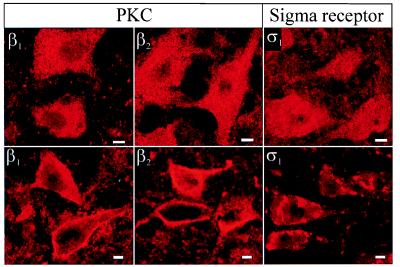
Figure 3.
Translocation from the cytoplasmic to the membrane compartments of β1 and β2 cPKC isoforms and of σ1 receptor in response to (+)pentazocine (100 nM). In basal conditions (Upper), β1 and β2 cPKC isoforms and σ1 receptor immunofluorescence staining were found in the cytoplasm. A long perfusion of (+)pentazocine (Lower) produced a selective translocation of β1 and β2 cPKC isoforms and of the σ1 receptor to the membrane vicinity. Neuronal images are single confocal sections. (Bars = 5 μm.)
Short (3-min) perfusions with the prototypic and selective σ1 ligands [(+)SKF-10,047 and PTZ] or with mixed σ1/σ2 drugs (DTG, haloperidol, BD-1047, and BD-1063) reduced the frequency of both hypoglossal (Fig. 1 C and D) and C1-C2 cervical (not shown) rhythmic activity, as indicated by an increase in the interburst duration. Burst duration and amplitude were not significantly affected. This effect was immediate, reversible, and concentration-dependent (Figs. 1 C and D). A fading of the effect on neural activity occurred only in response to successive, short perfusions of the σ1 ligands [PTZ and (+)SKF-10,047] (see below). We determined that the PTZ-induced XII response was mediated through the σ1 receptor by preventing or reversing the PTZ effect by NE-100 (100 nM), a selective σ1 antagonist (12) (Fig. 1E).
In previous pharmacological studies, the σ1 receptor has been linked to pertussis-sensitive heterotrimeric guanine nucleotide-binding (Gi/o) proteins (8–10) and reported to trigger inositol phospholipid hydrolysis (13) and the phosphorylation of proteins involved in exocytosis (14). To examine whether inositol lipids participate in σ1 receptor effects, we administered the selective PLC inhibitor U-73,122 (15) before (15 min) and during a short PTZ perfusion. Subsequent application of PTZ in the presence of U-73,122 had no effect on the XII in any of the preparations (n = 6/6; Fig. 1E). U-73,343, an inactive analog of U-73,122, did not affect the PTZ effect (n = 2/2). This indicates that the PTZ-induced response involves the activation of PLC. The specific biochemical action of U-73,122 [the uncoupling of heterotrimeric G proteins from the phospholipase C β isoform (15)] further supports the involvement of membrane-bound G proteins and, thus, a plasma membrane step in the cascade triggered by the σ1 receptor.
Desensitization of σ1 Receptors via PKC.
The above-mentioned fading response to selective σ1 ligands is suggestive of a desensitization process. Long (≈30 min) perfusions of PTZ resulted in a progressive rundown of its effect (n = 4/6; mean latency ± SE, 10.35 ± 1.7 min; Fig. 2A). A similar observation was made with (+)SKF-10,047 (n = 2/2).
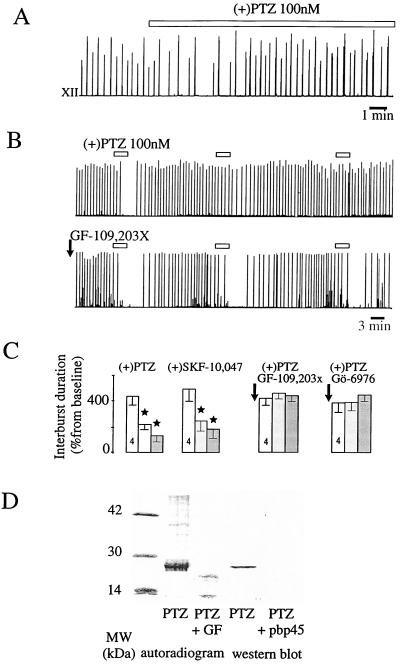
Figure 2.
Desensitization of the σ1 response via cPKC. (A) Desensitization of the σ1 effect on the integrated XII activity after 30 min of perfusion (open bar). (B) Desensitization induced by successive, short perfusions of PTZ (Upper) was prevented by a preperfusion of GF-109,203x (50 nM, 5 min; Lower). (C) Mean values of the effects of successive, short perfusions of σ1 in control conditions and after GF-109,203x and Gö-6976 perfusions, which prevented the PTZ-induced desensitization. (D) Autoradiogram after a long perfusion of PTZ revealed a phosphorylated, 28-kDa protein at the level of the σ1 receptor protein (revealed by Western blot analysis), which did not appear after GF-109,203x perfusion. In the Western blot, this band disappeared in the presence of the synthetic peptide anti-PBP45 (pbp-45) (3).
