Abstract
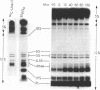
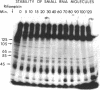
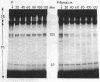
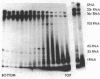
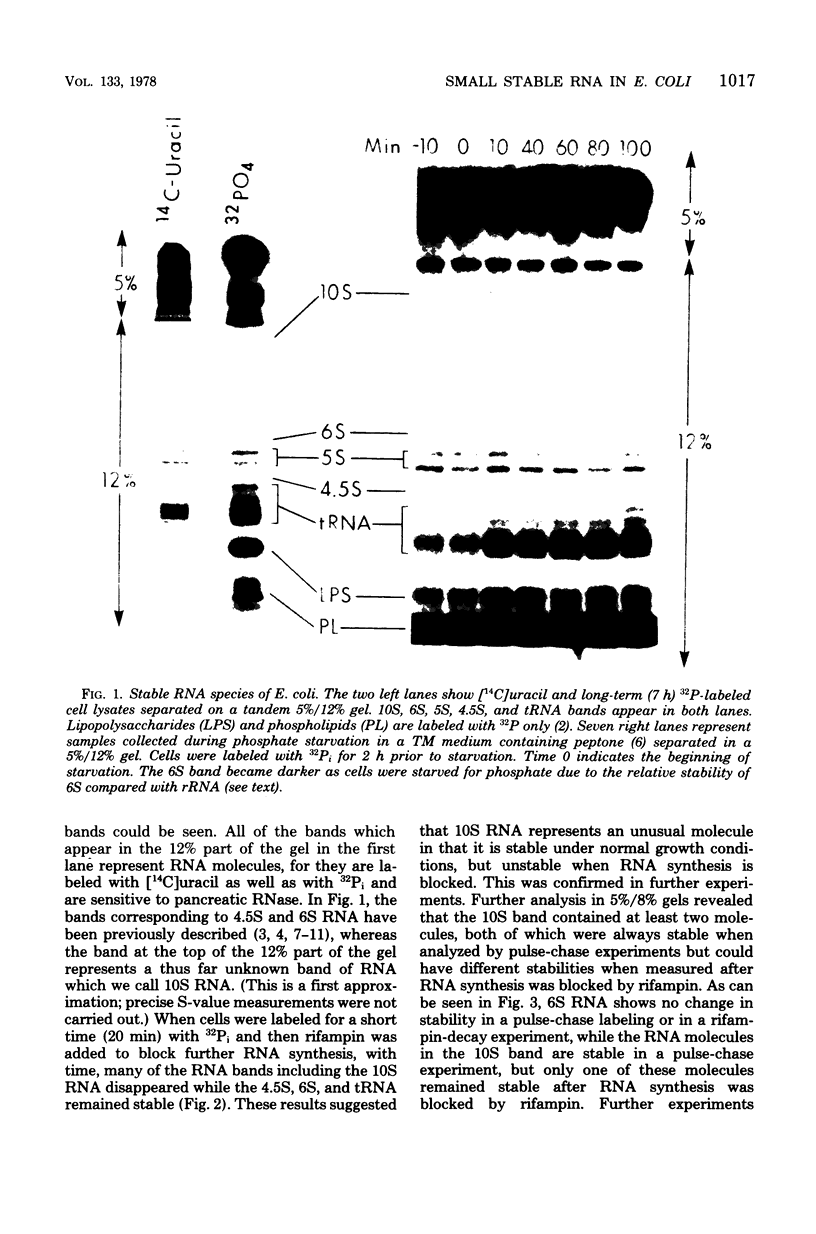
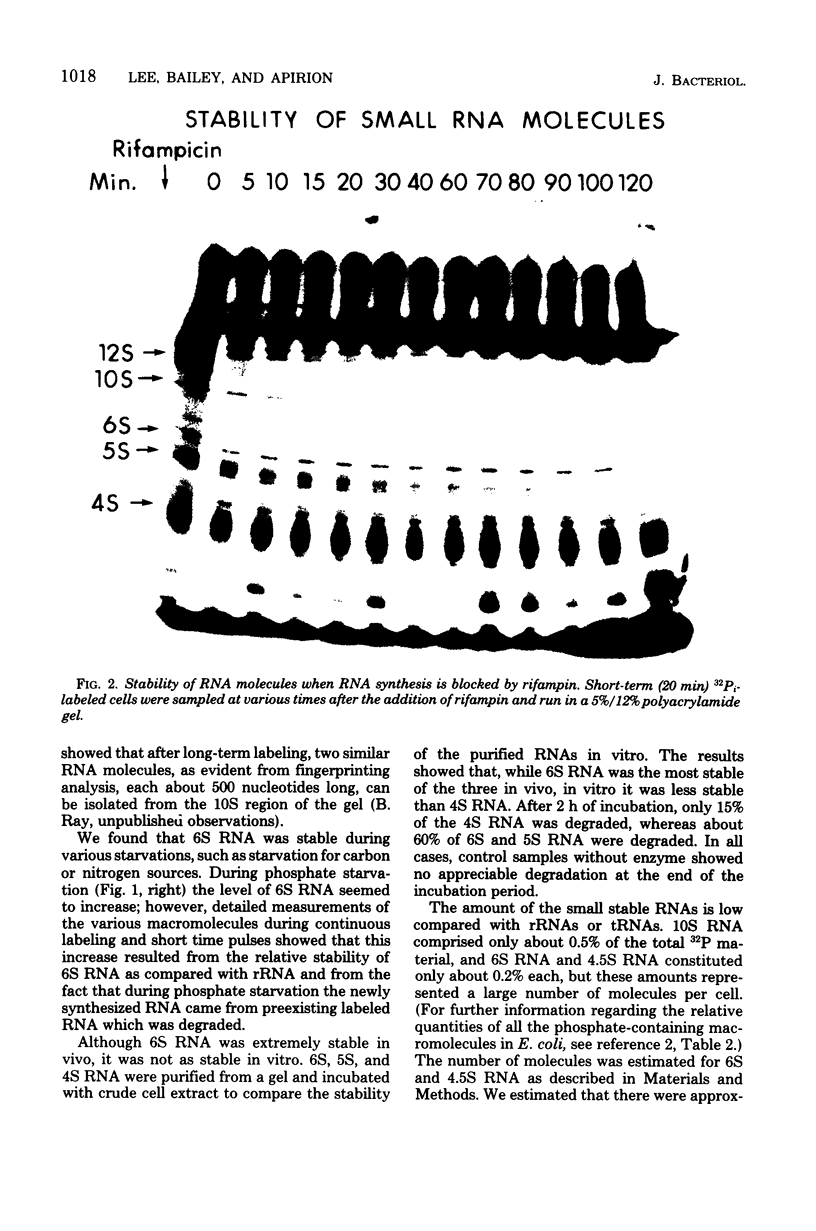
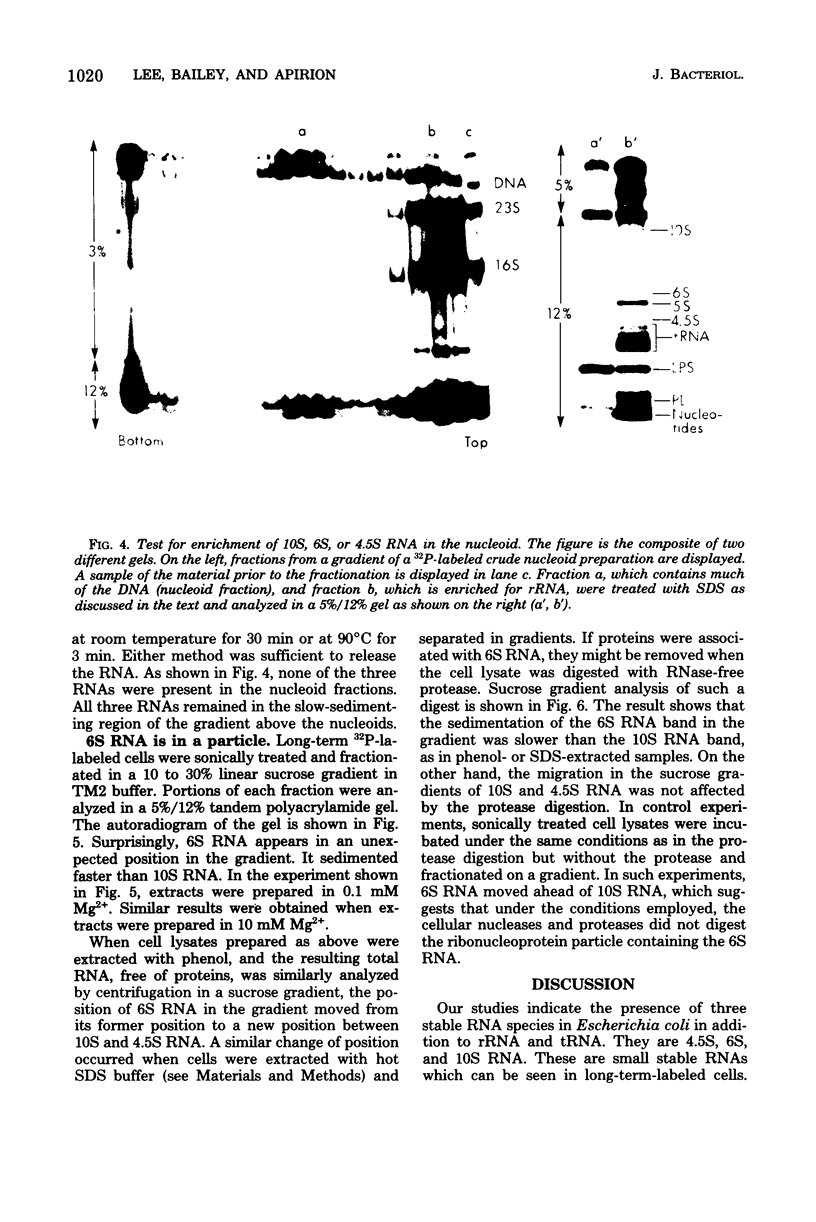
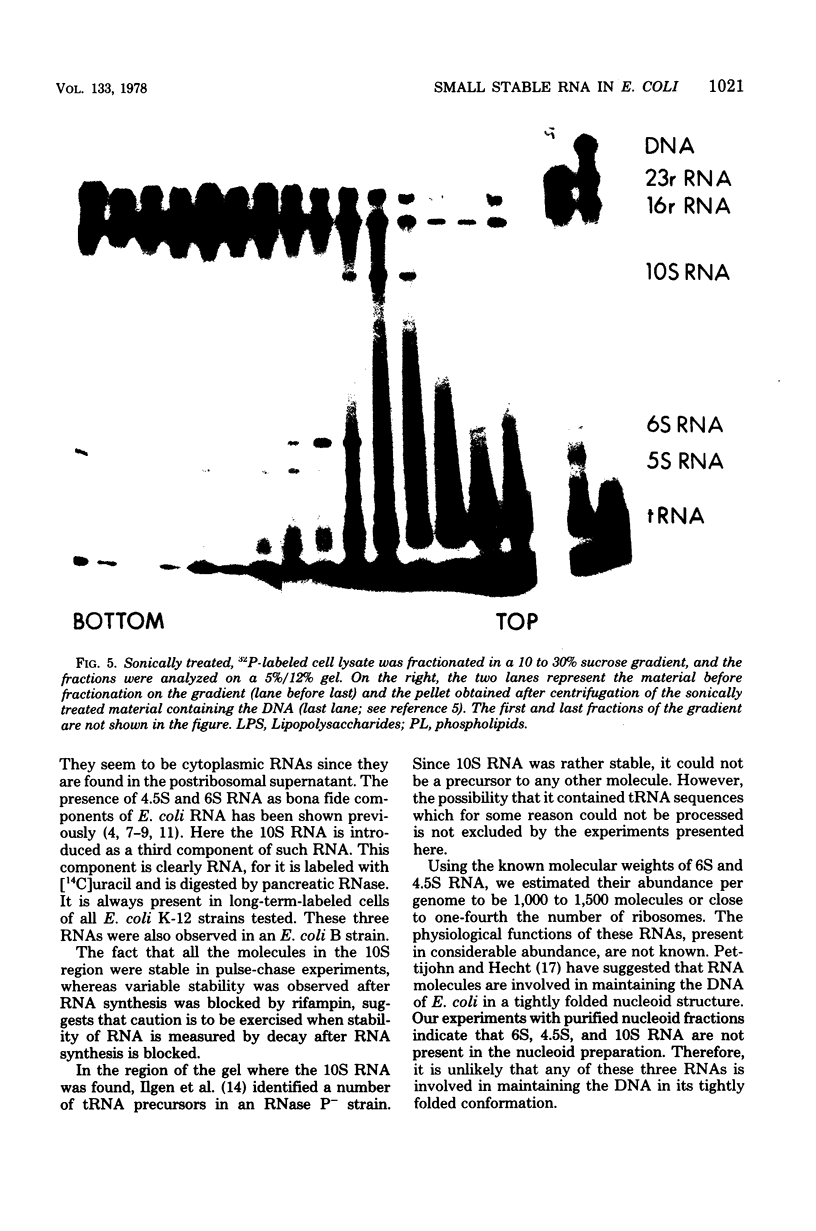
Small stable RNA molecules of Escherichia coli other than 5S (rRNA) and 4S (tRNA) were studied. Two of the molecules corresponded to 4.5S and 6S RNA, which have been reported previously. The third stable RNA molecule, 10S RNA, has not been described before. RNA labeled with 32Pi or [14C]uracil for a relatively long time, when separated in 5%/12% tandem polyacrylamide gels, displayed three bands corresponding to 10S, 6S, and 4.5S RNA in addition to rRNA and tRNA bands. These RNAs were stable in pulse-chase-labeling experiments. The amount of these RNAs was small, comprising only 0.2 to 0.5% of the total 32P incorporation. However, this amount represented a large number of molecules; for 6S and 4.5S, it was about 1,000/DNA molecule. These three