Fig. 2B illustrates that, in a protocol of successive, short (3-min) drug perfusions, the second application of the selective σ1 drug PTZ already induced desensitization. This could be quantified, as indicated on Fig. 2C. This desensitization lasted up to 5 hr.
The desensitization process may occur through different kinase cascades. H-7, a blocker of the regulatory and catalytic moieties (16) of protein kinases, was used. H-7 (1 μM) prevented the desensitization of the effect of both PTZ and (+)SKF-10,047, whereas it did not modify the effect of PTZ and (+)SKF-10,047 on the XII. Because H-7 also acts on various protein kinases, we tested H-1004 (1 μM), which inhibits the same protein kinases as does H-7, but is devoid of activity on PKC at the concentration used (17). The efficacy of H-1004 in preventing PKA activation was ascertained as it prevented the enhancement of XII frequency induced by isoproterenol (10 μM, n = 2/2). H-1004 had no effect on desensitization, indicating that PKC was the only protein kinase involved in the σ1 cascade. To investigate which member(s) of the PKC family was (were) involved, we used two selective and high-affinity PKC inhibitors that compete at the ATP-binding site, GF-109,203x and Gö-6976. Desensitization normally induced by successive perfusions of PTZ on XII was not seen after a 5-min application of either GF-109,203x [50 nM, which blocks both novel PKC and cPKC (18)] or Gö-6976 [50 nM, which selectively inhibits cPKC (19)] (Figs. 2 B and C). This indicates that cPKC, indeed, is involved in this process.
After PTZ-induced desensitization during orthophosphate H3[32P]O4 perfusion, autoradiograms revealed the phosphorylation of proteins, one of which was of the same molecular mass (28 kDa) as that expected for the cloned σ1 receptor (3–5) (Fig. 2D). Western blot analysis in the presence and absence of the synthetic peptide anti-PBP45 (3) further supports the notion that this protein corresponds to the σ1 receptor (Fig. 2D). The phosphorylation of this 28-kDa protein was selectively prevented by pretreatment with GF-109,203x, confirming that PKC was responsible for this phenomenon.
Translocation of cPKC and σ1 Receptor During Desensitization.
To identify the PKC isoforms activated during the σ1 desensitization, we visualized their location by immunofluorescence confocal microscopy by using specific antibodies directed against the four isoforms (α, β1, β2, and γ) of cPKC. Indeed, their activation follows their translocation from the cytoplasm to the plasma membrane (16). Fig. 3 illustrates that a long perfusion of PTZ induced such a translocation of the β1 and β2 isoforms without any change in the location of the isoforms α and γ.
We addressed the mismatch between the activation of the intracellular σ1 receptor and the subsequent recruitment of the cell membrane cascade (G proteins and PLC) by assessing the location of the σ1 receptor before and after its activation. Immunofluorescence staining under control conditions shows that the σ1 receptor is located in the cytoplasm (Figs. 1B and 3), which is in agreement with previous observations (3–5). After a perfusion of PTZ, the labeling shifted to the vicinity of the cytoplasmic membrane (Fig. 3).
DISCUSSION
This study shows that the intracellular σ1 receptor is expressed in the mammalian brainstem, where it modulates the hypoglossal motor activity within a few seconds. It acts through PLC, and its response desensitizes through cPKC. The shift of the σ1 receptor immunofluorescence staining is suggestive of its translocation from the cytoplasm to the membrane vicinity.
The present selective inhibition of the hypoglossal motor activity by several σ ligands is in keeping with their inhibitory effect, which was reported previously in in vivo and in vitro studies performed in the substantia nigra, the red nucleus, or brainstem motor nuclei, with equivalent doses administered either in situ, i.v., or i.p. (1, 20, 21). That all σ drugs were effective at doses within their respective IC50, as identified by binding studies (1, 2), and that a purported selective σ1 antagonist (12) (NE-100) blocked the action of (+)pentazocine in the present study provide additional support to the implication of σ1 receptors.
In neonate rat cardiomyocytes in culture (22), the effect of (+)pentazocine was also suggesting that the σ1 desensitization may also occur peripherally. The present desensitization of the hypoglossal motor response induced by nanomolar concentrations of the specific σ1 ligands also is consistent with their weak potencies to induce long-lasting (≈90-min) dystonia, in contrast to σ2 ligands (1, 20, 21). According to our data, it is likely, therefore, that after such latency, the σ1 response had already desensitized.
Implication of pertussis toxin-sensitive heterotrimeric Gi/o proteins has been proposed previously in the immediate in vivo and in vitro modulation by σ1 ligands of neuronal firing and potassium conductance (8–10). Here, we bring further support to this notion as we show that the intracellular steps of σ1 receptor activation result in the recruitment of the subsequent, calcium-dependent PLC/PKC cascade. However, the σ1 receptor differs (structurally and by its subcellular distribution) from conventional membrane-bound seven transmembrane-domain receptors known to interact with heterotrimeric G proteins and responsible for the rapid modulation of neuronal firing. Furthermore, the single-transmembrane-domain σ1 receptor also differs from cytoplasmic proteins/growth factors known to be active on monomeric G proteins and responsible for long-term metabolic activities (23). Altogether, this is suggestive of a novel mode of action of intracellular receptors. Because our results show a shift of the immunostaining of the σ1 receptor protein from the cytoplasm toward the cytoplasmic membrane in response to (+)pentazocine, it is probable that the activation of the σ1 receptor triggers its translocation from the endoplasmic reticulum to the cytoplasmic membrane to exert a subsequent, powerful, and rapid regulation of neural excitability through a heterotrimeric G protein/PLC/PKC cascade. This hypothesis is consistent with two very recent suggestions (24, 25) that a calcium efflux from the endoplasmic reticulum might contribute to the σ inducing effects.
These findings constitute evidence for a yet undescribed mode of rapid recruitment of membrane-bound, second-messenger cascade (here involving PLC and PKC) via activation of an intracellular, single-transmembrane-domain receptor (σ1 receptor). It is noteworthy that disturbances of the cranial nerve motor control occur spontaneously during schizophrenia in which σ1 binding is affected. Furthermore, involuntary tongue movements, a cardinal sign of tardive dyskinesia, are induced by many classical neuroleptics known to bind with high affinity to the σ1 receptor (1). Hence, the present findings at the cellular level may be related to these observations, which usually are ascribed to dopamine receptor activation despite weak evidence. Indeed, the mechanism described here might be of physiological and therapeutic importance in psychiatric, neurologic, digestive, immune, and endocrine systems, where the σ1 receptor is abundant and is known to regulate these functions (1, 6, 7).**
Acknowledgments
We thank P. Leclerc (Institut Fédératif de Recherche 21 “Hormones et Génétique” at Kremlin-Bicêtre) and G. Ghilini (Institut Alfred Fessard at Gif/Yvette) for their expertise in confocal microscopy imaging and immunofluorescence assays, respectively. We thank H. Glossmann and F. Moebius (Institut fur Biochemische und Pharmakologie, Innsbruck, Austria), A. S. Foutz and H. McLean (Institut Alfred Fessard, Gif sur Yvette, France), and J. M. Walker (Brown University, Providence, RI) for their helpful comments. F.P.M. was supported, in part, by the Amis des Sciences (Paris).
ABBREVIATIONS
- PLC
phospholipase C, cPKC, conventional protein kinase C
- (+)SKF-10,047
(+)N-allylnormetazocine
- PTZ
(+)pentazocine
- DTG
1,3-di-tolyl-guanidine
- BD-1047
N-[2-(3,4-dichlorophenyl) ethyl]-N-methyl-2-(dimethylamino)ethylamine
- BD-1063
1[2-(3,4-dichlorophenyl)ethyl]-4-methylpiperazine
- NE-100
N,N-dipropyl-2-[4-methoxy-3-(2,1,1phenylethoxy) phenyl]-ethylamine monohydrochloride
- U-73,122
1-[6-[[(17β)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexy]-1H-pyrrole-2,5-dione
- H-7
1-(5-isoquinolinesulfonyl)-2-methyl piperazine dihydrochloride
- H-1004
N-(2-guanidinoethyl)-5-isoquinolinesulfonamide hydrochloride
- GF-109,203x
3-[1-[3-(dimethylamino)propyl]-1H-indol-3yl]-4-(1H-indol-3yl)-1H-pyrrole-2,5-dione
- Gö-6976
12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo [2,3-a] pyrrolo [3,4-c] carbazole
- Gi/o proteins
guanine nucleotide-binding proteins
- XII
hypoglossal respiratory activity
Footnotes
Pande, A. C., Genève, J. & Scherrer, B. Congress of Collegium Internationale Neuro-Psychopharmacologicour, July 5–10, Glasgow, U.K., p. 30S, no. SM 0505.
Pande, A. C., Genève, J. & Scherrer, B. Congress of Collegium Internationale Neuro-Psychopharmacologicour, July 5–10, 1998, Glasgow, U.K., p. 30S, no. SM 0505.