RNAs were found in the postribosomal supernatant fraction. None of them was found in purified nucleoid fractions in which the tightly coiled DNA molecules were contained. Of these three RNAs, 6S RNA was unique in that it seemed to exist in a ribonucleoprotein particle. All these RNAs, as well as tRNA, were very stable in the cell under various physiological conditions. 5S RNA was less stable. On the other hand, purified 6S RNA was more susceptible than tRNA to cell nucleases when incubated with cell extracts, suggesting that, being in a particle, it is protected from cell nucleases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apirion D. Altered ribosomes in a suppressor strain of Escherichia coli. J Mol Biol. 1966 Apr;16(2):285–301. doi: 10.1016/s0022-2836(66)80173-0. [DOI] [PubMed] [Google Scholar]
- Bailey S. C., Apirion D. Identification of lipopolysaccharides and phospholipids of Escherichia coli in polyacrylamide gels. J Bacteriol. 1977 Jul;131(1):347–355. doi: 10.1128/jb.131.1.347-355.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell A. L., Garber R. L., Altman S. Nucleotide sequence and in vitro processing of a precursor molecule to Escherichia coli 4.5 S RNA. J Biol Chem. 1976 Dec 10;251(23):7709–7716. [PubMed] [Google Scholar]
- Brownlee G. G. Sequence of 6S RNA of E. coli. Nat New Biol. 1971 Feb 3;229(5):147–149. doi: 10.1038/newbio229147a0. [DOI] [PubMed] [Google Scholar]
- Caras M. A., Bailey S. C., Apirion D. The DNA of Escherichia coli can be displayed in a single band in polyacrylamide gels. FEBS Lett. 1977 Mar 1;74(2):283–286. doi: 10.1016/0014-5793(77)80865-x. [DOI] [PubMed] [Google Scholar]
- Goldstein J., Harewood K. Another species of ribonucleic acid in Escherichia coli. J Mol Biol. 1969 Jan;39(2):383–387. doi: 10.1016/0022-2836(69)90325-8. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Baillie D. L. Precursors of stable RNA accumulated in a mutant of E. coli. FEBS Lett. 1973 Aug 15;34(2):273–279. doi: 10.1016/0014-5793(73)80811-7. [DOI] [PubMed] [Google Scholar]