References
- 1.Walker J M, Bowen W D, Walker F O, Matsumoto R R, deCosta B R, Rice K C. Pharmacol Rev. 1990;42:355–402. [PubMed] [Google Scholar]
- 2.Quirion R, Bowen W D, Itzhak Y, Junien J L, Musacchio J M, Rothman R B, Su T P, Tam S W, Taylor D P. Trends Pharmacol Sci. 1992;13:85–86. doi: 10.1016/0165-6147(92)90030-a. [DOI] [PubMed] [Google Scholar]
- 3.Hanner M, Moebius F, Flandorfer A, Knaus H G, Striesnig J, Kempner E, Glossmann H. Proc Natl Acad Sci USA. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kekuda R, Prasad P D, Fei Y J, Leibach F H, Ganapathy V. Biochem Biophys Res Commun. 1996;229:553–558. doi: 10.1006/bbrc.1996.1842. [DOI] [PubMed] [Google Scholar]
- 5.Seth P, Fei Y-J, Li H W, Huang W, Leibach F H, Ganapathy V. J Neurochem. 1998;70:922–931. doi: 10.1046/j.1471-4159.1998.70030922.x. [DOI] [PubMed] [Google Scholar]
- 6.Su T P, Junien J L. In: Sigma Receptors. Itzhak Y, editor. London: Academic; 1994. pp. 21–44. [Google Scholar]
- 7.Maurice T, Bockaert B P. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:69–102. doi: 10.1016/s0278-5846(96)00160-1. [DOI] [PubMed] [Google Scholar]
- 8.Monnet F P, Blier P, Debonnel G, deMontigny C. Naunyn-Schmiedeberg’s Arch Pharmacol. 1992;346:32–39. doi: 10.1007/BF00167567. [DOI] [PubMed] [Google Scholar]
- 9.Monnet F P, Debonnel G, deMontigny C. Brit J Pharmacol. 1994;112:709–715. doi: 10.1111/j.1476-5381.1994.tb13134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soriani O, Vaudry H, AiMei Y, Roman F, Cazin L. J Pharmacol Exp Ther. 1998;286:163–171. [PubMed] [Google Scholar]
- 11.Morin-Surun M P, Boudinot E, Sarraseca H, Fortin G, Denavit-Saubié M. Exp Brain Res. 1992;90:375–383. doi: 10.1007/BF00227251. [DOI] [PubMed] [Google Scholar]
- 12.Okuyama S, Imagawa Y, Ogawa S I, Araki H, Ajima A, Tanaka M, Maramatsu M, Nakazato A, Yamagushi K, Yoshida M, Otomo S. Life Sci. 1993;53:PL285–PL290. doi: 10.1016/0024-3205(93)90588-t. [DOI] [PubMed] [Google Scholar]
- 13.Bowen W D, Kirschner B N, Newman A H, Rice K C. Eur J Pharmacol. 1988;149:399–400. doi: 10.1016/0014-2999(88)90678-4. [DOI] [PubMed] [Google Scholar]
- 14.Brent P J, Herd L, Saunders H, Sim A T R, Dunkley P R. J Neurochem. 1997;68:2201–2211. doi: 10.1046/j.1471-4159.1997.68052201.x. [DOI] [PubMed] [Google Scholar]
- 15.Bleasdale J E, Thakur N R, Gremban R S, Bundy G L, Fitzpatrick F A, Smith R J, Bunting S J. J Pharmacol Exp Ther. 1990;255:756–758. [PubMed] [Google Scholar]
- 16.Tanaka C, Nishizuka Y. Annu Rev Neurosci. 1994;17:551–567. doi: 10.1146/annurev.ne.17.030194.003003. [DOI] [PubMed] [Google Scholar]
- 17.Hidaka H, Inagaki M, Kawamoto S, Sasaki Y. Biochemistry. 1984;23:5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- 18.Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, et al. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- 19.Martiny-Baron G, Kazanietz M G, Mischak H, Blumberg P M, Kochs G, Hug H, Marme D, Schächtele C. J Biol Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- 20.Matsumoto R R, Walker J M. Brain Res Bull. 1992;29:419–425. doi: 10.1016/0361-9230(92)90078-c. [DOI] [PubMed] [Google Scholar]
- 21.Kim M B, Bickford P C. Neuropharmacology. 1992;31:77–83. doi: 10.1016/0028-3908(92)90164-k. [DOI] [PubMed] [Google Scholar]
- 22.Ela C, Hasin Y, Eilam Y. Eur J Pharmacol. 1996;295:275–280. doi: 10.1016/0014-2999(95)00750-4. [DOI] [PubMed] [Google Scholar]
- 23.York R D, Yao H, Dillon T, Ellig C L, Eckert S P, McCleskey E W, Stork J S. Nature (London) 1998;392:622–626. doi: 10.1038/33451. [DOI] [PubMed] [Google Scholar]
- 24.Su T P, Hayashi T, Maurice T. Soc Neurosci Abstr. 1998;24:2055. [Google Scholar]
- 25.Vilner B J, Bower W D. Soc Neurosci Abstr. 1998;24:1594. [Google Scholar]