- Griffin B. E. Separation of 32P-labelled ribonucleic acid components. The use of polyethylenimine-cellulose (TLC) as a second dimension in separating oligoribonucleotides of '4.5 S' and 5 S from E. coli. FEBS Lett. 1971 Jun 24;15(3):165–168. doi: 10.1016/0014-5793(71)80304-6. [DOI] [PubMed] [Google Scholar]
- Griffin B. E. Studies and sequences of Escherichia coli 4.5 S RNA. J Biol Chem. 1975 Jul 25;250(14):5426–5437. [PubMed] [Google Scholar]
- Hindley J. Fractionation of 32P-labelled ribonucleic acids on polyacrylamide gels and their characterization by fingerprinting. J Mol Biol. 1967 Nov 28;30(1):125–136. doi: 10.1016/0022-2836(67)90248-3. [DOI] [PubMed] [Google Scholar]
- Ikemura T., Dahlberg J. E. Small ribonucleic acids of Escherichia coli. I. Characterization by polyacrylamide gel electrophoresis and fingerprint analysis. J Biol Chem. 1973 Jul 25;248(14):5024–5032. [PubMed] [Google Scholar]
- Ikemura T., Dahlberg J. E. Small ribonucleic acids of Escherichia coli. II. Noncoordinate accumulation during stringent control. J Biol Chem. 1973 Jul 25;248(14):5033–5041. [PubMed] [Google Scholar]
- Ilgen C., Kirk L. L., Carbon J. Isolation and characterization of large transfer ribonucleic acid precursors from Escherichia coli. J Biol Chem. 1976 Feb 25;251(4):922–929. [PubMed] [Google Scholar]
- Kaplan R., Apirion D. The fate of ribosomes in Escherichia coli cells starved for a carbon source. J Biol Chem. 1975 Mar 10;250(5):1854–1863. [PubMed] [Google Scholar]
- Meyhack B., Meyhack I., Apirion D. Colicin E3: a unique endoribonuclease. Proc Natl Acad Sci U S A. 1973 Jan;70(1):156–160. doi: 10.1073/pnas.70.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettijohn D. E., Hecht R. RNA molecules bound to the folded bacterial genome stabilize DNA folds and segregate domains of supercoiling. Cold Spring Harb Symp Quant Biol. 1974;38:31–41. doi: 10.1101/sqb.1974.038.01.006. [DOI] [PubMed] [Google Scholar]
- Raj N. B., Ro-Choi T. S., Busch H. Nuclear ribonucleoprotein complexes containing U1 and U2 RNA. Biochemistry. 1975 Oct 7;14(20):4380–4385. doi: 10.1021/bi00691a006. [DOI] [PubMed] [Google Scholar]
- SCHAECHTER M., MAALOE O., KJELDGAARD N. O. Dependency on medium and temperature of cell size and chemical composition during balanced grown of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]